Health-related quality of life (HRQL) measurements enable us to take patient perception into account when evaluating treatment outcomes from clinical trials (CTs). The purpose of this study was to evaluate the use of HRQL questionnaires as a measurement of efficacy in CT design.
MethodsA duplicate systematic review of the CTs examined by a Clinical Research Ethics Committee between 1995 and 2006 was performed to check for use of HRQL. We gathered data concerning general aspects including medical specialty, drugs evaluated, methodological quality and inclusion of economic variables. For CTs including HRQL measurements, we analysed the type of questionnaire in use. Where there were no HRQL measurements, we analysed the methodological possibilities for including them, and the relevance of their absence.
ResultsA total of 242 CTs were analysed; 69 (28.5%) included HRQL measurements, and 10 CTs (4.1%) used them as a primary endpoint. Only 22 CTs used more than one questionnaire. Data analysis by therapeutic area showed that HRQL was most commonly studied in the fields of rheumatology, urology, psychiatry and oncology. Only 33 CTs included economic variables.
ConclusionsMeasurements based on clinical parameters are the most commonly used means of measuring efficacy. Only a small percentage of CTs take the patient's perception of his/her health into account, despite the increasing importance given to this parameter. Including HRQL questionnaires in CTs design is still far from common.
Las medidas de “calidad de vida relacionada con la salud” (CVRS) permiten incorporar la percepción del paciente en la evaluación de los resultados obtenidos en los ensayos clínicos (EC). El objetivo de este estudio fue evaluar el uso de cuestionarios de CVRS como medida de eficacia en el diseño de ECs.
MétodosSe lleva a cabo una revisión sistemática por duplicado de los ECs analizados por un Comité Ético de Investigación Clínica, entre los años 1995 y 2006. Se recogieron datos relativos a aspectos generales como la especialidad médica, fármacos evaluados, calidad metodológica o inclusión de variables económicas. Para aquellos ECs que incluían medidas de CVRS, se analizó el tipo de cuestionario utilizado. Para aquellos que no analizaban CVRS, se analizó la posibilidad metodológica de su análisis, así como la relevancia de su ausencia.
ResultadosSe analizaron un total de 242 ECs, 69 (28,5%) de los cuales incluyeron medidas de CVRS, 10 (4,1%) como variable primaria. Únicamente 22 ECs emplearon más de un cuestionario. El análisis de datos por áreas terapéuticas puso de manifiesto que reumatología, urología, psiquiatría y oncología, fueron las áreas donde la CVRS fue analizada en una mayor proporción. Únicamente 33 ECs incluyeron variables económicas.
ConclusionesLas medidas basadas en parámetros clínicos son las más usadas como medidas de eficacia. Una pequeña proporción de ECs considera la percepción del estado de salud del paciente, en contraste a la creciente importancia asignada a estos aspectos. La inclusión de cuestionarios de CVRS en el diseño de ECs, está lejos de ser una medida habitual.
The assessment of the efficacy and safety of drugs is the main goal of drugs clinical trials (CT). Traditionally, efficacy quantification has consisted of the identification of changes that treatments introduce in intermediate variables linked to clinical manifestations or to patients’ survival. Management of certain disorders, like cardiovascular or cancer, results in a reduction in their mortality rate, which translates into prolonged life expectancy.1,2 However, outcome measurements based on survival do not allow to assess the effect of treatment preventing disability due to disease development. Healthcare systems do definitely need tools allowing to accurately estimate the impact of interventions not aiming to prevent fatal events but rather to reduce suffering resulting from disabling disorders.3 “Quality adjusted life” expectancy has become a widely used measurement parameter, which simplifies comparison of the relative efficiency of the various healthcare interventions. This outcome unit involves measurement of preferences on health states that enable individuals themselves to set up the usefulness of the health profile throughout their lives.4,5
Over the last decades, estimation of quality of life (QOL) has been introduced as a new dimension of efficacy, which complements that based on life expectancy.6 The reasons for this change are various. On the one hand, the ongoing development of QOL evaluation as a scientific discipline, which has enabled the design and use of standardised questionnaires with proved metric properties and with a degree of reliability and validity similar to measurements performed in the laboratory or through clinical observation.7 On the other hand, epidemiology has emphasised the growing importance of chronic disorders whose main social repercussion results from them being a source of disability and worsening of HRQL rather than mortality.8
Physicians have been well aware of these changes and HRQL questionnaires have become instruments of a potential usefulness in medical clinics. In routine practice, there is also an ever increasing demand from patients that medical actions improve their QOL, which entails the need to have indicators of healthcare outcome related to that variable.9 Regulatory Agencies, as well as other organizations committed to recommend standards of use of health technologies, have admitted its relevance by incorporating it to the evaluation process of new treatments.10,11
Among ‘patient-reported outcome’ measures, HRQL instruments are the most complex involving a multidomain concept comprising physical, psychological, and social components. The notion of multidimensionality is a key component of definition of HRQL. A single domain, e.g., physical functioning or fatigue, is not considered as a HRQL measure, even though it is a patient-reported outcome. Additionally, HRQL instruments attempt to measure both the effectiveness and the side effects of treatments.12,13
Use of HRQL measurement might be thought to be a definitely established practice, which can be broadly found in the scientific literature. Nevertheless, there are many published studies on healthcare intervention outcomes that confine to efficacy measurement in terms of survival or illness symptoms, even in disorders where HRQL is particularly relevant.14
The objective of this study is to evaluate the use of HRQL questionnaires as an efficacy measure in the design of CTs.
MethodsWe performed an observational, descriptive, quantitative evaluation study aimed to carry out HRQL measurements in CTs by their review. The analysis units were the drug CT protocols evaluated by the Ethics Committee of Clinical Research (ECCR) of the health areas of Burgos and Soria (Spain) between 1995 and 2006. This ECCR covers 4 public general hospitals and a web of health centres of the areas mentioned above. Research protocols not related to drug or not being an actual CT were excluded.
Every CT was analysed by two researchers. Discrepancies found were sorted out by discussion between both peer reviewers until consensus was reached. If lack of agreement persists, the opinion of a third researcher was sought.
A CT was considered to use HRQL measurements when properly validated measurement questionnaires were used and their application was mentioned in the ‘Method’ section. The questionnaire used must be explicitly quoted. Additionally, HRQL analysis must be the primary or secondary endpoint of the trial.
Variables recorded for each CT evaluated are as follows: researcher, sponsor, disorder on which the CT is based, evaluated drug and comparator, primary efficacy variable, secondary efficacy variables and potential assessment of economic variables (resource consumption analysis). Primary efficacy variables separated protocols in four groups: HRQL, survival measures, self-functioning scales and clinical variables. Given that we focussed on the use of HRQL tools in terms of multidomain questionnaires we separated them from other patient-reported outcomes as ability to carry out activities of daily living (self-functioning scales) or the report of the presence of symptoms as for example pain or dyspnoea (clinical variables). The group of clinical variables included also physician and nurse recorded scores as blood pressure figure or biochemical determinations. Methodological quality of the protocol using the Jadad score was also evaluated, in order to analyse potential differences between the CTs group that include HRQL measures and those which do not.15 For CTs considering HRQL measurements, data on questionnaire/s used and type of questionnaire (generic/specific) were recorded. For CTs not considering HRQL measurements, data on the methodological potential of considering such measurements, their relevance and the type of condition for which health intervention were assessed (chronic disorders, influence on daily life activities, etc.). Previous statements are based on the fact that HRQL measurements do not have similar relevance in all CTs. The importance of not including HRQL measurements in CTs entails a subjective component, which might be considered as a limitation. Even though evaluation of this measurement can be arbitrary, we regarded it as important to be taken into account in order to judge if its omission was due to a methodological limitation or to its irrelevancy. For such analysis two researchers carried out a separate gauge, which sorted such lack of evaluation as warranted or unwarranted. The differences found were reported to the rest of researchers in order to achieve a consensus following thorough discussion. The research team considered the evaluation performed as acceptable when the discrepancy rate among researchers was <20%.
A subanalysis was carried out excluding those CTs in which HRQL estimations were not considered methodologically possible.
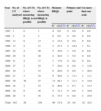
ResultsA review of 242 CT was performed, 69 (28.5%) of which included HRQL measurements as efficacy measurement variables. This was the primary evaluated endpoint in 14.5% (10 CTs, 4% among all CTs reviewed) of the studies estimating HRQL, whereas it was a secondary endpoint in 85.5%. Among the 10 CTs where HRQL was the primary endpoint, 3 of them belonged to the rheumatology area (arthritis), 2 to psychiatry (schizophrenia), 2 to pneumology (asthma and COPD), 1 to urology (benign prostate hyperplasia), and 2 analysed side effects of drugs (one from infectious diseases and the other one from the gastroenterology group). Only the two CTs aimed to assess treatments of schizophrenia included both ‘generic’ and ‘specific’ scales. The rest of CTs used a single questionnaire, which was ‘specific’ in all but one case. Sixty-eight percent of the CTs measuring HRQL (47 CTs) used a single questionnaire whereas 32% (22 CTs) used two or more. Less than 14% of the CTs considered economic and resource consumption data (table 1). Five of the protocols analysed mentioned HRQL measurement, but did not specify the questionnaire used.
Global results of the main variables analysed by year
| Year | No. of CT analysed | Measure HRQL | HRQL primary end point | Use more than one scale | Economic variables analysed | Methodological quality point¿for CT measuring HRQL | Methodological quality point¿for CT not measuring HRQL | ||||
| N° | (%) | N° | (%) | N° | (%) | N° | (%) | ||||
| 1993 | 1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | – | 4.00 |
| 1994 | 2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 50.0 | – | 2.50 |
| 1995 | 13 | 2 | 15.4 | 0 | 0.0 | 0 | 0.0 | 2 | 15.4 | 3.50 | 4.64 |
| 1996 | 13 | 3 | 23.1 | 2 | 15.4 | 1 | 7.7 | 2 | 15.4 | 4.00 | 4.00 |
| 1997 | 24 | 5 | 20.8 | 1 | 4.2 | 0 | 0.0 | 1 | 4.2 | 3.40 | 3.95 |
| 1998 | 20 | 4 | 20.0 | 1 | 5.0 | 0 | 0.0 | 3 | 15.0 | 5.00 | 4.00 |
| 1999 | 21 | 3 | 14.3 | 0 | 0.0 | 0 | 0.0 | 4 | 19.0 | 5.00 | 3.50 |
| 2000 | 27 | 7 | 25.9 | 0 | 0.0 | 3 | 11.1 | 4 | 14.8 | 3.29 | 3.70 |
| 2001 | 16 | 6 | 37.5 | 0 | 0.0 | 3 | 18.8 | 1 | 6.3 | 2.33 | 4.00 |
| 2002 | 21 | 9 | 42.9 | 2 | 9.5 | 2 | 9.5 | 6 | 28.6 | 4.00 | 3.08 |
| 2003 | 30 | 13 | 43.3 | 3 | 0.0 | 4 | 13.3 | 5 | 16.7 | 3.54 | 2.88 |
| 2004 | 18 | 6 | 33.3 | 1 | 5.6 | 2 | 11.1 | 2 | 11.1 | 3.33 | 3.42 |
| 2005 | 16 | 5 | 31.3 | 0 | 0.0 | 3 | 18.8 | 0 | 0.0 | 3.80 | 3.36 |
| 2006 | 20 | 6 | 30.0 | 0 | 0.0 | 4 | 20.0 | 2 | 10.0 | 4.00 | 3.21 |
| Total | 242 | 69 | 28.5 | 10 | 4.1 | 22 | 9.1 | 33 | 13.6 | 3.67¿¿ | 3.61¿¿ |
CT: clinical trial; HRQL: health-related quality of life.
Mean score corresponding to methodological quality (Jadad score) was 3.63 points (0–5). Concerning CTs measuring HRQL mean score was 3.67 whereas it was 3.61 in those who did not perform such measurement; no significant differences were found between these two groups (p=0.818). The rate of protocols including HRQL ranged between 15–30% from 1995 to 2000, and this percentage increased to 30–40% between 2001 and 2006; a growing tendency in the use of these questionnaires was therefore noticed during the recent years (Table 1).
Phase analysis of CTs elicited the following results: 30 CTs were analysed in phase II, 6 of which (20%) measured HRQL; 159 were analysed in phase III with 52 (33%) of them measuring HRQL; additional 53 CTs were analysed in phase IV out of which 11 measured HRQL. These data regarding phase IV studies are remarkable since of those studies only 11 CTs (11/53–21%) include HRQL measurement, 8 (8/53–15%) estimate resource consumption and 2 (2/53–4%) evaluate both.
Table 2 shows the distribution of CTs by therapeutic areas, the number of CTs measuring HRQL, as well as of those which did not but in which such evaluation would have been desirable. A detailed analysis of therapeutic areas proved that only in rheumatology, urology, psychiatry and oncology, HRQL measurement was used in over 40% of analysed protocols. Following analyses of protocols not measuring HRQL, it was observed according to the researchers’ criterion that this parameter would have added relevant information in over 50% of CTs concerning the areas of nephrology, infectious diseases, allergy, cardiovascular system, psychiatry and gastroenterology (Table 2).
HRQL measurement analysis by therapeutic areas
| Area | Total number of CTs | Number of CTs evaluating HRQL | No. of CTs not evaluating HRQL in which it would have been relevant | ||
| N° | (%) | N° | (%) | ||
| Rheumatology | 15 | 10 | 66.7 | 5 | 33.3 |
| Urology | 13 | 8 | 61.5 | 4 | 30.8 |
| Psychiatrics | 29 | 12 | 41.4 | 16 | 55.2 |
| Oncology | 32 | 13 | 40.6 | 16 | 50.0 |
| Allergy | 5 | 2 | 40.0 | 3 | 60.0 |
| Neurology | 15 | 5 | 33.0 | 6 | 36.4 |
| Endocrinology | 11 | 3 | 27.3 | 4 | 47.4 |
| Digestive | 19 | 5 | 26.3 | 9 | 33.3 |
| Pneumology | 30 | 7 | 23.3 | 10 | 66.7 |
| Infectious diseases | 12 | 2 | 16.7 | 8 | 33.3 |
| Haematology | 9 | 1 | 11.1 | 3 | 58.3 |
| Cardiovascular | 36 | 1 | 2.8 | 21 | 0.0 |
| Gynaecology | 1 | 0 | 0.0 | 0 | 80.0 |
| Nephrology | 5 | 0 | 0.0 | 4 | 0.0 |
| Nutrition | 1 | 0 | 0.0 | 0 | 50.0 |
| Ophthalmology | 2 | 0 | 0.0 | 1 | 0.0 |
| Paediatrics | 5 | 0 | 0.0 | 0 | 0.0 |
| Vaccines | 2 | 0 | 0.0 | 0 | 30.8 |
| Total | 242 | 69 | 28.5 | 110 | 45.5 |
HRQL: health-related quality of life.
Our review found 26 CTs (10.8%) that used scales that could be considered as related to HRQL measurements, like those analysing performance status or functional capacity (Karnofsky, Barthel, Rankin, etc.). However, to our view, these questionnaires do not include all aspects of what can be regarded as HRQL. The rest of CTs used survival or clinical variable scores, these latter being clearly predominant (Table 3).
Inter-observer agreement was higher than 80% in all the variables evaluated. The opinion of a third observer in order to reach an agreement was not required in any case.
Following the criterion of the evaluating researchers, HRQL could not have been measured in 26 CTs (11% of all CTs analysed). Subanalysis performed considering this issue would have increased the rate of use of HRQL measurement to 31.9%, which is slightly higher than 28.5% found in the initial analysis carried out (Table 4).
CTs analysis by year according to HRQL assessment characteristics
| Year | No. of CT analysed | No. of CTs where measuring HRQL is not possible | No. of CTs where measuring HRQL is possible | Measure HRQL | Primary end point | Use more than one scale | |||
| N° | (%)¿¿ | N° | (%)¿¿ | N° | (%)¿¿ | ||||
| 1993 | 1 | 0 | 1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 1994 | 2 | 0 | 2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 1995 | 13 | 0 | 13 | 2 | 15.4 | 0 | 0.0 | 0 | 0.0 |
| 1996 | 13 | 2 | 11 | 3 | 27.3 | 2 | 18.2 | 1 | 9.1 |
| 1997 | 24 | 4 | 20 | 5 | 25.0 | 1 | 5.0 | 0 | 0.0 |
| 1998 | 20 | 0 | 20 | 4 | 20.0 | 1 | 5.0 | 0 | 0.0 |
| 1999 | 21 | 4 | 17 | 3 | 17.6 | 0 | 0.0 | 0 | 0.0 |
| 2000 | 27 | 2 | 25 | 7 | 28.0 | 0 | 0.0 | 3 | 12.0 |
| 2001 | 16 | 1 | 15 | 6 | 40.0 | 0 | 0.0 | 3 | 20.0 |
| 2002 | 21 | 3 | 18 | 9 | 50.0 | 2 | 11.1 | 2 | 11.1 |
| 2003 | 30 | 36 | 27 | 13 | 48.1 | 3 | 11.1 | 4 | 14.8 |
| 2004 | 18 | 3 | 15 | 6 | 40.0 | 1 | 6.7 | 2 | 13.3 |
| 2005 | 16 | 0 | 16 | 5 | 31.3 | 0 | 0.0 | 3 | 18.8 |
| 2006 | 20 | 4 | 16 | 6 | 37.5 | 0 | 0.0 | 4 | 25.0 |
| Total | 242 | 26 | 216 | 69 | 31.9 | 10 | 4.6 | 22 | 10.2 |
CT: clinical trial; HRQL: health-related quality of life.
Consistently with the results from our study, HRQL still plays a minor role in the design of CTs. Although 28.5% of the protocols include HRQL measurements, only 4% use them as a primary endpoint of efficacy measurement. The fact that only a minority of CTs take into account self-perception of the patients’ own health state, contrasts with the growing importance given to their contribution to health-related decision making. However, this issue has not been very frequently addressed in the scientific literature. Some studies evaluate the relevance of HRQL measurements but limit to specific disorders or populations.16–18 Our study evaluates through a quantitative analysis the relevance of HRQL as an efficacy measurement in the design of drug CTs.
The results obtained show that intermediate variables (like certain clinical parameters) are the most widely used with regard to others like HRQL and survival. Although CT is the available tool giving a higher level of evidence for clinical decision making, only final outcome measurements allow comparison among treatments. Therefore, their ability to report decisions involving comparisons is limited by generalized lack of results in the same terms, especially in the context of cost-effectiveness studies.
Methodological quality of the CT protocols as assessed with the Jadad score, does not seem to play an important role in the use of HRQL measurements, since differences between both groups are not significant. On the contrary, when tracking its use over time a rise over the last years can be noticed. This appears logical since the methodological aspects related to HRQL measurement are in a continuous development and CT designers are fostering a growing knowledge about their usefulness. When an estimation of use rate of HRQL measurement is carried out on the basis of a particular trial phase, it becomes evident that CTs in phase IV do not use such parameter as much as desirable. As they are aimed to evaluate the optimal use of drugs in already approved indications, analysis of variables like HRQL or resource consumption would be of special interest.
The improvement in the efficiency of the use of healthcare resources is an outstanding criterion to be considered before approval of a new drug. Nevertheless, the low rate of CTs recording economic data (only 13.6%) proves that most of them do not perform prospective cost-effectiveness evaluations. This leads to the subsequent need of cost estimations, which will be included in a model which will not benefit from the availability of cost or effectiveness data at a patient level.19 The main advantage of this type of analyses would be a higher validity because they are found in the patients enrolled themselves.
In our opinion, HRQL measurement does not always have the same importance in the design of a CT. Non-evaluation of HRQL might be warranted in certain CTs like those performed in paediatric disorders or critical patients in whom a short-term intervention is assessed. The re-calculation of the proportion of studies analysing HRQL, once the protocols unable to estimate that parameter are excluded, would elicit a rate of 31.9% which does not mean a great difference with regard to 28.5% obtained without exclusion of those CTs (Table 4). Considering that evaluation of this issue is largely subjective, our results show that in about 3 out of every 4 clinical trials HRQL measurement would add some relevant information. This proportion shows the potential increase that HRQL measurement might mean in a close future. Besides, there is a definite need to measure the patients’ perception in CTs involving chronic disorders, which affect activities of daily living like for example urine incontinence, Crohn's disease, asthma or chronic arthritis. Lack of HRQL measurement in drug CTs concerning those disorders might be considered an important limitation, especially in the case of newly developed drugs aiming to prove clinically relevant achievements. However, its absence as an explicit registration criterion by regulatory agencies can partly explain why CTs do not include such evaluations in their design.
The lack of HRQL measurements in certain therapeutic areas in which it would have been clinically relevant (like 58% of CTs from the cardiovascular area or 50% from oncology) is particularly striking. By contrast, in the rheumatology area this can be observed in only 33% of the studies. This difference may be due to the link between rheumatologic treatments and the pain relief they aim to achieve since, individuals generally adapt to the various manifestations of the disease with the exception of pain.20,21 Thus, treatments followed by a pain relieve like hip replacement, often show larger effect sizes than other treatments which translate into a loss of social life like for example sleep apnoea.22–24 In order to know the baseline value and be able to evaluate the effect size, which allows to estimate the real impact of a certain therapy in terms of improvement in the QOL, Sprangers25 has suggested the ‘then test’ as an alternative. The challenge of this technique is to resolve methodological problems and incorporate the patient's perception into the CT outcome measurements in a more optimal way.
Our study has differentiated between HRQL and functional level since we have considered them as two separate concepts and consequently the autonomy level scales have not been recorded as HRQL questionnaires. According to our results, 10.8% of CTs include this type of questionnaires as primary efficacy variables evaluating the persons’ ability to carry out daily life activities. The use of these tools is mainly indicated for the evaluation of treatment of disability-generating disorders like neurological conditions. This differentiation is of a great importance since treatments like thrombolysis for stroke use this type of scores with the aim of estimating the improvement in their prognosis in terms of both mortality and disability.26 Use of HRQL questionnaires is the main way to determine utilities according to the degree of disability for subsequent consideration in cost-effectiveness studies from this type of conditions.23 A conceptual model, which enables the researcher to understand the relationship between these two concepts has been proposed.27 Consistently with them, the “functional status level” plays a mediating role between diseases and HRQL. Both dimensions play a key role for investigation of the efficacy of treatments, but HRQL is mostly oriented to evaluate the outcomes from the patient perception perspective and functional scales in turn to care planning.
HRQL measurements are important to estimate healthcare outcomes and their usefulness can be more accurately assessed as complementary to survival variable analysis. The results from our study show that at present this is still far from being the case. Continual use of this efficacy measurement might result in an adequate assessment of chronic disorders whose burden of disease is due to disability.
The inclusion of HRQL measurement in CTs for the assessment of efficacy of new drugs is for the time being underused. Systematic incorporation of this parameter to CT protocols would translate into a more accurate and broader estimation of the efficacy of new treatments, what would make its comparison with other drugs or healthcare interventions much easier. It would also entail direct use of the results from CTs for economic evaluation of drugs.
Conflict of interestsAuthors have no conflict of interests to declare.