Off-label use of medication is common in hospital clinical practice and should be applied together with follow-up of a healthcare treatment protocol and in compliance with a procedure which ensures that the patient is informed and that he or she provides informed consent.
A review of the literature on controlling 310 disorders showed that off-label use was indicated for 69 of them (22.3%) with the minimum required scientific evidence.
It would be useful for the Pharmacy and Therapeutics Committee to have a list of the disorders that can be controlled using off-label drugs, providing a reference to those disorders which must follow a healthcare treatment protocol.
A list of the mentioned characteristics is also useful for the hospital pharmacist for validating prescriptions, as it would provide a reference for assessing prescriptions which at first sight could seem questionable.
Finally, this list would be very useful if a search index of all the drugs by disorder was to be included in the Pharmacotherapeutic Guide. It would complement the usual indices which include active ingredients and specialities.
El uso de medicamentos en indicaciones no aprobadas en sus fichas técnicas (indicaciones off-label) es común en la práctica clínica hospitalaria y su aplicación debería ir unida al seguimiento de un protocolo terapéutico asistencial y al cumplimiento de un procedimiento que garantice la información y el consentimiento informado del paciente.
La revisión bibliográfica sobre manejo de 310 enfermedades puso de manifiesto que en 69 de ellas (22,3%) está descrito el uso de medicamentos en indicaciones off-label con un mínimo exigible de aval científico.
Disponer de esta lista de enfermedades en cuyo manejo se usan medicamentos off-label puede resultar de utilidad a la Comisión de Farmacia y Terapéutica al ofrecerle una referencia de aquellas enfermedades en las que sería prioritario monitorizar la existencia de protocolos terapéuticos asistenciales.
Una lista de las mencionadas características también es útil al farmacéutico de hospital en su función de validar prescripciones médicas, pues le ofrece una referencia para evaluar prescripciones que a primera vista pueden ser cuestionables.
Finalmente, tal lista es muy útil si se pretende añadir a la guía farmacoterapéutica del hospital un índice de búsqueda de medicamentos usados por enfermedades, que complemente los índices habituales de principios activos y especialidades.
Spanish Royal Decree 1015/2009,1 which regulates the availability of medications in special situations, involves the following three components:
- –
Compassionate use of medications in research: The use of a medication before it is authorised for commercial use in Spain in patients with chronic or severely debilitating diseases or life-threatening conditions that cannot be satisfactorily treated using authorised medications. The medication must be in the process of authorisation for commercialisation, or at least be subject to clinical trials.
- –
Use of medications under conditions other than those authorised: The use of a medication under conditions other than those authorised in its summary of product characteristics (which is referred to in the scientific literature as “off-label” use).
- –
Access to medications that are not authorised in Spain: The use of medications that are authorised in other countries, but not in Spain, when these do not fall within the definition of compassionate use of medication in research, as previously described.
With regard to access to medications in conditions different from those indicated in the summary of product characteristics, article 13.1 of the Royal Decree states that: The use of authorised medications for uses other than those established in the summary of product characteristics will be exceptions to the rule, and this will be limited to those cases in which authorised therapeutic alternatives are not available to any given patient, while respecting any restrictions that may have been established with regard to the prescription and/or dispensing of the medication and the health care protocol at the hospital. The prescribing physician must explain the need for using the medication in the clinical history, and must inform the patient as to the possible benefits and potential risks of treatment, and obtain informed consent as per Law 41/2002, of November 14.
Under these conditions, the use of a medication under circumstances other than those indicated in the summary of product characteristics has been established in common medical practice. Health care protocols also establish the possible alternatives for treatment and the order in which they may be used, and thus the attending physician must follow a protocol or series of restrictions for the prescription of these types of medications. Strict compliance is also needed with the law of patient autonomy with regard to obtaining informed consent.2–4
We should consider that the approved indications in the summary of product characteristics established by the European Medicines Agency (EMEA) or the Spanish Agency for Medicines and Healthcare Products (AEMPS, for its initials in Spanish) do not necessarily coincide with those approved by other health agencies such as the Food and Drug Administration (FDA).
The off-label use of a medication can range from being the first option to being the last line of treatment. The conditions for the use of each medication must be well defined, since, if a drug is a more effective and/or safer therapeutic alternative than other, it may be the treatment of choice for the disease in question, regardless of the presence of its use for said condition on the summary of product characteristics.
Information is very scarce regarding the off-label use of medications. Some studies report that certain drugs are frequently used off-label. In the USA, the use of medications not approved by the FDA has been estimated at 21% of all prescriptions, although this percentage can be higher for certain drugs, such as gabapentin (83%), amitriptyline (81%), oral dexamethasone (79%), isosorbide mononitrate (75%), rituximab (75%), risperidone (66%) and digoxin (66%). According to the scientific literature, the same restrictions or level of evidence are not required for the off-label use of all medications, and although the off-label use of isosorbide mononitrate and digoxin is sufficiently accredited, the same cannot be said for gabapentin, risperidone, or amitriptyline.5,6
On the other hand, the off-label use of drugs can create conflicts for the different expectations held by certain interested parties: health administrators, physicians, the pharmaceutical industry, and patients. In this manner, health administrators may question the need for paying for unapproved drugs in the treatment of a disease, physicians may desire the freedom to prescribe whatever drugs they deem fit, the pharmaceutical industry may seek to expand the market with new drugs and new uses for established drugs, and finally, patients desire safe, effective, and accessible medications.7
When evaluating the off-label inclusion of a drug in treatment protocols, several different important aspects must be taken into account8,9:
- –
If the drug in question is new, the scientific evidence that endorses its off-label use may not be based on high-quality trials, and only limited information may be available regarding the safety of using the drug.
- –
If the drug in question has been commercialised for some time, its off-label use may involve widely ranging circumstances, varying from cases in which, due to a reduced price of the drug, the clinical costs for obtaining a new indication are not offset by the benefits acquired from a hypothetical authorisation of the new use, to those cases of a presumed lack of interest in obtaining authorisation for a new indication, such as in the use of bevacizumab in the treatment of age-related macular degeneration.10–12
- –
In the case of the off-label use of drugs with established severe adverse reactions, patient safety requires special attention.
- –
The off-label use of high-cost medications requires reasonable expectations with regard to clinical results.
- –
In all cases, the off-label use of medications requires some minimum level of scientific evidence that endorses said use.
With the objective of evaluating the off-label use of medications, we reviewed recent editions of two different Spanish manuals, the Cliniguía13 (clinical guidelines) and Normas de actuación en urgencias14 (emergency medicine guidelines). They offer basic information regarding the management of different diseases in adults and are extensively used among physicians both in the primary care setting and specialised medicine. We also carried out a Medline literature search regarding the treatment of several different diseases, focusing on data compiled in clinical guidelines and recently published systematic reviews, and in their absence, publications that have been widely cited in medical literature.
We considered those drugs whose use is recommended for the treatment of certain diseases in the cited sources and is not included in the summary of product characteristics detailed in the EMEA or AEMPS.
For the inclusion of a drug in this context, we evaluated the available evidence for its off-label use, according to the following criteria:
- –
We did not include those drugs that the consulted medical texts mentioned as having a doubtful efficacy in the disorder in question.
- –
We did not include those drugs for which the consulted sources indicated the scarcity or deficiency of scientific literature endorsing their use for the disease in question.
- –
We did include drugs that have been recommended in all or most of the consulted sources.
- –
We did not consider off-label indications those diseases in which a proprietary drug did not include that particular indication but another drug exists (whether commercialised in Spain or not) with the same active ingredient for which the EMEA had approved said indication (for example, the indication for the treatment of sickle cell anaemia has not been approved for the brand name Hydrea®, but this indication has been approved by the EMEA for another proprietary drug that also contains hydroxyurea, Siklos®).
- –
We also did not consider off-label use those cases in which the drug is employed using the conditions of administration and dosage established in the summary of product characteristics for certain approved indications which constitute a symptom or a sub-pathology of the primary disease (for example, the use of propranolol in porphyria for the control of crises of tachycardia and hypertension, or drugs such as clozapine and quetiapine in Huntington's chorea for the treatment of associated psychosis).
- –
We did consider off-label use that, in the treatment of these sub-pathologies, the drug is administered at a dosage or form of administration different from that recommended in the summary of product characteristics (for example, omeprazole at high doses in continuous intravenous perfusion for upper gastrointestinal bleeding).
- –
We also considered off-label use that a drug is approved for any given indication by the FDA, but not by the EMEA or the AEMPS.
- –
For those drugs that had not been commercialised in Spain, we only considered off-label use to have occurred in those diseases that were not authorised as an indication in the summary of product characteristics from the country of origin.
We reviewed manuals and protocols published on the management of 310 different diseases/treatment circumstances corresponding to the following medical specialties: neurology/psychiatry: 52 (16.8%), endocrinology/metabolism/nephrology: 47 (15.2%), digestive tract: 33 (10.6%), rheumatology/traumatology: 30 (9.7%), cardiovascular: 26 (8.4%), dermatology: 23 (7.4%), haematology: 23 (7.4%), gynaecology: 20 (6.4%), emergency medicine/ICU: 18 (5.8%), and other (surgery, ophthalmology, ENT, pneumology, urology): 38 (12.3%).
In 69 of these diseases (22.3%), the off-label use of the drug was described.
The results detailed in our review are summarised in Tables 1–9. The column observations summarises the data that are especially relevant with regard to the off-label use of each drug. In addition to the bibliographic references, we mention the most relevant aspects of each use, i.e., the mechanism of action of the drug for the off-label indication, the line of treatment of the drug within the treatment plan, the approval of using the drug for a given pathology by the FDA (if the indication was not approved by the EMEA or the AEMPS), dosage (if the off-label indication is different from the approved uses), and any other aspect considered to be unusual in the off-label use of each drug.
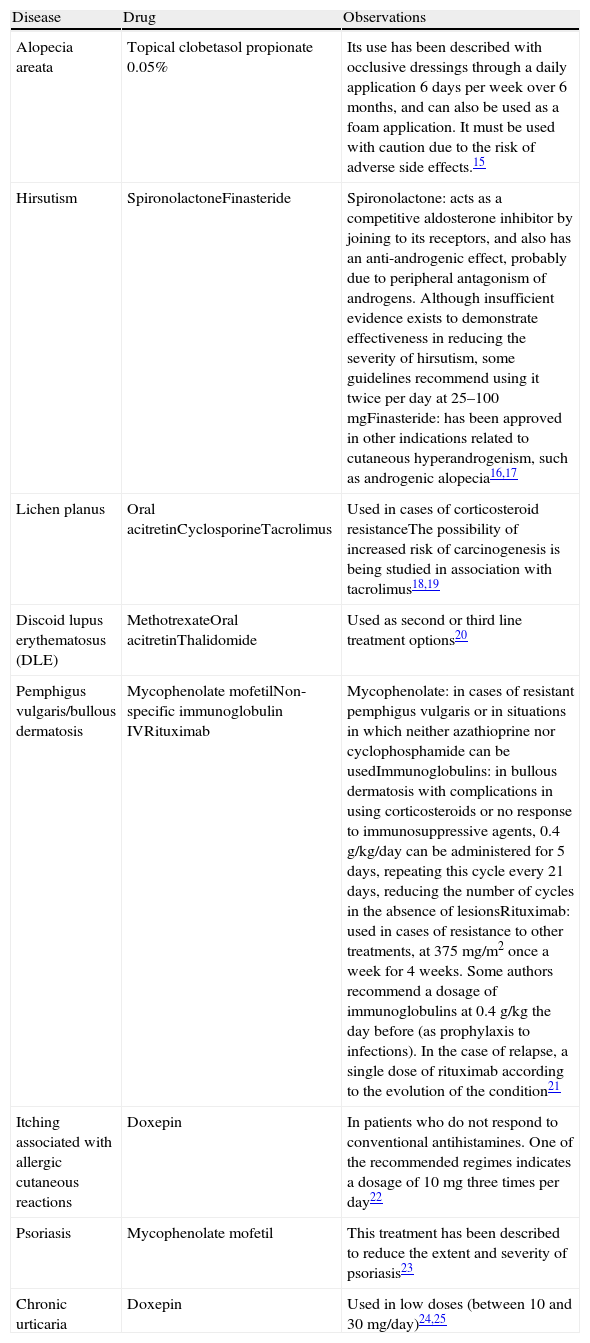
Drugs Used in Dermatology Under Indications Are Not Included in Their Summary of Product Characteristics.
| Disease | Drug | Observations |
| Alopecia areata | Topical clobetasol propionate 0.05% | Its use has been described with occlusive dressings through a daily application 6 days per week over 6 months, and can also be used as a foam application. It must be used with caution due to the risk of adverse side effects.15 |
| Hirsutism | SpironolactoneFinasteride | Spironolactone: acts as a competitive aldosterone inhibitor by joining to its receptors, and also has an anti-androgenic effect, probably due to peripheral antagonism of androgens. Although insufficient evidence exists to demonstrate effectiveness in reducing the severity of hirsutism, some guidelines recommend using it twice per day at 25–100mgFinasteride: has been approved in other indications related to cutaneous hyperandrogenism, such as androgenic alopecia16,17 |
| Lichen planus | Oral acitretinCyclosporineTacrolimus | Used in cases of corticosteroid resistanceThe possibility of increased risk of carcinogenesis is being studied in association with tacrolimus18,19 |
| Discoid lupus erythematosus (DLE) | MethotrexateOral acitretinThalidomide | Used as second or third line treatment options20 |
| Pemphigus vulgaris/bullous dermatosis | Mycophenolate mofetilNon-specific immunoglobulin IVRituximab | Mycophenolate: in cases of resistant pemphigus vulgaris or in situations in which neither azathioprine nor cyclophosphamide can be usedImmunoglobulins: in bullous dermatosis with complications in using corticosteroids or no response to immunosuppressive agents, 0.4g/kg/day can be administered for 5 days, repeating this cycle every 21 days, reducing the number of cycles in the absence of lesionsRituximab: used in cases of resistance to other treatments, at 375mg/m2 once a week for 4 weeks. Some authors recommend a dosage of immunoglobulins at 0.4g/kg the day before (as prophylaxis to infections). In the case of relapse, a single dose of rituximab according to the evolution of the condition21 |
| Itching associated with allergic cutaneous reactions | Doxepin | In patients who do not respond to conventional antihistamines. One of the recommended regimes indicates a dosage of 10mg three times per day22 |
| Psoriasis | Mycophenolate mofetil | This treatment has been described to reduce the extent and severity of psoriasis23 |
| Chronic urticaria | Doxepin | Used in low doses (between 10 and 30mg/day)24,25 |
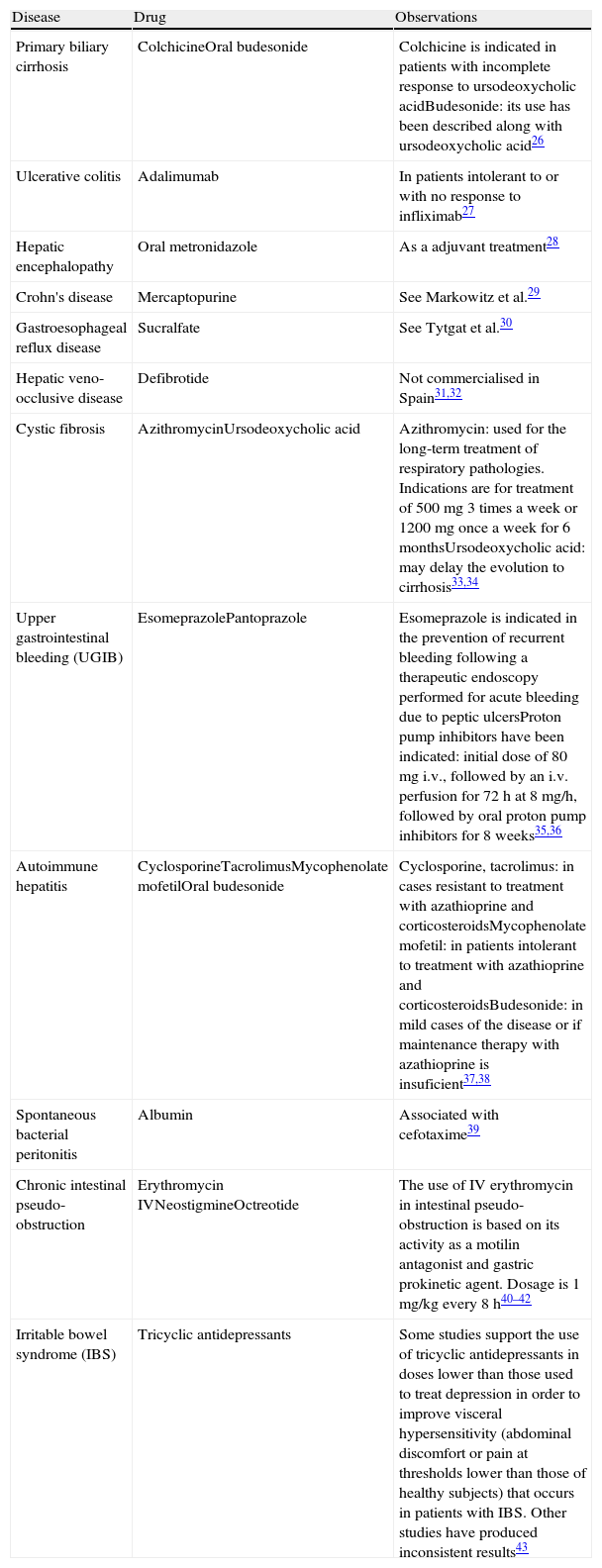
Drugs Used in the Gastrointestinal System Under Indications Not Included in Their Summary of Product Characteristics.
| Disease | Drug | Observations |
| Primary biliary cirrhosis | ColchicineOral budesonide | Colchicine is indicated in patients with incomplete response to ursodeoxycholic acidBudesonide: its use has been described along with ursodeoxycholic acid26 |
| Ulcerative colitis | Adalimumab | In patients intolerant to or with no response to infliximab27 |
| Hepatic encephalopathy | Oral metronidazole | As a adjuvant treatment28 |
| Crohn's disease | Mercaptopurine | See Markowitz et al.29 |
| Gastroesophageal reflux disease | Sucralfate | See Tytgat et al.30 |
| Hepatic veno-occlusive disease | Defibrotide | Not commercialised in Spain31,32 |
| Cystic fibrosis | AzithromycinUrsodeoxycholic acid | Azithromycin: used for the long-term treatment of respiratory pathologies. Indications are for treatment of 500mg 3 times a week or 1200mg once a week for 6 monthsUrsodeoxycholic acid: may delay the evolution to cirrhosis33,34 |
| Upper gastrointestinal bleeding (UGIB) | EsomeprazolePantoprazole | Esomeprazole is indicated in the prevention of recurrent bleeding following a therapeutic endoscopy performed for acute bleeding due to peptic ulcersProton pump inhibitors have been indicated: initial dose of 80mg i.v., followed by an i.v. perfusion for 72h at 8mg/h, followed by oral proton pump inhibitors for 8 weeks35,36 |
| Autoimmune hepatitis | CyclosporineTacrolimusMycophenolate mofetilOral budesonide | Cyclosporine, tacrolimus: in cases resistant to treatment with azathioprine and corticosteroidsMycophenolate mofetil: in patients intolerant to treatment with azathioprine and corticosteroidsBudesonide: in mild cases of the disease or if maintenance therapy with azathioprine is insuficient37,38 |
| Spontaneous bacterial peritonitis | Albumin | Associated with cefotaxime39 |
| Chronic intestinal pseudo-obstruction | Erythromycin IVNeostigmineOctreotide | The use of IV erythromycin in intestinal pseudo-obstruction is based on its activity as a motilin antagonist and gastric prokinetic agent. Dosage is 1mg/kg every 8h40–42 |
| Irritable bowel syndrome (IBS) | Tricyclic antidepressants | Some studies support the use of tricyclic antidepressants in doses lower than those used to treat depression in order to improve visceral hypersensitivity (abdominal discomfort or pain at thresholds lower than those of healthy subjects) that occurs in patients with IBS. Other studies have produced inconsistent results43 |
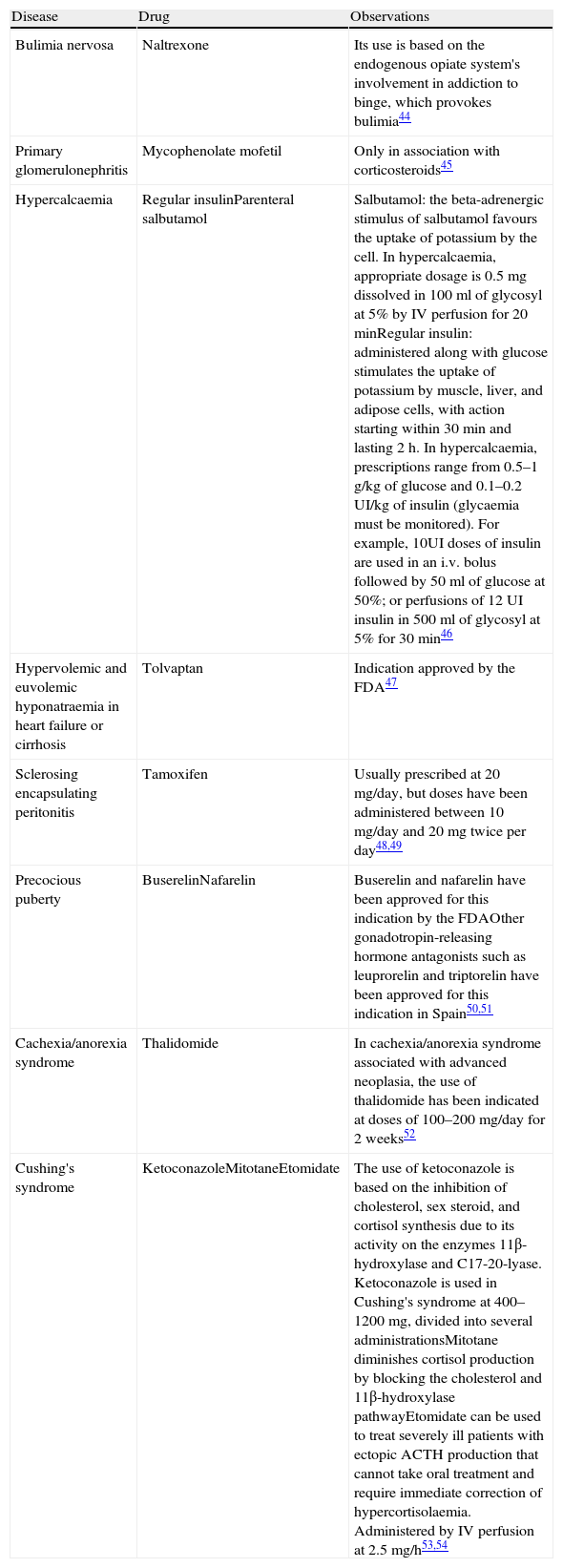
Drugs Used in Endocrinology/Metabolism/Nephrology Under Indications Are Not Included in Their Summary of Product Characteristics.
| Disease | Drug | Observations |
| Bulimia nervosa | Naltrexone | Its use is based on the endogenous opiate system's involvement in addiction to binge, which provokes bulimia44 |
| Primary glomerulonephritis | Mycophenolate mofetil | Only in association with corticosteroids45 |
| Hypercalcaemia | Regular insulinParenteral salbutamol | Salbutamol: the beta-adrenergic stimulus of salbutamol favours the uptake of potassium by the cell. In hypercalcaemia, appropriate dosage is 0.5mg dissolved in 100ml of glycosyl at 5% by IV perfusion for 20minRegular insulin: administered along with glucose stimulates the uptake of potassium by muscle, liver, and adipose cells, with action starting within 30min and lasting 2h. In hypercalcaemia, prescriptions range from 0.5–1g/kg of glucose and 0.1–0.2UI/kg of insulin (glycaemia must be monitored). For example, 10UI doses of insulin are used in an i.v. bolus followed by 50ml of glucose at 50%; or perfusions of 12UI insulin in 500ml of glycosyl at 5% for 30min46 |
| Hypervolemic and euvolemic hyponatraemia in heart failure or cirrhosis | Tolvaptan | Indication approved by the FDA47 |
| Sclerosing encapsulating peritonitis | Tamoxifen | Usually prescribed at 20mg/day, but doses have been administered between 10mg/day and 20mg twice per day48,49 |
| Precocious puberty | BuserelinNafarelin | Buserelin and nafarelin have been approved for this indication by the FDAOther gonadotropin-releasing hormone antagonists such as leuprorelin and triptorelin have been approved for this indication in Spain50,51 |
| Cachexia/anorexia syndrome | Thalidomide | In cachexia/anorexia syndrome associated with advanced neoplasia, the use of thalidomide has been indicated at doses of 100–200mg/day for 2 weeks52 |
| Cushing's syndrome | KetoconazoleMitotaneEtomidate | The use of ketoconazole is based on the inhibition of cholesterol, sex steroid, and cortisol synthesis due to its activity on the enzymes 11β-hydroxylase and C17-20-lyase. Ketoconazole is used in Cushing's syndrome at 400–1200mg, divided into several administrationsMitotane diminishes cortisol production by blocking the cholesterol and 11β-hydroxylase pathwayEtomidate can be used to treat severely ill patients with ectopic ACTH production that cannot take oral treatment and require immediate correction of hypercortisolaemia. Administered by IV perfusion at 2.5mg/h53,54 |
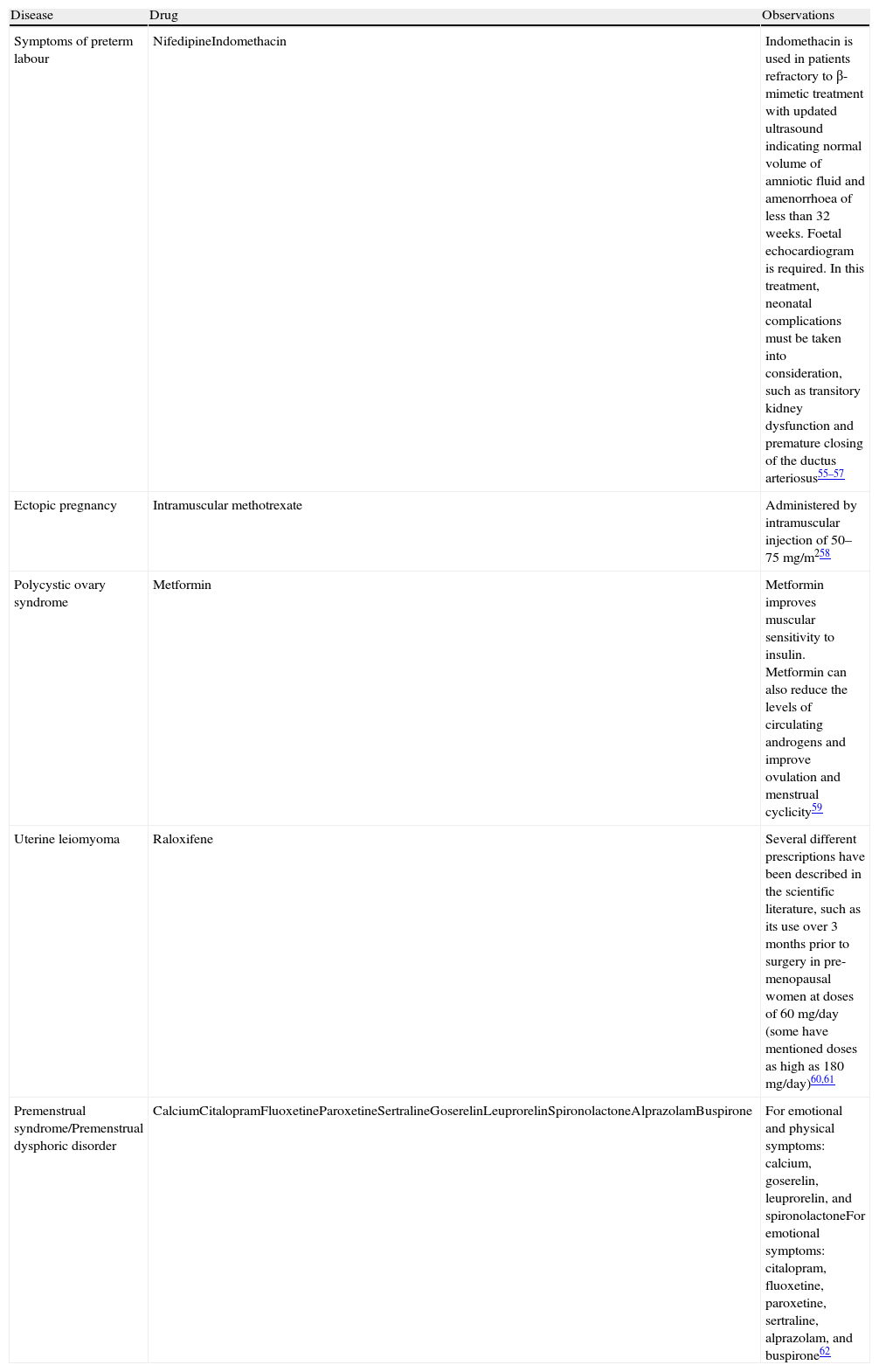
Drugs Used in Obstetrics and Gynaecology Under Indications Not Included in Their Summary of Product Characteristics.
| Disease | Drug | Observations |
| Symptoms of preterm labour | NifedipineIndomethacin | Indomethacin is used in patients refractory to β-mimetic treatment with updated ultrasound indicating normal volume of amniotic fluid and amenorrhoea of less than 32 weeks. Foetal echocardiogram is required. In this treatment, neonatal complications must be taken into consideration, such as transitory kidney dysfunction and premature closing of the ductus arteriosus55–57 |
| Ectopic pregnancy | Intramuscular methotrexate | Administered by intramuscular injection of 50–75mg/m258 |
| Polycystic ovary syndrome | Metformin | Metformin improves muscular sensitivity to insulin. Metformin can also reduce the levels of circulating androgens and improve ovulation and menstrual cyclicity59 |
| Uterine leiomyoma | Raloxifene | Several different prescriptions have been described in the scientific literature, such as its use over 3 months prior to surgery in pre-menopausal women at doses of 60mg/day (some have mentioned doses as high as 180mg/day)60,61 |
| Premenstrual syndrome/Premenstrual dysphoric disorder | CalciumCitalopramFluoxetineParoxetineSertralineGoserelinLeuprorelinSpironolactoneAlprazolamBuspirone | For emotional and physical symptoms: calcium, goserelin, leuprorelin, and spironolactoneFor emotional symptoms: citalopram, fluoxetine, paroxetine, sertraline, alprazolam, and buspirone62 |
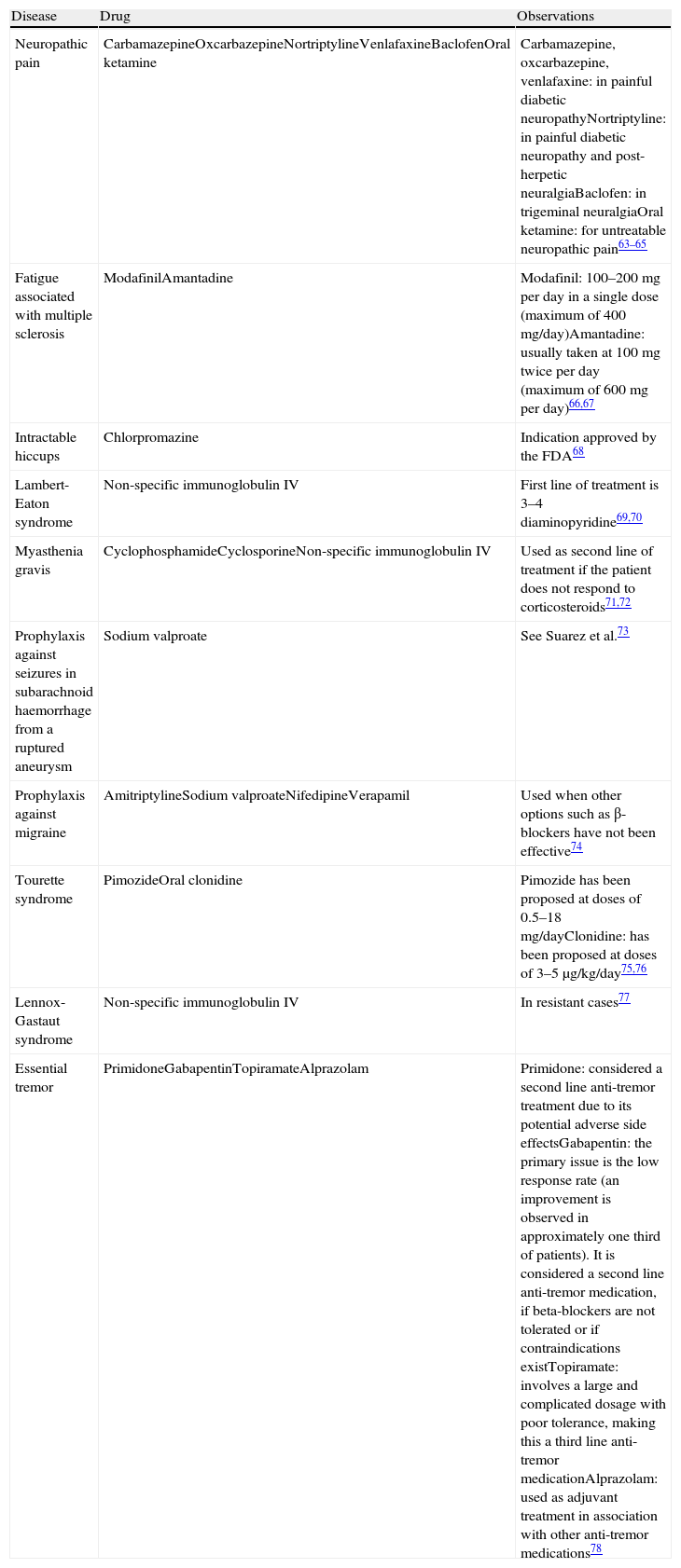
Drugs Used in Neurology Under Indications Not Included in Their Summary of Product Characteristics.
| Disease | Drug | Observations |
| Neuropathic pain | CarbamazepineOxcarbazepineNortriptylineVenlafaxineBaclofenOral ketamine | Carbamazepine, oxcarbazepine, venlafaxine: in painful diabetic neuropathyNortriptyline: in painful diabetic neuropathy and post-herpetic neuralgiaBaclofen: in trigeminal neuralgiaOral ketamine: for untreatable neuropathic pain63–65 |
| Fatigue associated with multiple sclerosis | ModafinilAmantadine | Modafinil: 100–200mg per day in a single dose (maximum of 400mg/day)Amantadine: usually taken at 100mg twice per day (maximum of 600mg per day)66,67 |
| Intractable hiccups | Chlorpromazine | Indication approved by the FDA68 |
| Lambert-Eaton syndrome | Non-specific immunoglobulin IV | First line of treatment is 3–4 diaminopyridine69,70 |
| Myasthenia gravis | CyclophosphamideCyclosporineNon-specific immunoglobulin IV | Used as second line of treatment if the patient does not respond to corticosteroids71,72 |
| Prophylaxis against seizures in subarachnoid haemorrhage from a ruptured aneurysm | Sodium valproate | See Suarez et al.73 |
| Prophylaxis against migraine | AmitriptylineSodium valproateNifedipineVerapamil | Used when other options such as β-blockers have not been effective74 |
| Tourette syndrome | PimozideOral clonidine | Pimozide has been proposed at doses of 0.5–18mg/dayClonidine: has been proposed at doses of 3–5μg/kg/day75,76 |
| Lennox-Gastaut syndrome | Non-specific immunoglobulin IV | In resistant cases77 |
| Essential tremor | PrimidoneGabapentinTopiramateAlprazolam | Primidone: considered a second line anti-tremor treatment due to its potential adverse side effectsGabapentin: the primary issue is the low response rate (an improvement is observed in approximately one third of patients). It is considered a second line anti-tremor medication, if beta-blockers are not tolerated or if contraindications existTopiramate: involves a large and complicated dosage with poor tolerance, making this a third line anti-tremor medicationAlprazolam: used as adjuvant treatment in association with other anti-tremor medications78 |
Drugs Used in Ophthalmology and ENT Under Indications Are Not Included in Their Summary of Prudct Characteristics.
| Disease | Drug | Observations |
| Conjunctivitis (viral, bacterial, and irritability) | Diclofenac drops | See Arcos González and Castro Delgado79 and Galvez et al.80 |
| Neovascular (wet) age-related macular degeneration (AMD) | Intravitreal bevacizumab | The cumulative experience and results from several scientific studies have shown that bevacizumab can provide similar benefits to ranibizumab (which is approved for intraocular use under the indications for AMD). When considering this alternative, we must not overlook the substantially lower annual cost of treatment with bevacizumab as compared to ranibizumabIn AMD, bevacizumab is administered intravitrea at 1.25mg every 4 weeks10–12 |
| Uveitis | MethotrexateInfliximab | Methotrexate is used as a second or third line treatment optionThe use of infliximab has been described for controlling ocular inflammation in children with non-infectious chronic uveitis associated with resistant juvenile idiopathic arthritis; also for uveitis and uveoretinitis associated with Behçet's disease81–83 |
| Recurrent aphthous stomatitis | ColchicineDapsonePentoxifyllineSucralfateThalidomide | See Mimura et al.,84 Altengurg et al.,85 Pizarro et al.,86 Rattan et al.,87 and Bonnetblanc et al.88 |
| Sudden hearing loss | PiracetamNimodipinePentoxifyllineMethylprednisolone IV | An example of the treatment protocol for sudden hearing loss mentions the use of IV methylprednisolone at 40–80mg/day for 5 days, along with B1B6B12 complex by intramuscular injection, IV piracetam at 3g every 8h, and IV perfusion of nimodipine at 15–30μg/kg/h, passing on later to oral treatment for 15 days of methylprednisolone at 40mg/day (progressively reducing dosage), B-complex pills, 800mg of piracetam every 8h and 30mg of nimodipine every 8h89,90 |
| Vertigo | Parenteral haloperidol | Its use has been indicated in the acute phase (until symptoms disappear), and treatment must be suspended when the vertigo ceases91 |
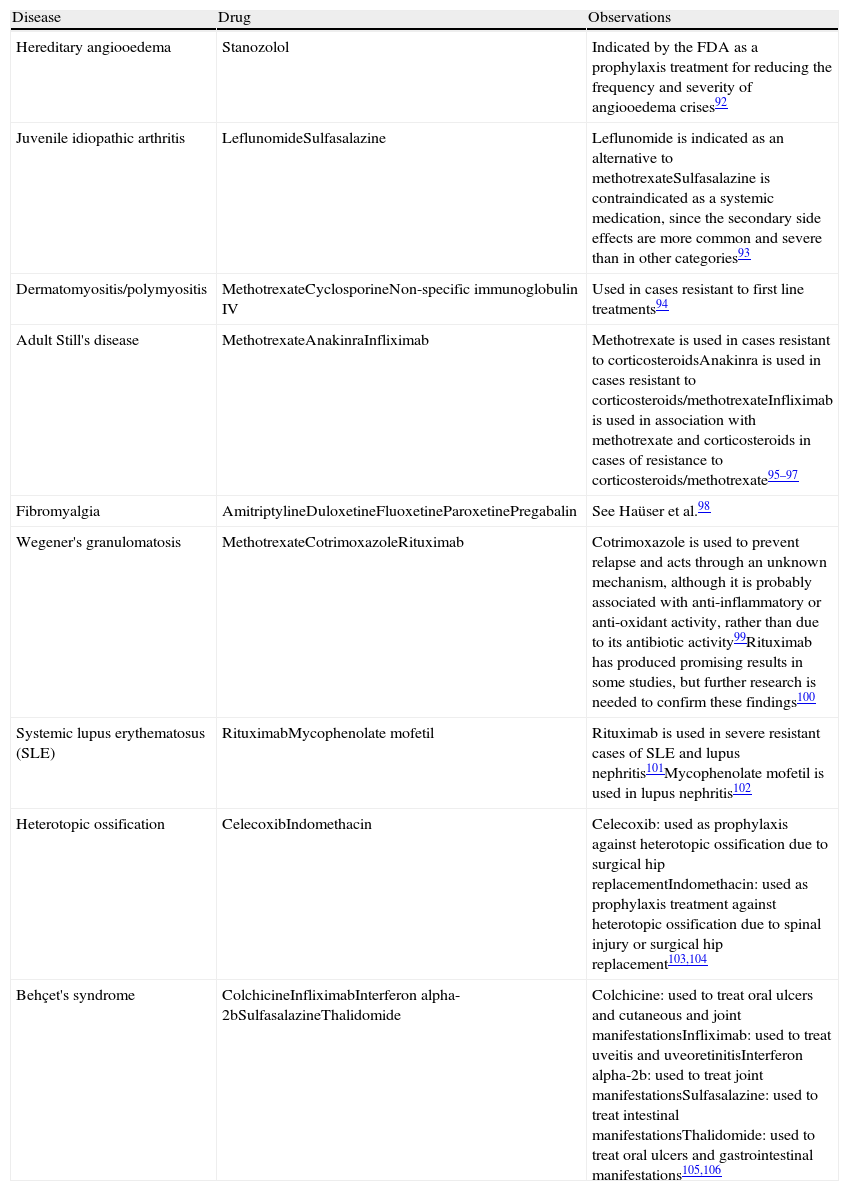
Drugs Used in Rheumatology Under Indications Not Included in Their Summary of Product Characteristics.
| Disease | Drug | Observations |
| Hereditary angiooedema | Stanozolol | Indicated by the FDA as a prophylaxis treatment for reducing the frequency and severity of angiooedema crises92 |
| Juvenile idiopathic arthritis | LeflunomideSulfasalazine | Leflunomide is indicated as an alternative to methotrexateSulfasalazine is contraindicated as a systemic medication, since the secondary side effects are more common and severe than in other categories93 |
| Dermatomyositis/polymyositis | MethotrexateCyclosporineNon-specific immunoglobulin IV | Used in cases resistant to first line treatments94 |
| Adult Still's disease | MethotrexateAnakinraInfliximab | Methotrexate is used in cases resistant to corticosteroidsAnakinra is used in cases resistant to corticosteroids/methotrexateInfliximab is used in association with methotrexate and corticosteroids in cases of resistance to corticosteroids/methotrexate95–97 |
| Fibromyalgia | AmitriptylineDuloxetineFluoxetineParoxetinePregabalin | See Haüser et al.98 |
| Wegener's granulomatosis | MethotrexateCotrimoxazoleRituximab | Cotrimoxazole is used to prevent relapse and acts through an unknown mechanism, although it is probably associated with anti-inflammatory or anti-oxidant activity, rather than due to its antibiotic activity99Rituximab has produced promising results in some studies, but further research is needed to confirm these findings100 |
| Systemic lupus erythematosus (SLE) | RituximabMycophenolate mofetil | Rituximab is used in severe resistant cases of SLE and lupus nephritis101Mycophenolate mofetil is used in lupus nephritis102 |
| Heterotopic ossification | CelecoxibIndomethacin | Celecoxib: used as prophylaxis against heterotopic ossification due to surgical hip replacementIndomethacin: used as prophylaxis treatment against heterotopic ossification due to spinal injury or surgical hip replacement103,104 |
| Behçet's syndrome | ColchicineInfliximabInterferon alpha-2bSulfasalazineThalidomide | Colchicine: used to treat oral ulcers and cutaneous and joint manifestationsInfliximab: used to treat uveitis and uveoretinitisInterferon alpha-2b: used to treat joint manifestationsSulfasalazine: used to treat intestinal manifestationsThalidomide: used to treat oral ulcers and gastrointestinal manifestations105,106 |
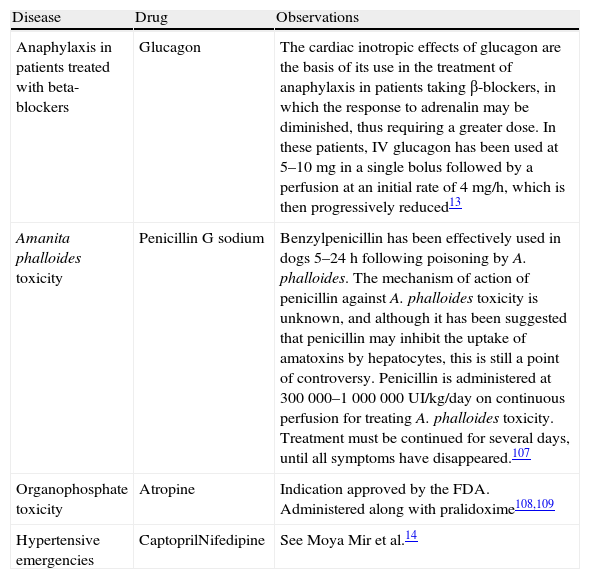
Drugs Used in Emergency Medicine Under Indications Not Included in Their Summary of Product Characteristics.
| Disease | Drug | Observations |
| Anaphylaxis in patients treated with beta-blockers | Glucagon | The cardiac inotropic effects of glucagon are the basis of its use in the treatment of anaphylaxis in patients taking β-blockers, in which the response to adrenalin may be diminished, thus requiring a greater dose. In these patients, IV glucagon has been used at 5–10mg in a single bolus followed by a perfusion at an initial rate of 4mg/h, which is then progressively reduced13 |
| Amanita phalloides toxicity | Penicillin G sodium | Benzylpenicillin has been effectively used in dogs 5–24h following poisoning by A. phalloides. The mechanism of action of penicillin against A. phalloides toxicity is unknown, and although it has been suggested that penicillin may inhibit the uptake of amatoxins by hepatocytes, this is still a point of controversy. Penicillin is administered at 300 000–1 000 000UI/kg/day on continuous perfusion for treating A. phalloides toxicity. Treatment must be continued for several days, until all symptoms have disappeared.107 |
| Organophosphate toxicity | Atropine | Indication approved by the FDA. Administered along with pralidoxime108,109 |
| Hypertensive emergencies | CaptoprilNifedipine | See Moya Mir et al.14 |
Drugs Used in Haematology and Other Diseases Under Indications Not Included in Their Summary of Product Characteristics.
| Disease | Drug | Observations |
| Aplastic anaemia | Cyclosporine | Used along with anti-thymocyte immunoglobulin (from rabbits and horses) and corticosteroids110 |
| Autoimmune haemolytic anaemia | Non-specific immunoglobulin IVCyclophosphamideCyclosporineMycophenolate mofetilRituximab | Autoimmune haemolytic anaemia (AIHA) includes 2 types: warm agglutinin AIHA (WA-AIHA), which is mediated by IgG autoantibodies and usually responds to corticosteroids and immunosuppressants, and cold agglutinin AIHA (CA-AIHA), which is mediated by IgM autoantibodies, although the efficacy of this treatment is not well establishedAssociation of IV Ig and corticosteroids is frequently used as the first line of treatment for WA-AIHARituximab is the only drug that may have activity against CA-AIHA111 |
| Cystic fibrosis | AzithromycinUrsodeoxycholic acid | See Table 232,33 |
| Acute pericarditis | Acetylsalicylic acid (aspirin)NSAID (ibuprofen, indomethacin)ColchicineCorticosteroids | Acetylsalicylic acid is used at high doses (500–1000mg every 6h), and when pain and fever cease the prescription can be gradually reduced (for example, 500mg every 8h for one week, then 250mg every 8–12h for 2 weeks)If the patient does not respond or if contraindications exist for acetylsalicylic acid, ibuprofen (1200–1800mg/day) or indomethacin (75–150mg/day) may be usedCorticosteroids may be necessary at moderate doses (for example prednisone at 0.2–0.5mg/kg/day)Colchicine (0.5–1.2mg/day) is useful for preventing relapse112,113 |
| Idiopathic thrombocytopaenic purpura (ITP) | Anti-D immunoglobulinRituximab | Anti-D immunoglobulin: used to increase platelet count in adult patients that are Rho (D)-positive and with no history of splenectomy. This indication is approved by other non-Spanish medicines agencies for the same medication that has been commercialised in Spain114Rituximab: normally administered in doses of 375mg/m2 1 day per week for 4 weeks100 |
| Thrombotic thrombocytopaenic purpura (TTP) | RituximabVincristineCyclosporineDefibrotide | Rituximab: normally prescribed at 375mg/m2 one day per week for 4 weeksVincristine: for cases of TTP resistant to other treatmentsCyclosporine: used to prevent relapse of TTP in cases resistant to other treatmentsDefibrotide: not commercialised in Spain115,116 |
| Unclogging venous catheters | Urokinase | Applied through an injection into the catheter117 |
| Kawasaki disease | Acetylsalicylic acid (ASA) | This indication is not included in the summary of product characteristics for ASA, but the concomitant use of immunoglobulin is mentioned in that for immunoglobulin IVASA is indicated in Kawasaki disease at initial doses during the acute phase of 80–100mg/kg/day (divided into 4 doses) for 14 days or up until 48–72h after the patient's fever has ceased, and afterwards, at 3–5mg/kg/day for 6–8 weeks or until platelet levels have normalised118–120 |
The off-label use of a drug can be based on any of the following circumstances:
- –
The assumption that a given drug will have a certain activity that is known to be present in other drugs of the same class (for example, the summary of product characteristics for buserelin and nafarelin do not mention indications for use in precocious puberty, which is an indication that has been approved for leuprorelin and triptorelin; the same goes for oral metronidazole as a adjuvant treatment in hepatic encephalopathy, which is an approved indication in other oral antibiotics that act on the intestines, such as paromomycin, neomycin, and rifaximin).
- –
An extension of the indications for a given drug to others that are ‘related’ to the already approved uses in the summary of product characteristics (for example, the off-label use of methotrexate in different auto-immune diseases, or the use of captopril or nifedipine in hypertensive emergencies, or adalumimab, which is approved for use in Crohn's disease and has an off-label use for ulcerative colitis).
- –
The use of a drug's mechanism of action in order to obtain a beneficial effect in an off-label indication (for example, using insulin to treat hypercalcaemia is based on the stimulation of potassium uptake in muscle, liver, and adipose cells; the use of ketoconazole in Cushing's syndrome is based on the inhibition of cholesterol, sex steroid, and cortisol synthesis due to its activity on the enzymes 11β-hydroxylase and C17-20-lyase; the use of intravenous erythromycin in intestinal pseudo-obstruction is based on its action as a motilin antagonist and gastric prokinetic agent; the activity of metformin to improve peripheral uptake of insulin is the basis for its off-label use in polycystic ovary syndrome).
- –
In certain cases, the mechanism of action in the off-label indication is completely unknown (for example, the use of penicillin G to treat Amanita phalloides toxicity).
In the hospital environment, medications are frequently used off-label, and these situations should be compiled into health care protocols. The physician should comply with these protocols, and the Pharmacy and Therapeutics Committee should play an important role in their control.
A list of all diseases or treatment situations in which the scientific literature recommends the off-label use of drugs would also help the Pharmacy and Therapeutics Committee to provide reference information on the diseases for which drugs are frequently prescribed outside of the realm of their approved indications, and/or regarding those that should be used with special caution under a specific health care protocol.
Additionally, having a reference list for drugs used off-label could help hospital pharmacists who must validate medical prescriptions in understanding and justifying certain prescriptions that at first glance might seem unreasonable (for example, prescribing ketoconazole in doses as high as 1200mg/day to treat Cushing's syndrome and not a fungal infection; insulin can be used to treat hypercalcaemia even though the patient may not be diabetic; an intravenous dose of erythromycin as low as 1mg/kg/8h would indicate that the patient is being treated for intestinal pseudo-obstruction; a prescription of 500mg of azithromycin three times per week or 1200mg once per week over 6 months would indicate that the patient has cystic fibrosis; the use of tamoxifen in a male patient would indicate that the patient does not have breast cancer, but rather a complication of peritoneal dialysis, such as sclerosing encapsulating peritonitis).
Furthermore, a list of off-label indications for medications would provide a significant help in incorporating a search index by disease, including a list of drugs used for each disease, in a hospital Pharmacotherapy Guide or any other database. This would provide incomplete information if only those indications approved by each medication's summary of product characteristics were to be included.121
Conflict of InterestThe authors declare that they have no conflict of interest.
Please cite this article as: García-Sabina A, et al. Revisión sobre el uso de medicamentos en condiciones no incluidas en su ficha técnica. Farm Hosp. 2011. doi:10.1016/j.farma.2010.06.011.