The Community Pharmacy Survey on Patient Safety Culture (CPSOPSC) is a tool created by the Agency for Healthcare Research and Quality and used in the United States to assess the patient safety culture among community pharmacy workers. This survey has been adapted for use in hospital pharmacies in other countries. However, it has not yet been implemented in Spanish hospital pharmacies due to the lack of an applicable version in Spain. This project aims to adapt and reach a consensus on the CPSOPSC for its subsequent use as a tool to improve patient safety in hospital pharmacies in Spain.
MethodsThis non-clinical study will be developed in different phases: obtaining the necessary permissions, reviewing the literature to identify studies on the use of the CPSOPSC in hospital pharmacies, adapting the survey's questions to the sociocultural context, reaching a consensus on the questions using the Delphi-Rand/UCLA methodology with a panel of patient safety experts. These experts, who are hospital pharmacists, will evaluate the adapted survey in several rounds, using a Likert scale and telematic workshops to adjust the questions. Finally, a software application will be developed for the implementation, completion, and data management of the survey.
DiscussionAdapting the CPSOPSC to hospital pharmacies in Spain may be a useful tool for measuring the patient safety culture in this context. Through the Delphi-Rand/UCLA methodology, expert consensus and the relevance of the survey are ensured. Additionally, the creation of a computer application will facilitate data collection and analysis, promoting its use among professionals. The resulting survey from this project can identify specific needs and areas for improvement in Spanish hospital pharmacies, being useful for future actions aimed at improving patient safety.
El Community Pharmacy Survey on Patient Safety Culture (CPSOPSC) es una herramienta creada por la Agency for Healthcare Research and Quality y utilizada en Estados Unidos para evaluar la cultura de seguridad de paciente entre los trabajadores de las farmacias comunitarias. Este cuestionario ha sido adaptado para su utilización en el contexto de los Servicios de Farmacia Hospitalaria (SFH) en otros países. Sin embargo, aún no se ha implementado en los SFH españoles por no disponer de una versión aplicable en España. Se plantea este proyecto para adaptar y consensuar el CPSOPSC para su posterior utilización como herramienta de mejora en la seguridad del paciente de las farmacias hospitalarias en España.
MétodosEstudio no clínico a desarrollar en diferentes fases: Solicitud de permisos correspondientes, revisión de la literatura para identificar estudios sobre el uso del CPSOPSC en farmacias hospitalarias, adaptación de las preguntas del cuestionario al contexto sociocultural, consenso de las preguntas mediante metodología Delphi-Rand/UCLA con un panel de expertos en seguridad del paciente. Los expertos son farmacéuticos hospitalarios que evaluarán el cuestionario adaptado en varias rondas, utilizando una escala de Likert y talleres telemáticos para ajustar las preguntas. Finalmente, se desarrollará una aplicación informática para la implementación, cumplimentación y explotación de datos del cuestionario.
DiscusiónLa adaptación del CPSOPSC a las farmacias hospitalarias en España puede constituir una herramienta útil para medir la cultura de seguridad del paciente en este contexto. A través de la metodología Delphi-Rand/UCLA, se asegura un consenso experto y la relevancia del cuestionario. Por otra parte, la creación de una aplicación informática facilitará la recolección y el análisis de datos, promoviendo su uso entre los profesionales. El cuestionario obtenido tras este proyecto podrá identificar necesidades específicas y áreas de mejora en las farmacias hospitalarias españolas, siendo útil para futuras acciones de mejora en la seguridad del paciente.
Patient safety is a fundamental aspect of healthcare delivery, encompassing pharmaceutical services. Research and development of tools to assess and improve patient safety culture has increased in recent years.1,2 One such example is the Community Pharmacy Survey on Patient Safety Culture (CPSOPSC), a questionnaire developed in the United States by the Agency for Healthcare Research and Quality (AHRQ) to assess patient safety culture in community pharmacies.3 A systematic review of its published results showed that, although pharmacy staff highly value patient safety, more than half of the survey participants identified areas for improvement.4
Nevertheless, while the CPSOPSC has been translated into Spanish by AHRQ, it has never been used in the field of hospital pharmacy in Spain. In order to apply the questionnaire in a different setting, it is essential to adapt it to ensure that the questions and concepts accurately reflect the socio-cultural and organisational realities. Several authors have already adapted and used the CPSOPSC in hospital pharmacies in other countries. According to research conducted in Saudi Arabia and Kuwait, this tool is reliable and reveals areas for improvement in safety culture.5–7 An adaptation of the questionnaire showed mostly favourable attitudes towards patient safety culture in China, highlighting its importance and global application.8
The main objective of this study is to adapt and reach a consensus on the CPSOPSC questionnaire for implementation in hospital pharmacy services (HPS) in Spain. The Delphi-Rand/UCLA methodology, known for its effectiveness in achieving consensus among experts, will be used to ensure that the adaptations of the questionnaire are validated by experts in patient safety.9 In addition, the creation of a computer programme for the collection of the final questionnaire will improve the accessibility and use of the questionnaire by allowing data collection and analysis in an efficient, organised and convenient way.
This adaptation of the CPSOPSC questionnaire could contribute to improving patient safety culture by raising staff awareness, assessing the current status, identifying strengths and areas for improvement, examining trends in culture change over time, and evaluating the impact of initiatives and interventions implemented, thus promoting continuous action on patient safety.
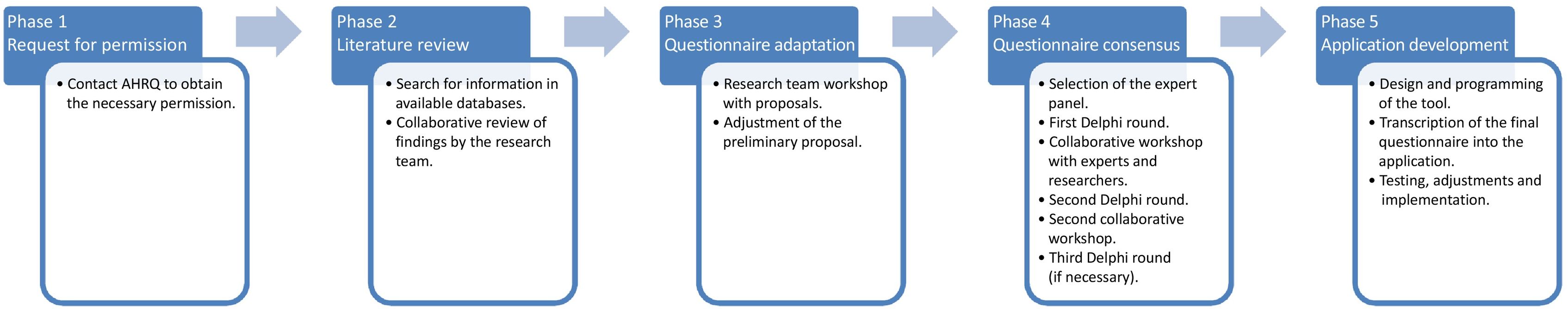
MethodsThis project is developed through the following non-clinical phases (Fig.1).
Phase 1. Request for permission: Contact AHRQ to obtain the necessary permission to modify the CPSOPSC questionnaire.
Phase 2. Literature review: Identification of research that has applied CPSOPSC in HPS. Project researchers will search PubMed and Web of Science databases. The keywords «Hospital Pharmacy» and «Community Pharmacy Survey on Patient Safety» will be used, with no restrictions on publication date or language. After the selection of relevant studies, data will be extracted in terms of country, methodology of questionnaire adaptation, participants, and conclusions. These results will be jointly reviewed by the research team and taken into account in the adaptation of the CPSOPSC.
Phase 3. Questionnaire adaptation: Socio-cultural adaptation of CPSOPSC questions through research team teleconference meetings, to obtain a preliminary proposal as a starting point for consensus.
Phase 4. Questionnaire consensus: The Delphi-Rand/UCLA9 methodology will be used to achieve consensus among a panel of hospital pharmacy experts who meet the following criteria: (1) Currently practising professionals. (2) At least 5&#¿;years of professional experience in the field of patient safety. (3) Relevant education in patient safety. (4) Maximum geographical distribution (professionals from different Autonomous Regions). A total of 12 experts will be selected to ensure a diversity of opinions and to facilitate interaction during the meetings.
Several rounds of evaluation will be carried out using the Redcap® application. In the first round, the experts will receive a preliminary version of the adapted questionnaire and rate each question for relevance and clarity using a 5-point Likert scale (1—question not appropriate and/or unnecessary, to 5—question very appropriate and/or necessary). They will be asked to provide additional comments or improvements to the questions if deemed necessary. After the first round, a telematic workshop will be scheduled with the researchers and the expert panel to present the results and assess the adaptation of the wording of the questions according to the panellists' input. During the workshops, the experts will not be aware of the ratings of the other panel members, thus ensuring that the process is blinded.
In the second round, the revised version of the questionnaire will be presented for further evaluation, with the aim of reaching a final consensus on the questions of doubtful eligibility or those included after evaluation in the first-round workshop. The results will be presented in a second telematic workshop. A third round of consensus will only take place if there is no agreement on any of the questions added after the first round.
Phase 5. Application development: This phase involves transcription of the finalised questionnaire into a computer application to facilitate its implementation and completion.
It will also include the design and testing of the application. The application will allow data to be collected, stored, and analysed efficiently and securely, ensuring accessibility, and use by HPS staff.
Statistical analysisThe analysis of the data obtained through the Delphi-Rand/UCLA9 methodology will focus on assessing the degree of consensus among the experts on each question of the questionnaire. Questions that receive a score of 4 or more from at least 75% of the panel members will be considered appropriate and will be included in the final questionnaire. For questions where consensus is not reached in the first round, the standard deviation or interquartile range of the responses will be calculated to measure the discrepancy. Questions that receive a score of 2 or less from at least 75% of the panel will be considered inappropriate and will be removed from the final questionnaire.
DiscussionThe availability of an adaptation of the CPSOPSC for use in the Spanish HPS may improve the culture of patient safety in this setting. After reviewing the literature, we found no evidence of the use of the questionnaire in this context, which highlights the importance and novelty of this study.
The CPSOPSC has been adapted in other countries. In Kuwait, Abdallah et al.5,6 used a methodology that included initial translation into Arabic, followed by review by a panel of bilingual experts and pilot testing to ensure comprehension and relevance of the questions. Similarly, in Saudi Arabia, Al-Surimi et al.7 applied a translation and back-translation methodology to ensure the linguistic equivalence of the questionnaire. Subsequently, a group of experts reviewed and adjusted the questionnaire to ensure its cultural and contextual appropriateness. Jia et al.8 followed the same approach in China with a review by a panel of experts and a pilot test to assess comprehension and appropriateness. In our case, we have the advantage that the CPSOPSC is translated into Spanish by AHRQ itself. Therefore, only a cultural adaptation and expert consensus using the Delphi-Rand/UCLA9 methodology will be necessary to obtain a questionnaire that reflects the specific realities and needs of our setting.
A noteworthy innovation of this study is the creation of a computer programme for the use of the questionnaire. This tool will allow the collection of data in an easy, simple, and secure way, enabling its analysis in a complete and accurate manner. In addition, the digitalisation of the questionnaire will help to promote its use among professionals. Another added benefit is that it will allow users to make both aggregated and anonymised comparisons with hospitals of similar complexity, allowing them to identify the strengths and weaknesses of pharmacy services, both individually and collectively. It is worth emphasising that there are published safety questionnaires that successfully use this type of application, such as the Self-Assessment Questionnaire on the Safe Use of Medicines in Hospitals, which reinforces its usefulness.10
There are some limitations that should be considered. The inherent subjectivity of the Delphi-Rand/UCLA9 methodology may influence the results, as it depends on the opinions of the panel members. The selection criteria for the experts were designed to reduce this variability. In addition, restricting participation in the expert panel to hospital pharmacists may introduce bias by excluding the perspectives of other professionals involved in HPS, such as nurses or pharmacy technicians. In the event of a blind break in the assessment of panel members, the event will be documented and potential bias will be assessed to take corrective action, such as revising the affected questions. On the other hand, the use of the software application, although innovative, may present a technological barrier to access. A user guide will be developed to minimise this limitation.
In conclusion, the adaptation of this already consolidated tool to the Spanish HPS can help to identify specific needs and areas for improvement. This could be useful for proposing future projects aimed at improving patient safety.
Ethical responsibilitiesThe rules of good clinical practice and Organic Law 3/2018, on the protection of personal data and guarantee of digital rights, will be applied to the conduct of the study. Personal data will be handled in accordance with current confidentiality regulations. The promoter is the sole owner of the study data and no data will be shared with third parties without his consent. The principal investigator and the designated statisticians will have access to the original data stored in the Redcap® server of the Spanish Society of Hospital Pharmacy. As a non-clinical study, it does not need to be reviewed by a research ethics committee; however, it will follow the relevant ethical standards.
FundingProject funded by the Spanish Hospital Pharmacy Foundation as part of the Grants to Working Groups of the Spanish Society of Hospital Pharmacy. Clinical Safety Working Group. Call 2023–2024.
Submission to conferencesOn behalf of the authors, I declare that the article is original and has not been published or is under consideration by any other journal, and I grant the journal Farmacia Hospitalaria the exclusive rights to edit, publish, reproduce, distribute copies, create derivative works in paper, electronic or multimedia formats, and include the article in national and international indexes or databases.
Statement on the use of generative and AI-assisted AI technologies in the drafting process
During the preparation of this paper, the authors used ChatGPT (OpenAI) to improve language and readability. After using this tool, the authors reviewed and edited the content as necessary and take full responsibility for the content of the publication.
CRediT authorship contribution statementJuan Manuel Rodríguez-Camacho: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. José Manuel Caro-Teller: Writing – review & editing, Validation, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. Sergio Plata-Paniagua: Writing – review & editing, Validation, Project administration, Methodology, Funding acquisition. Juan Alfredo Montero-Delgado: Writing – review & editing, Validation, Software, Formal analysis, Data curation. Inés Jiménez-Lozano: Writing – review & editing, Validation, Methodology. Carmen María Cuadros-Martínez: Writing – review & editing, Validation.
We would like to thank the expert panel for their disinterested participation in this project: Pilar Alonso Castell, Hospital Sant Joan de Déu, Barcelona. Helena Esteban Cartelle, Complejo Hospitalario Universitario de Santiago de Compostela. María José Fernández Megía, Hospital Universitario y Politécnico La Fe, Valencia. Andrea Lázaro Cebas, Hospital Universitario Santa Lucía, Cartagena. Victoria Lerma Gaude, Complejo Hospitalario Universitario de Albacete. Irene Mangues Befalluy, Hospital Universitari Arnau de Vilanova, Lleida. Marta Moro Agud, Hospital Universitario La Paz, Madrid. María José Otero López, Complejo Asistencial Universitario de Salamanca. Montserrat Pérez Encinas, Hospital Universitario Fundación Alcorcón, Madrid. Juan Francisco Rangel Mayoral, Complejo Hospitalario Universitario de Badajoz, Begoña Tortajada Goitia, Hospital Regional Universitario de Málaga, Montserrat Vilanova Boltò, Hospital Universitario Son Llàtzer, Palma.