To describe and organize the current information available on binary, ternary and/or quaternary mixtures used in opioid-free anesthesia (OFA), as well as their physicochemical stability, in order to facilitate its correct administration, optimize its use, and prevent potential effectiveness and safety issues.
MethodA systematic review of the literature on OFA was conducted in PubMed/Medline, Trissel, Micromedex, Lexicomp, www.ahfsdruginformation.com, ASHP's Extended Stability for Parenteral Drugs, and www.stabilis.org. Only articles published in English or Spanish until May 2020 and with access to full text were considered. MeSH terms used included: “drug incompatibility” AND “opioid-free anesthesia” AND “administration, intravenous” AND “dexmedetomidine” AND “lidocaine” AND “ketamine” AND “magnesium sulphate” OR “infusions, intravenous. A first search was carried out in PubMed/Medline that included OFA clinical cases. The results obtained were collected in a database. A second search was carried out on the incompatibilities of intravenous mixtures. Information was compiled on mutually-compatible/incompatible drugs, reference concentrations, stability time at room temperature (23 ± 2 °C) and under refrigeration (4 ± 2 ºC), type of administration recommended, and relevant results and conclusions. Two two-dimensional tables on the compatibility of each drug combination were created for administration as Y-site infusion or as a mixture in a single solution.
ResultsSeven hundred and eighty articles were identified, with the full text of 203 being accessed. A total of 4,762 cases treated with OFA protocols were chronologically collected from 32 different publications. Administration of two concomitant drugs was the most usual regimen (42.4%). The most frequently drugs were dexmedetomidine (25 studies), ketamine hydrochloride (25 studies) and lidocaine (14 studies). Compatibility/incompatibility data was collected for 11 drugs, associated to 7 pharmacological groups; compatibility with Y-site administration was found in 43 of 55 combinations (78.18%) and with integration into one single solution in 13 of 55 drug combinations (23.63%). None of the sources reviewed reported any adverse results related to potential pharmacological incompatibilities.
ConclusionsDespite the availability of multiple OFA protocols, few studies analyze the compatibility between binary drug mixtures. No information exists as yet regarding compatibilities in the context of ternary and quaternary mixtures.
Describir y estructurar la información actual disponible sobre mezclas binarias, ternarias y/o cuaternarias empleadas en una “anestesia libre de opiáceos”, así como su estabilidad fisicoquímica, para facilitar su correcta administración, optimizar su uso y prevenir posibles problemas de efectividad o seguridad.
MétodoRevisión sistemática de la literatura sobre anestesia libre de opiáceos en PubMed/Medline, Trissel, Micromedex, Lexicomp, AHFS Drug Information, Extended Stability for Parenteral Drugs y Stabilis Web. Artículos publicados en inglés o español hasta mayo de 2020 y con acceso a texto completo. Se emplearon los términos MeSH: “Drug Incompattbility” AND “Opioid Free Anesthesia” AND “Administraron, Intravenous” AND “Dexmedetomidine” AND “Lidocaine” AND “Ketamine” AND “Sulphate Magnesium” OR “Infusions, Intravenous”. Se realizó una primera búsqueda en PubMed/Medline incluyendo casos clínicos de anestesia general tipo anestesia libre de opiáceos. Los resultados obtenidos se estructuraron en una base de datos. La segunda búsqueda fue sobre incompatibilidades de las mezclas intravenosas. Se recogieron medicamentos compatibles/incompatibles; concentraciones de referencia; tiempo de estabilidad a temperatura ambiente (23 ± 2 °C) y en refrigeración (4 ± 2 °C); tipo de administración recomendada y resultados y conclusiones relevantes. Se crearon dos tablas bidimensionales de la compatibilidad de cada combinación de fármacos para la administración en Y o en mezcla en una sola solución.
ResultadosSe identificaron 780 artículos; se accedió al texto completo de 203. Se recogieron de forma cronológica los 4.762 casos tratados en 32 diferentes publicaciones con protocolos de anestesia libre de opiáceos. El uso de dos fármacos fue la asociación más frecuente (42,4%). Los fármacos más empleados fueron dexmedetomidina (25 trabajos), clorhidrato de ketamina (25 trabajos) y lidocaína (14 trabajos). Se recopiló información de compatibilidad/incompatibilidad de 11 medicamentos, asociados a 7 grupos farmacológicos, encontrándose compatibilidad en Y en 43 de 55 combinaciones (78,18%) y en mezcla en una sola solución en 13 de 55 combinaciones de fármacos (23,63%). En ningún trabajo publicado se expone algún tipo de evento adverso en relación con una posible incompatibilidad farmacológica.
ConclusionesExisten múltiples protocolos de anestesia libre de opiáceos, pero los estudios de compatibilidad entre las diferentes mezclas de fármacos empleadas son muy limitados cuando se trata de mezclas binarias, y no existe información en el caso de mezclas ternarias y cuaternarias.
Anesthesiology departments register a high incidence of errors in the administration of drugs, due to the gravity and complexity of anesthetic-surgical procedures’. During anesthetic processes, patients receive an average of fifteen drugs more or less simultaneously2. The morbimortality associated with these errors varies depending on the drug, the dose, the administration pathway and the characteristics of the patient3. In addition, there are multiple factors that alter the physicochemical compatibility of drugs when given together: pH, temperature, concentration, ion link, packaging, infusion time and exposure to light4. Different authors highlight the importance of performing compatibility studies of drugs, in order to obtain information that prevents adverse effects and guarantees the patient's safety5. The strategies for reducing the risk of incompatibility are well known, and include standardization of concentrations, reduction of drug mixtures in perfusion packs and/or pumps, reference to existing compatibility databases, use of multiple-lumen catheters or infusion lines, and/or filters in vascular lines6,7.
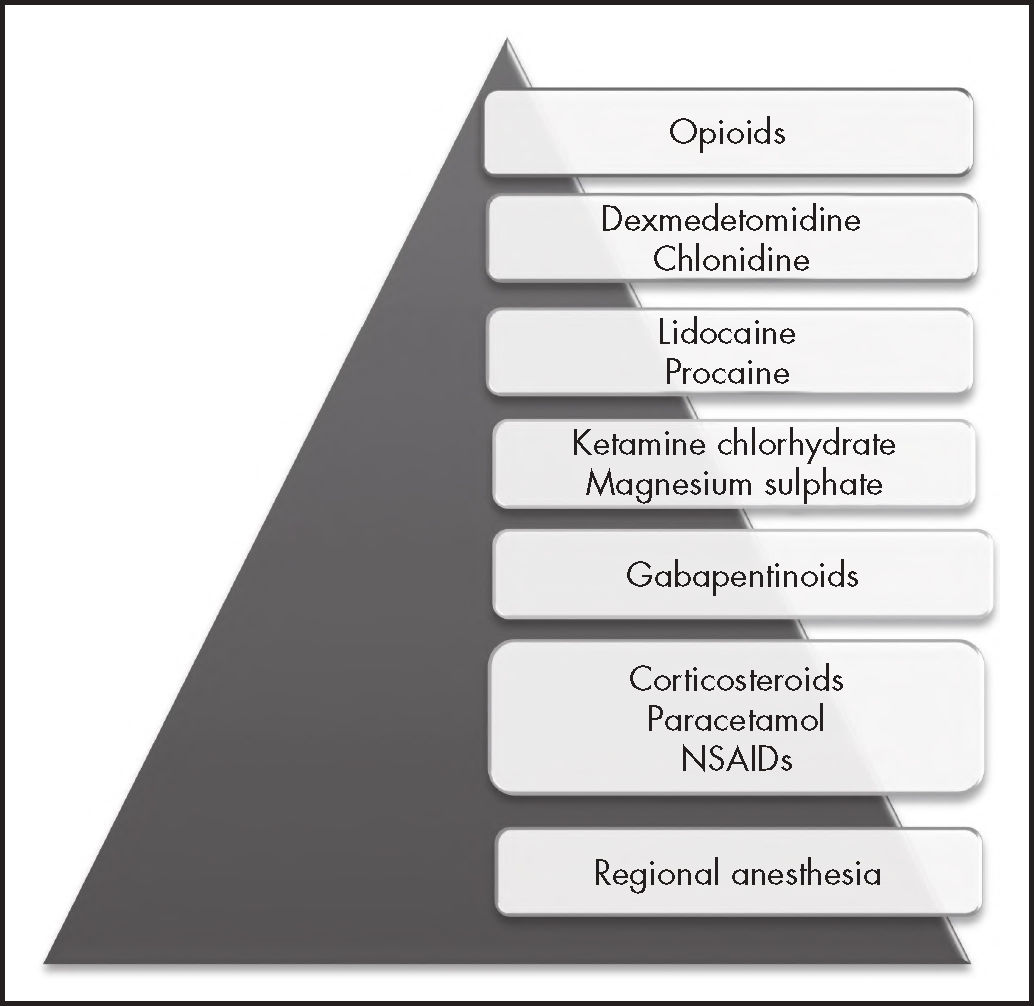
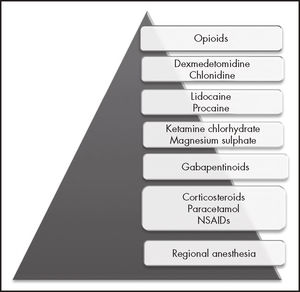
Among the drugs that are traditionally employed in anesthesiology we find opioids, which are associated with a high rate of adverse effects, and whose addiction-related issues are today a world emergency, the number of deaths from opioid overdose having increased exponentially in the last decade8. One of the pillars of primary prevention is the judicious use of these drugs during the perioperative period. In this regard, a new anesthe-siologic mode, known as opioid-free anesthesia (OFA) has become popular in recent years. OFA is a type of multimodal anesthesia that avoids the use of intraoperative opioids at systemic, neuraxial or intracavitary level, and is based on a series of drugs of a different nature (Figure 1)9. Multiple OFA protocols have been published, all of which include the use of a high number of drugs, leading to a dramatic reduction in requirements for postoperative analgesic opioids.
The safe use of drugs and the implementation of safe practices are a priority in healthcare. The present paper is aimed at offering an update on the current situation and systematizing the information available in different databases regarding the mutual compatibility/incompatibility of the different binary, ternary and/or quaternary mixtures employed in OFA protocols, with a view to facilitating its proper administration, optimizing its use and preventing potential issues of effectiveness or safety in its pharmacotherapy.
MethodsA review of the literature on previously published OFA studies was undertaken. An initial search of primary bibliography involving clinical cases of general anesthesia using OFA was conducted on PubMed/Medline. The search strategy was multiple and systematic, and reviewed by an IT specialist. The search only included the words “opioid-free anesthesia”, with no time limits and in all languages. The authors of medical and veterinary papers were subsequently contacted by e-mail, in order to establish whether any kind of mixture was used in their work, and –if so– whether they were aware of, and/or had used, stability studies. The information pertaining to the selected papers was organized in a database, including the following information: main author, journal, and year of publication; number of cases; type of surgical procedure; drugs that were assessed; mixture of drugs and postoperative analgesia. The second bibliographical search focused on studies of intravenous drug incompatibilities on Pub/Medline, Trissel, Micromedex, Lexicomp, www.ahfsdruginformation.com, ASHP's Extended Stability for Parenteral Drugs, and www.stabilis.org. Only articles published in English or Spanish up until May of 2020, with access to the full texts were included. The search was performed using the following MeSH terms: “Drug Incompatibility” AND “Opioid Free Anesthesia” AND “Administration, Intravenous” AND “Dexmedetomidine” AND “Lidocaine” AND “Ketamine” AND “Magnesium Sulphate” OR “Infusions, Intravenous”. Papers covering information on compatibility/incompatibility of intravenously administered drugs were included. The following data were collected: compatible/incompatible drugs; reference concentrations; stability time at room temperature (23 ± 2 °C) and under refrigeration (4 ± 2 °C); type of recommended administration; and main results and conclusions. Information on the pharmacological compatibility of Y-site administration was also collected, as well as on different anesthetic mixtures in single solution. The search was supplemented with articles classed as relevant and referenced in the papers found.
Finally, two bidimensional tables were created, indicating whether each combination of drugs is compatible or incompatible with Y-site administration or with administration in the form of a single-solution mixture. In cases where discrepancies arose regarding compatibility/incompatibility as per the different databases, the combinations were deemed incompatible, to avoid confusion.
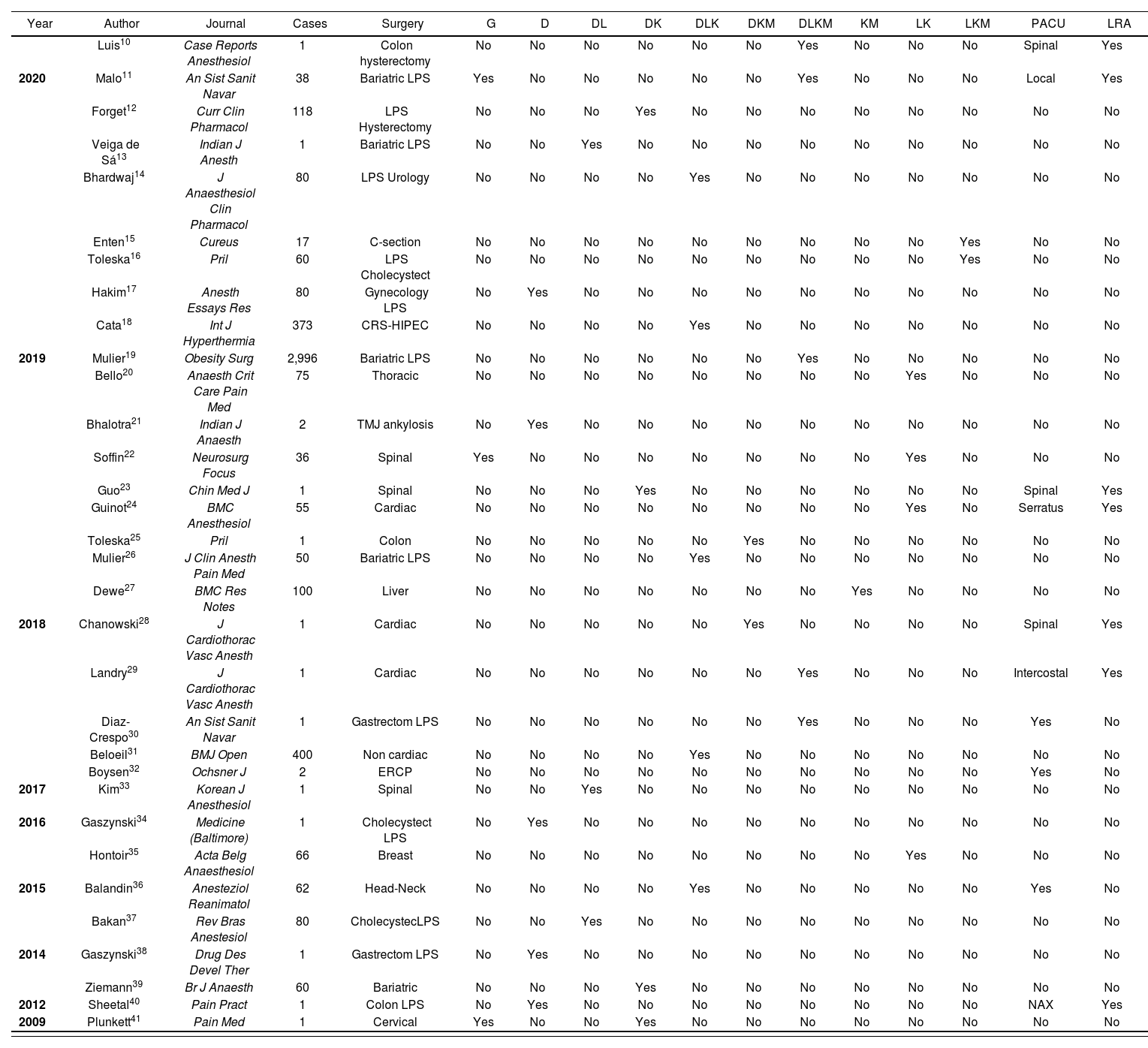
ResultsSeven hundred and eighty papers were identified using the first search strategy. Studies of clinical cases or case series were included. Duplicated or redundant papers were excluded, as were those published in languages other than English or Spanish, and a relevance analysis was performed by reviewing titles and abstracts. A total number of 203 papers allowing access to full text was obtained. Table 1 includes papers in which the type of surgery and the drugs employed are specified, and offers a chronological presentation of the 4,762 cases treated with OFA protocols in 32 different studies. The use of two drugs was the most frequent association (42.4%). The most frequently used drugs were dexmedetomidine (25 papers) and lidocaine (14 papers). The most frequent pharmacological combinations were the ternary mixture of dexmedetomidine, lidocaine and ketamine (5 papers) and the binary mixture of lidocaine and ketamine (4 papers). In only 12.5% of the studies (4/32) were these protocols maintained during the postoperative period, in the course of which some form of locoregional analgesia was the most commonly employed regime. In none of the studies was it explained whether the administration of multiple drugs took the form of mixtures in solution, in spite of the fact that several mixture protocols exist (Table 2). Of the 52 emails sent to the different authors, 27 received a response (51.9%). Only 2 authors of medical studies and 6 authors of veterinary studies replied that they had used ternary mixtures, but none of them were aware of compatibility/incompatibility of mixtures. None of the published papers describe any kind of adverse event due to potential pharmacological incompatibility.
Case reports on opioid-free anesthesia (OFA)
| Year | Author | Journal | Cases | Surgery | G | D | DL | DK | DLK | DKM | DLKM | KM | LK | LKM | PACU | LRA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Luis10 | Case Reports Anesthesiol | 1 | Colon hysterectomy | No | No | No | No | No | No | Yes | No | No | No | Spinal | Yes | |
| 2020 | Malo11 | An Sist Sanit Navar | 38 | Bariatric LPS | Yes | No | No | No | No | No | Yes | No | No | No | Local | Yes |
| Forget12 | Curr Clin Pharmacol | 118 | LPS Hysterectomy | No | No | No | Yes | No | No | No | No | No | No | No | No | |
| Veiga de Sá13 | Indian J Anesth | 1 | Bariatric LPS | No | No | Yes | No | No | No | No | No | No | No | No | No | |
| Bhardwaj14 | J Anaesthesiol Clin Pharmacol | 80 | LPS Urology | No | No | No | No | Yes | No | No | No | No | No | No | No | |
| Enten15 | Cureus | 17 | C-section | No | No | No | No | No | No | No | No | No | Yes | No | No | |
| Toleska16 | Pril | 60 | LPS Cholecystect | No | No | No | No | No | No | No | No | No | Yes | No | No | |
| Hakim17 | Anesth Essays Res | 80 | Gynecology LPS | No | Yes | No | No | No | No | No | No | No | No | No | No | |
| Cata18 | Int J Hyperthermia | 373 | CRS-HIPEC | No | No | No | No | Yes | No | No | No | No | No | No | No | |
| 2019 | Mulier19 | Obesity Surg | 2,996 | Bariatric LPS | No | No | No | No | No | No | Yes | No | No | No | No | No |
| Bello20 | Anaesth Crit Care Pain Med | 75 | Thoracic | No | No | No | No | No | No | No | No | Yes | No | No | No | |
| Bhalotra21 | Indian J Anaesth | 2 | TMJ ankylosis | No | Yes | No | No | No | No | No | No | No | No | No | No | |
| Soffin22 | Neurosurg Focus | 36 | Spinal | Yes | No | No | No | No | No | No | No | Yes | No | No | No | |
| Guo23 | Chin Med J | 1 | Spinal | No | No | No | Yes | No | No | No | No | No | No | Spinal | Yes | |
| Guinot24 | BMC Anesthesiol | 55 | Cardiac | No | No | No | No | No | No | No | No | Yes | No | Serratus | Yes | |
| Toleska25 | Pril | 1 | Colon | No | No | No | No | No | Yes | No | No | No | No | No | No | |
| Mulier26 | J Clin Anesth Pain Med | 50 | Bariatric LPS | No | No | No | No | Yes | No | No | No | No | No | No | No | |
| Dewe27 | BMC Res Notes | 100 | Liver | No | No | No | No | No | No | No | Yes | No | No | No | No | |
| 2018 | Chanowski28 | J Cardiothorac Vasc Anesth | 1 | Cardiac | No | No | No | No | No | Yes | No | No | No | No | Spinal | Yes |
| Landry29 | J Cardiothorac Vasc Anesth | 1 | Cardiac | No | No | No | No | No | No | Yes | No | No | No | Intercostal | Yes | |
| Diaz-Crespo30 | An Sist Sanit Navar | 1 | Gastrectom LPS | No | No | No | No | No | No | Yes | No | No | No | Yes | No | |
| Beloeil31 | BMJ Open | 400 | Non cardiac | No | No | No | No | Yes | No | No | No | No | No | No | No | |
| Boysen32 | Ochsner J | 2 | ERCP | No | No | No | No | No | No | No | No | No | No | Yes | No | |
| 2017 | Kim33 | Korean J Anesthesiol | 1 | Spinal | No | No | Yes | No | No | No | No | No | No | No | No | No |
| 2016 | Gaszynski34 | Medicine (Baltimore) | 1 | Cholecystect LPS | No | Yes | No | No | No | No | No | No | No | No | No | No |
| Hontoir35 | Acta Belg Anaesthesiol | 66 | Breast | No | No | No | No | No | No | No | No | Yes | No | No | No | |
| 2015 | Balandin36 | Anesteziol Reanimatol | 62 | Head-Neck | No | No | No | No | Yes | No | No | No | No | No | Yes | No |
| Bakan37 | Rev Bras Anestesiol | 80 | CholecystecLPS | No | No | Yes | No | No | No | No | No | No | No | No | No | |
| 2014 | Gaszynski38 | Drug Des Devel Ther | 1 | Gastrectom LPS | No | Yes | No | No | No | No | No | No | No | No | No | No |
| Ziemann39 | Br J Anaesth | 60 | Bariatric | No | No | No | Yes | No | No | No | No | No | No | No | No | |
| 2012 | Sheetal40 | Pain Pract | 1 | Colon LPS | No | Yes | No | No | No | No | No | No | No | No | NAX | Yes |
| 2009 | Plunkett41 | Pain Med | 1 | Cervical | Yes | No | No | Yes | No | No | No | No | No | No | No | No |
CRS-HIPEC: cyloreduclive surgery-hyperthermic intraperitoneal chemotherapy; D: dexmedetomidine; DK: dexmedetomidine and ketamine chlorhydrate; DKM: dexmedetomidine, ketamine chlorhydrate and magnesium sulphate; DL: dexmedetomidine and lidocaine; DLK: dexmedetomidine, lidocaine and ketamine chlorhydrate; DLKM: dexmedetomidine, lidocaine, ketamine chlorhydrate and magnesium sulphate; ERCP: endoscopic retrograde cholangiopancreatography; G: oral gabapentinoids; KM: ketamine chlorhydrate and magnesium sulphate; LK: lidocaine and ketamine chlorhydrate; LKM: lidocaine, ketamine chlorhydrate and magnesium sulphate; LRA: locoregional anesthesia; LPS: laparoscopic; NAX: neuraxial; PACU: postoperative maintenance of analgesia.
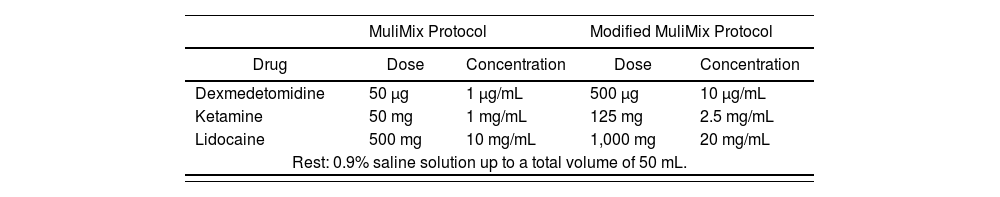
ISMA technique (infusion technique and maintenance of analgesia) or AAultimix protocol and Modified Mulimix protocol
| MuliMix Protocol | Modified MuliMix Protocol | |||
|---|---|---|---|---|
| Drug | Dose | Concentration | Dose | Concentration |
| Dexmedetomidine | 50 μg | 1 μg/mL | 500 μg | 10 μg/mL |
| Ketamine | 50 mg | 1 mg/mL | 125 mg | 2.5 mg/mL |
| Lidocaine | 500 mg | 10 mg/mL | 1,000 mg | 20 mg/mL |
| Rest: 0.9% saline solution up to a total volume of 50 mL. | ||||
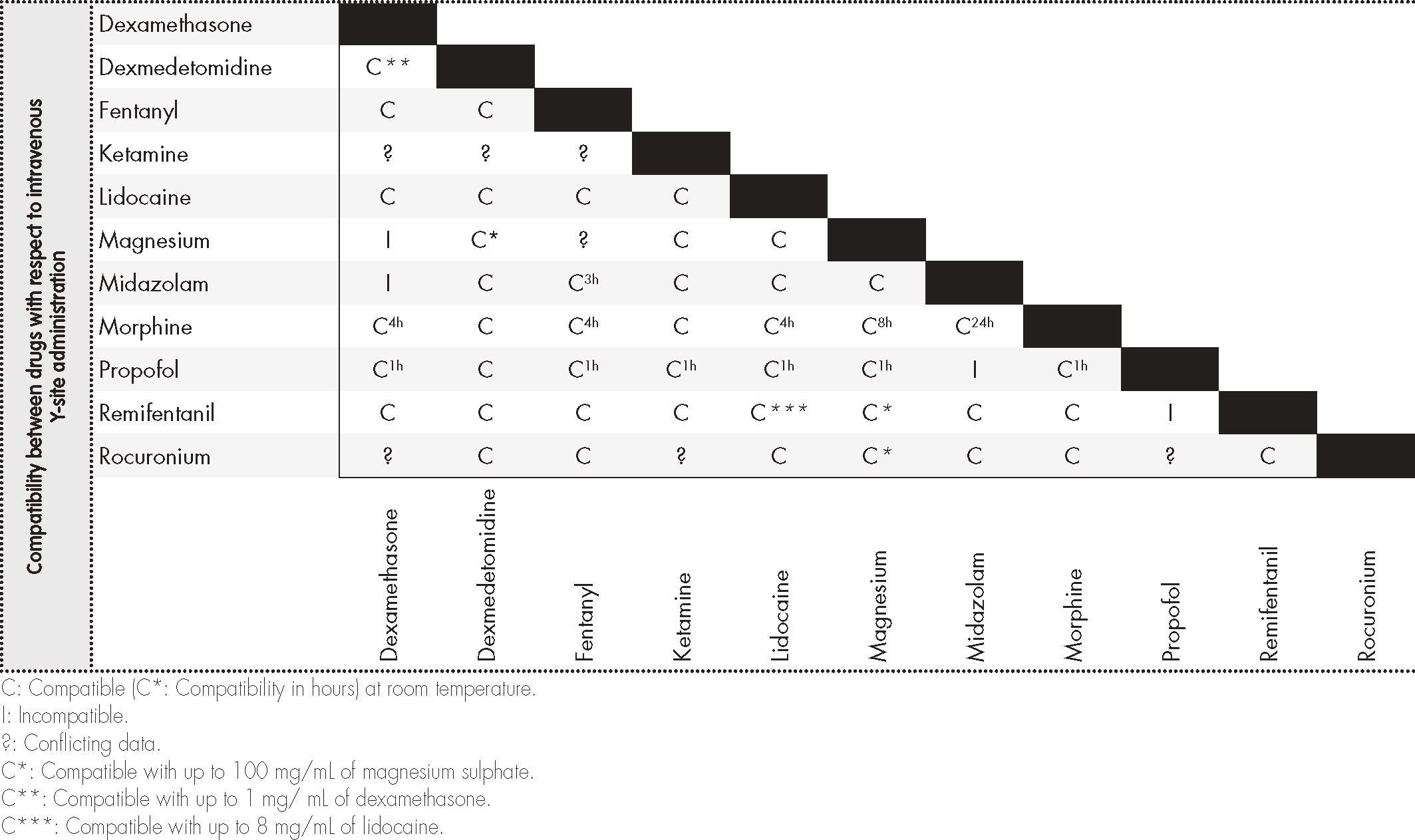
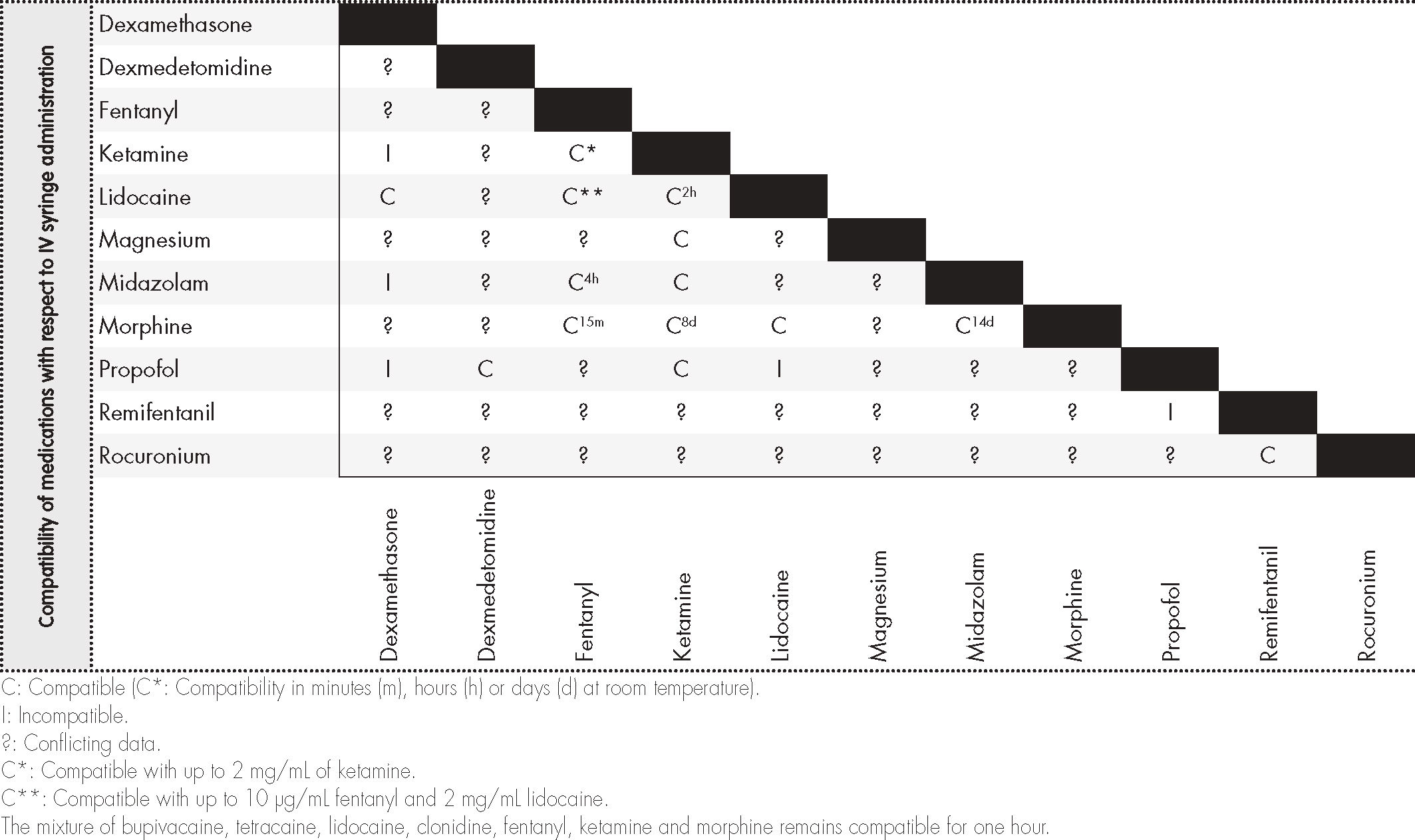
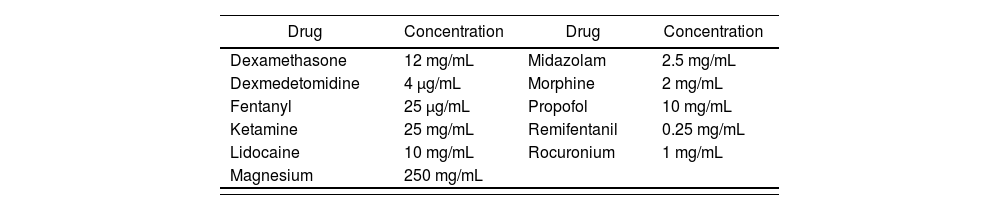
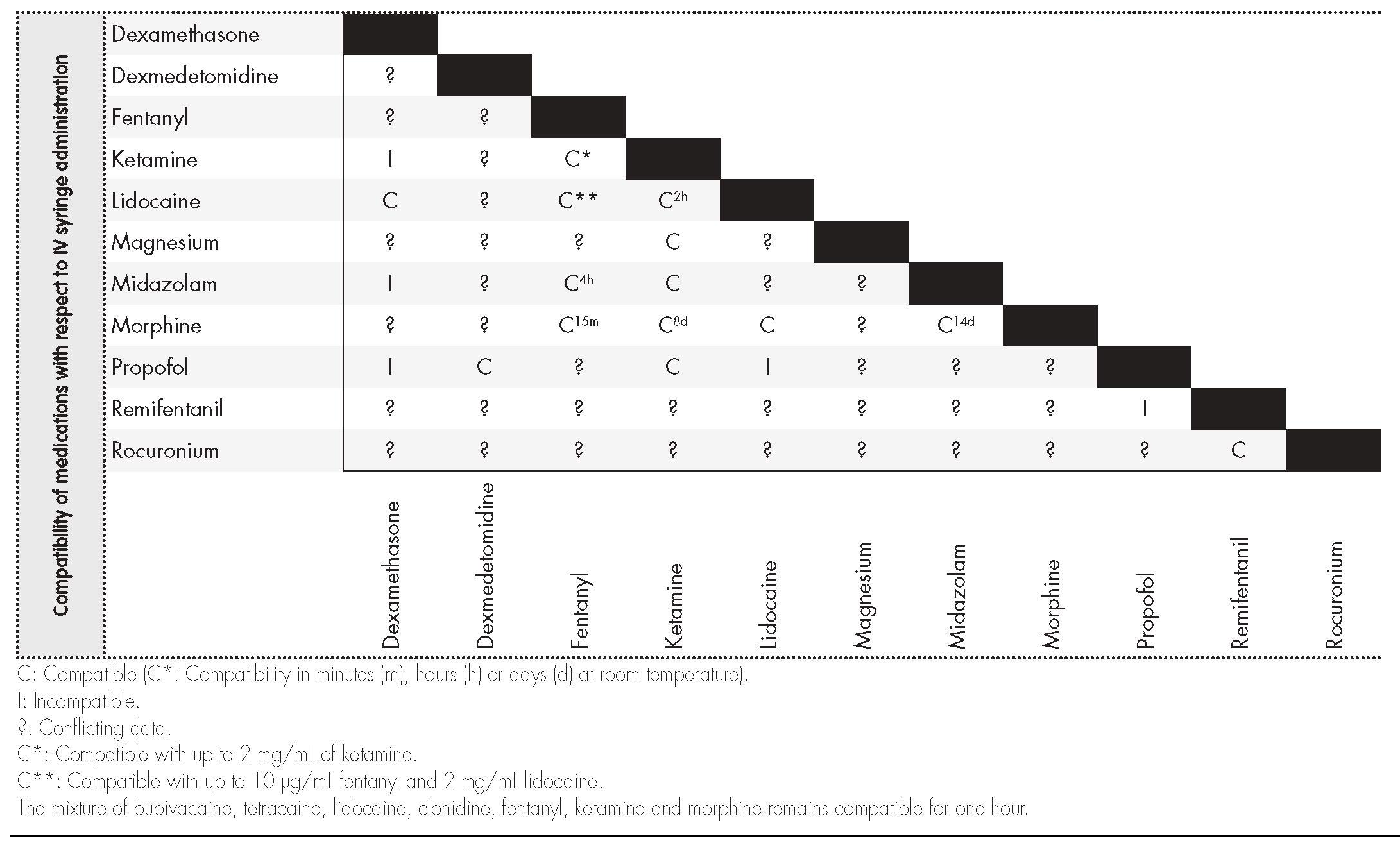
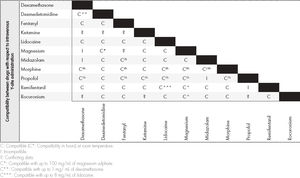
Information on compatibility/incompatibility was collected for 11 drugs, which were associated with 7 pharmacological groups. Table 2 show the main mixtures employed in OFA protocols. Table 3 shows the minimum concentration levels at which compatibility between drugs was studied, with subsequent reference being made to exceptions, in which the concentration was lower. Table 4 and 5 present summarized information, identifying the total amount of drugs on which information was collected regarding compatibility/incompatibility, in the form of Y-site infusion using the same line and continuous perfusion, as compared to other drugs that are commonly used in the OFA protocols. All of the compatibilities presented were recorded at room temperature.
Drugs analyzed and reference concentrations used
| Drug | Concentration | Drug | Concentration |
|---|---|---|---|
| Dexamethasone | 12 mg/mL | Midazolam | 2.5 mg/mL |
| Dexmedetomidine | 4 μg/mL | Morphine | 2 mg/mL |
| Fentanyl | 25 μg/mL | Propofol | 10 mg/mL |
| Ketamine | 25 mg/mL | Remifentanil | 0.25 mg/mL |
| Lidocaine | 10 mg/mL | Rocuronium | 1 mg/mL |
| Magnesium | 250 mg/mL |
Multiple OFA protocols have been published, and the use of this form of anesthesia is increasing every year, as shown in Table 1. The number of drugs commonly used in these protocols is high. Among the most frequently used non opioid agents are lidocaine, dexmedetomidine, dexamethasone and ketamine42, which are also part of the main mixture protocols (Table 2). One of these is MuliMix, used for induction and maintenance of anesthesia, which is prepared in a syringe and administered by means of direct infusion.
During the perioperative period the number of vascular points of access is usually limited, and the administration of drug mixtures by means of infusion or simultaneous (Y-site) administration is a common and often necessary practice. Pharmacological incompatibilities, physical or chemical in nature, may develop immediately after the mixture is prepared, sometimes without becoming evident2. Medical evidence suggests that the likelihood of mutual incompatibility increases with the number of associated drugs, and may vary depending on different circumstances2. Information on ternary and quaternary mixtures, or mixtures involving multiple drugs, is very limited43. In general, the preparation and administration of these drug mixtures requires knowledge regarding compatibility/incompatibility of the products involved. Micromedex, Lexicomp, Trissel, AHFS Drug Information, Extended Stability for Parenteral Drugs and Stabilis Web may be used indistinctly for the purpose of determining suitability.
The information on tables 4 and 56 of our paper can be used as a fast guide of reference to optimize and speed up the work of surgical anesthesiology and nursing teams, especially to avoid administering combinations that are not physicochemically compatible. In most cases there is not enough time to search the available databases, and it is much more useful to refer to the kind of document we present, in the form of a table, thereby minimizing the issues that result from infusing mutually incompatible drugs. These tables lack data on several drugs that are commonly used in OFA protocols, particularly the drugs dexmedetomidine and magnesium sulphate in continuous perfusion.
As an example, we may cite the work of Masaki et al.44, which shows that the popular and quite common addition of lidocaine to propofol to reduce injection-site pain (through the kallikrein-kinin and bradykinin system) produces an increase in oily vesicle diameter, and that this mixture is therefore physicochemically unstable over time and is associated with a risk of pulmonary embolism. In contrast, Gersonde et al.45 have shown that the mixture of propofol, dexmedetomidine and sufentanil is stable for its administration in continuous perfusion. Recently, Beiler et al.46 confirmed that a mixture of lidocaine (20 mg/mL) and ketamine (2.5 mg/mL), in a polypropylene syringe that is protected from the light, is stable for 48 hours at 28 °C.
We must stress the fact that propofol loses a great deal of its potency in PVC plastic packs, when diluted with glucose at 5%, but not in glass or polypropylene (PP) containers, and is also affected by exposure to light or storage at room temperature47. Furthermore, it is associated with many of the anesthetic incompatibilities in critical care48. In our opinion, it would be advisable to use halogenated anesthetics instead of propofol as a hypnotic agent in OFA protocols, given propofol's significant incompatibilities, the high number of intravenous drugs employed in these procedures, and the potential for pulmonary embolism and hepatic events.
Regarding OFA protocols, the work of Cohen et al. has revealed the high rate of errors in the dispensation, by pharmacy departments, of the drugs dexmedetomidine and dexamethasone (both of which are commonly used in OFA protocols) due to confirmation bias resulting from the similarity in the names of these drugs, and highlighted the need to develop scanning protocols for drug identification purposes, and to avoid storage of different agents in close proximity to each other49. The above author proposes the use of premixed dexmedetomidine, if available, to avoid mistakes, since its direct administration can cause cardiac arrest.
Despite the numerous intravenous mixtures that are prepared and administered, the present paper evidences the fact that there is a lack of information about the compatibility of the ternary and quaternary mixtures employed in current clinical practice, and not enough physicochemical studies of binary mixtures, which are the most frequently used. Ideally, the most common binary mixtures in single solution should be standardized, as should their methods of preparation in clean-rooms of hospital pharmacy departments, in accordance with Good Practice Guides50. This would guarantee the sterility of mixtures and their safe administration. Such an approach would undoubtedly increase the workload of pharmacy departments. We would propose considering low-risk preparations, with a 14-day period of microbiological stability under refrigeration, to alleviate the workload of nursing personnel while at the same time minimizing potential errors. An example would be the preparation and dispensation of a morphine hydrochloride and midazolam mixture, which is physicochemically stable for 14 days, or a morphine and ketamine mixture, which is stable for 8 days. However, we do consider that the lack of studies regarding most of the mixtures that are currently employed is a limitation in terms of assessing such preparation and storage operations by pharmacy departments. On the other hand, one of the significant limitations of the present paper lies in the fact that the most readily accessible databases –such as Medline– are heavily skewed towards publications from the English-speaking world.
In conclusion, obtaining access to the main databases on drug compatibilities should be a priority in anesthesiology departments, given the availability of different fast-reference resources, such as Micromedex, Trissel or Lexicomp, which make it possible to determine intravenous drug compatibility. The pharmaceutical industry does not generally recommend the simultaneous infusion of several different drugs using the same line, and this field should therefore be researched further, particularly as regards the drug mixtures that are in most common use in medicine. On the basis of the available medical evidence, we advise against the preparation of some binary mixtures and all ternary and quaternary mixtures in the different OFA protocols until physicochemical stability studies have been carried out.
FundingNo funding.
Conflict of interestNo conflict of interests.