In recent years, there has been a considerable increase in clinical trials in the field of oncohematology, an area in which new drugs are constantly emerging. In addition to the large number of available clinical trials, the growing complexity of the treatments for cancer is also a relevant factor. This situation has required a notable evolution and specialization of the functions and responsibilities of pharmacy technicians within the Clinical Trials Unit of the Pharmacy Service.
This article describes the role of the pharmacy technician in a Clinical Trials Unit of tan oncohematological hospital pharmacy service, with over 20 years of experience in clinical trials.
En los últimos años ha habido un aumento considerable de ensayos clínicos en el campo de la oncohematología, donde continuamente aparecen nuevos fármacos. Además del elevado número de ensayos clínicos disponibles, es también relevante la creciente complejidad de los tratamientos desarrollados para el tratamiento del cáncer. Esta situación ha requerido una evolución y especialización notable de las funciones y responsabilidades del personal técnico de farmacia en las unidades de ensayos clínicos de los servicios de farmacia.
En el presente artículo se describen las funciones del personal técnico de farmacia en una unidad de ensayos clínicos del servicio de farmacia de un hospital oncohematológico, con una trayectoria de más de 20 años de experiencia en ensayos clínicos.
Clinical trials (CTs) are research studies that evaluate a wide variety of health interventions or technologies. Some trials are designed to compare or demonstrate the effectiveness of different surgical techniques, medical devices, preventive methods, or health strategies, among other interventions. However, the most common CTs are those aimed at developing new drugs or new uses for existing drugs.1
This is particularly relevant in the field of oncohaematology, given that, according to the Spanish Registry of Clinical Trials, more than a third of the CTs authorised in 2023 were for cancer treatment.2 In this field, new drugs are constantly emerging. These drugs often form part of complex and extremely costly therapies that require rigorous control over their preparation and administration.
In this regard, the role of pharmacy services (PSs) in supporting and assisting CTs has evolved considerably in recent years, in line with the increased number and complexity of CTs. This has resulted in an increase in the number and variety of activities performed by pharmacy technicians (PTs) in the CT units in hospital centres.3
Pharmacy technicians play a key role in patient care. Their role has evolved and become fully integrated into pharmacy practice. They are trained staff who collaborate closely with pharmacists, assume various functions and responsibilities. They also have specific skills needed to address the pharmacotherapeutic needs of patients.4,5 These developments have resulted in CT pharmacy technicians (CTPTs) becoming highly specialised in this area.
The CTPTs prepare and manage medications for both inpatients and outpatients, dispensing oral or home-administered drugs directly to patients enrolled in CTs. They also play a key role in developing CTs in PSs, offering valuable support and expertise in areas such as monitoring, document management, stock control and expiry date tracking, therapeutic adherence (TA) evaluations, indicator follow-up, and CT unit quality management.
Therefore, it is essential to promote the education and training of CTPTs to ensure the safety and quality of all processes related to the medicines prescribed and administered in the context of CTs. This approach will help to ensure compliance with the relevant protocols and standards of good clinical practice.6
This study describes the main functions and responsibilities of the CTPT working in CTs within the PS of an oncohaematological hospital. The aim is to improve understanding of the activities they perform within their area of work, enabling other developing CT units to integrate these activities into their workflows.
Work modelThe CT unit of the PS was established towards the end of the 1990s. Its activity has increased steadily, over the last 5 years in particular, with the number of active CTs rising from 278 to 479. The growing number of active CTs in the field of oncohaematology has led to an increase in their complexity, involving highly varied and complex treatments such as Advanced Therapy Medicinal Products (ATMPs), including Chimeric Antigen Receptor T-cell therapy, tumour-infiltrating lymphocytes, and mesenchymal cells, as well as bispecific antibodies and targeted drugs.
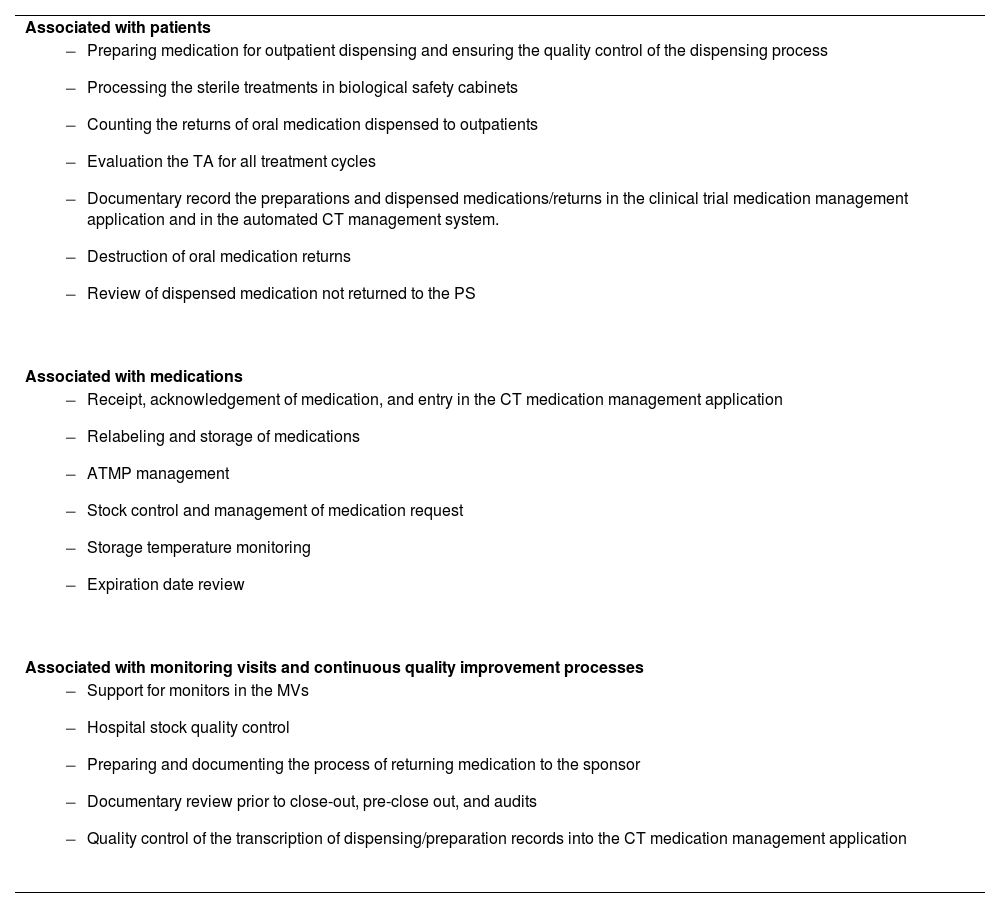
Over the past two decades of experience, the functions and responsibilities of the CTPT have been defined and expanded, in line with the evolving requirements of CTs. This change in the profile of the CTPT represents a qualitative leap, providing improved management and monitoring of CT processes, and bringing practice more closely into line with the quality standards set out in the published guidelines on CTs within hospital pharmacy.7–9 The tasks that can potentially be delegated to the CTPT can be categorised into three groups, based on their scope of application (Table 1).
Roles and responsibilities of clinical trial pharmacy technicians in the clinical trials unit of the pharmacy service.
| Associated with patients |
|
| Associated with medications |
|
| Associated with monitoring visits and continuous quality improvement processes |
|
TA, therapeutic adherence; CT, clinical trial; ATMP, advanced therapy medicinal product; PS, pharmacy service; MV, monitoring visits.
Each of the functions delegated to the CTPT of the CT unit of an oncohaematological hospital are detailed below.
Roles and responsibilities of clinical trial pharmacy technicians associated with patientsPreparation of medication for outpatient dispensing and subsequent quality control of the dispensing processThe entire chemotherapy process is managed by a computer application covering medical prescriptions, pharmaceutical validation, preparation, dispensing, distribution, and administration. This system is used for all oncospecific or supportive care and research treatments.
One of the main functions of PSs is to ensure the correct dispensing of medication to both inpatients and outpatients. This process requires the creation of a specific medication dispensing circuit that includes both care-level and CT medications.
In the case of outpatient-dispensed CT medication, an accurate record is essential to ensure the traceability required in CTs. Once the treatment has been validated by the pharmacist, the CTPT is responsible for preparing the relevant medication. This allows a second technician, from the outpatient dispensing area, to dispense it to patients more safely following a second review. The outpatient dispensing PT records the drug, quantity, lot, and expiry date of the CT medication dispensed in the chemotherapy application, as well as the kit number if necessary (for double-blind CT or numbered medication).
At the end of each day, the CTPT is responsible for reviewing all CT oral medication dispensed throughout the day. During this process, they check again that patients have received the correct medication (drug, dose, batch number, expiry date, and kit number), in accordance with the pharmacist's validation. They also check that this information has been correctly recorded in the chemotherapy application.
Processing of sterile treatments in biological safety cabinetsCytostatics are cytotoxic substances that must be prepared in vertical laminar flow biological safety cabinets (Class II, Type B) within negative-pressure clean rooms due to their potential mutagenic, teratogenic and carcinogenic effects on handlers. This approach ensures staff safety, maintains drug integrity, and prevents cross-contamination. In addition to cytostatics, other antineoplastic drugs with different safety profiles are also handled. For this reason, the PT responsible for handling these drugs must be trained and qualified for the tasks.10
This training should cover knowledge on the characteristics and nature of cytostatics, exposure risks, protective measures, handling techniques, work methodology, and the actions to take in the event of drug exposure.
Using personal protective equipment (PPE) correctly is one of the most effective ways to prevent exposure to hazardous products, so wearing the right clothes is essential. This includes closed footwear with non-slip soles as well as other available PPE such as caps, shoe covers, masks, gowns, sterile and nonsterile gloves, and protective goggles.
The PT of the preparation unit is trained and qualified to prepare intravenous treatments for CTs. If more complex preparations or non-standard procedures are required by some CTs, the designated reference CTPT is always assigned to undertake them or provide support.
At the end of each working day, the CTPT reviews the reports in the chemotherapy application reports relating to CT preparations carried out that day. This review verifies that the drug, dose and number of vials used are correct, as well as the kit number of the vials if applicable. This ensures the traceability of all CT treatments.
Counting the returns of oral medications dispensed to outpatientsOn the first day of each cycle, patients included in CTs involving oral drugs return any leftover medication from the previous cycle to the PS. When dispensing medication, the PT in the outpatient area records the return of any oral medication from the previous cycle in the chemotherapy application. They indicate the drug name, the number of returned packs, the kit number (if applicable), the batch number, expiry date, and the return date.
Subsequently, the CTPT counts the units returned in each pack in an area with air extraction, while wearing PPE, and records this information in the dispensing/return record. Another technician then reviews this process.
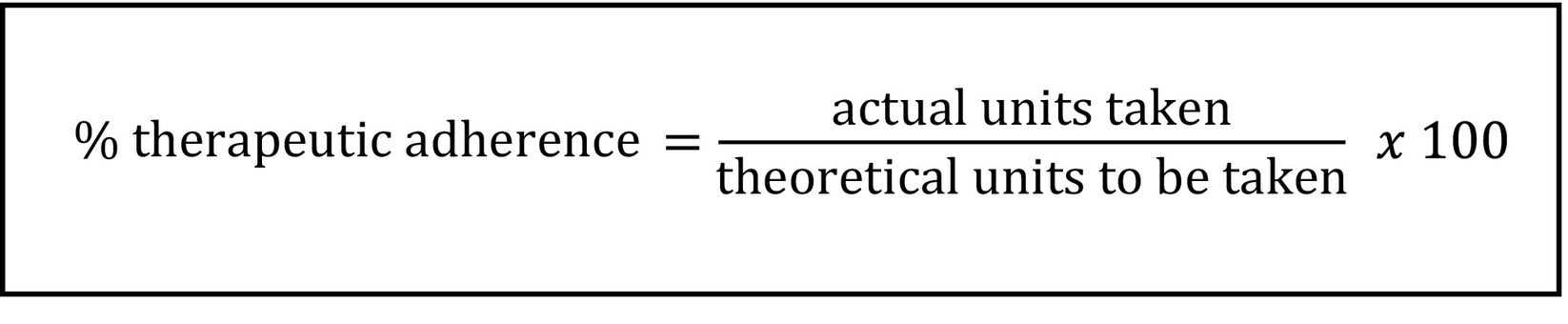
Evaluation of TA for all treatment cyclesIt is important to know the degree of TA among patients when assessing the effectiveness and safety of pharmacological treatments. For example, in CTs involving orally administered drugs, for example, TA is essential to ensure the validity of the efficacy results.
The CTPT calculates TA by counting the units returned by patients after each cycle of oral medication treatment. This is done by recording the dispensing and return dates, dosage, and the number of units dispensed and returned in the CT medication management application, and applying the formula shown in Fig. 1.
If inadequate TA is identified—that is, if a result is outside the 90%-110% range, or if adherence cannot be evaluated due to incomplete medication return—and if a justified reason for the discrepancy has been ruled out by reviewing the patient's medical record, the CTPT informs the CT pharmacist. The pharmacist then performs a second review and implements corrective measures or improvement actions. This includes communicating the information to the medical team by recording it in the patient's clinical history and reinforcing the dosage instructions with the patient, among other measures.
The involvement of the CTPT in calculating TA is essential for detecting possible episodes of inadequate TA and promptly reinforce patient health education when necessary.
Documentary record of preparations and dispensed medications/returns in the clinical trial medication management application and in the automated clinical trial management systemAccurate document control is essential for ensuring the quality and traceability of CTs. Here, the CTPT is responsible for transcribing the medication preparation and dispensing/return records for all CT treatments from the chemotherapy application to the CT medication management application. Specifically, some CTs have an automated management system (Interactive Response Technology [IRT]) available, which facilitates patient data management and assists centres and the CT sponsor in monitoring medication supply and controlling inventory. The CTPT is also responsible for recording any additional preparation, dispensing or return information in the automated system when required.
Destruction of oral medication returnsUsed investigational drugs are accounted for, managed, and disposed of in PSs under the appropriate safety conditions recommended by the National Institute for Occupational Safety and Health11 and in accordance with the quality standards set out in the United States Pharmacopoeia's (USP)12 Chapter 800 on the handling of hazardous drugs.
Thus, empty or partially empty packs are deposited in the cytostatic waste containers (Group IV) once the oral CT medication returned by patients in each treatment cycle has been accounted for and TA has been calculated.11 The CTPT records this procedure in the CT medication management application and records the destruction on the relevant certificate, which is signed by both the CTPT and a CT pharmacist.
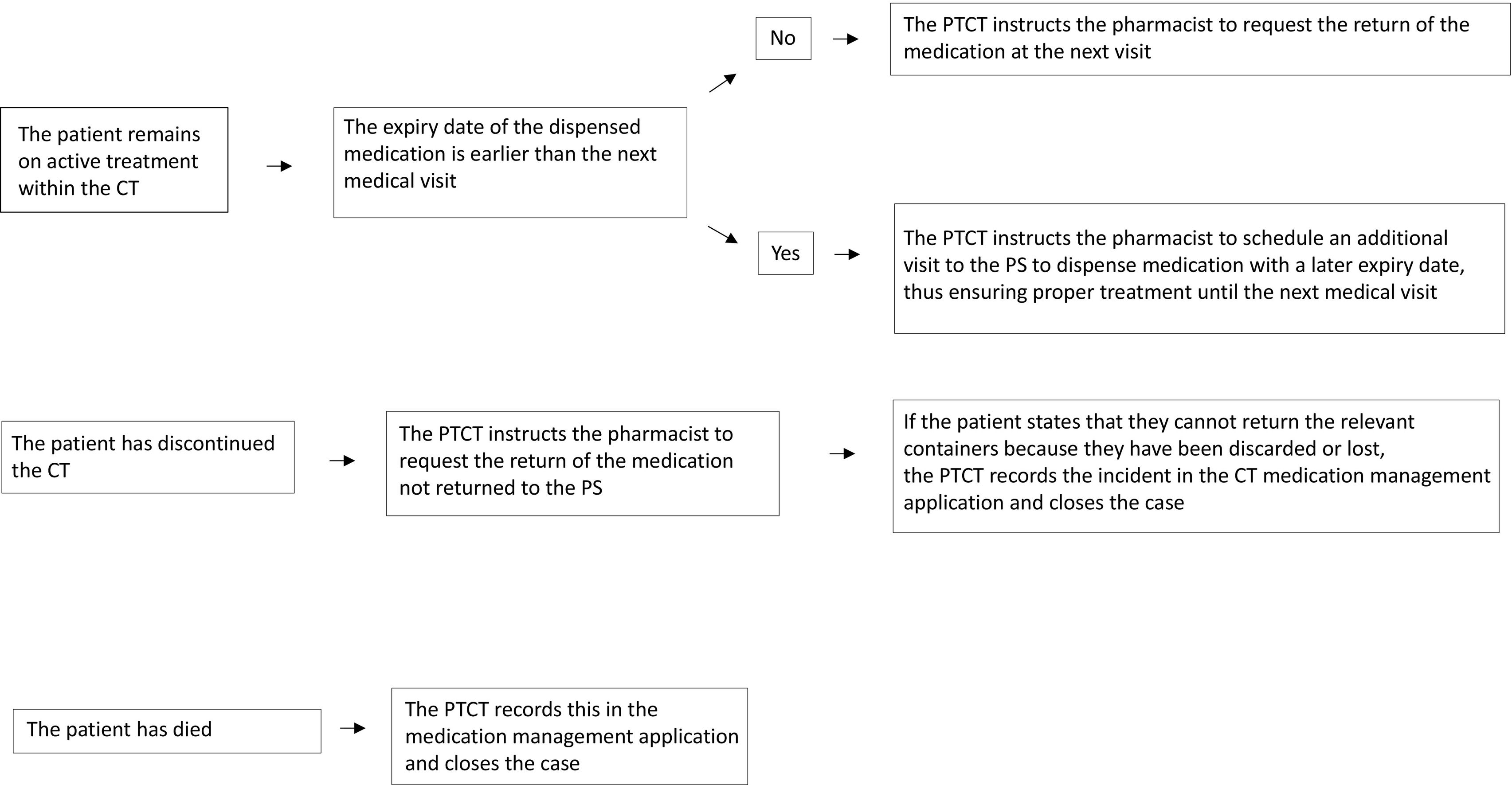
Review of dispensed medication not returned to the pharmacy serviceA quality circuit has been established, led by the CTPT, in order to improve the control of all CT medication dispensed to outpatients that has not been returned to the PS. As medications are only dispensed for a maximum of 3 months, the CTPT conducts a monthly review of all CT medication that was dispensed more than 4 months ago—or due to expire at the end of the current month—which has not been returned to the pharmacy service.
The CTPT reviews the clinical history of each identified patient to determine whether they are still undergoing active treatment within the CT, have discontinued treatment, or have died. Depending on each patient's situation, they proceed according to the flowchart shown in Fig. 2.
CT, clinical trial; CTPT, clinical trial pharmacy technician; PS, pharmacy service.
This circuit improves the quality of the CT documentation, minimising missing data in the accountability records. It also promotes the safe use of medication, reducing the risk of taking expired medication or the accumulation of oncospecific medication containers in patients' homes.
Roles and responsibilities of clinical trial pharmacy technicians associated with medicationsReceipt, acknowledgement of medication, and entry in the clinical trial medication management applicationAll CT samples received at the PS must be reviewed to ensure they are suitable for addition to the centre's CT stock. The CTPT is responsible for identifying the CT medication received by checking the following information on the delivery note: tthe medication received, the corresponding CT, quantity, the batch number, the expiry date, the kit number (if applicable), and the storage temperature during transport. If any discrepancies are found, the CTPT quarantines the medication according to its conservation characteristics and informs the relevant CT monitor.
Once the medication has been received, the CTPT acknowledges receipt via an automated IRT system or email, depending on the circumstances. The medication is then entered into the CT medication management application.
Relabeling and storage of medicationsClinical trial medication should be stored in a restricted access area according to the conditions established by the CT pharmacy protocol or manual, and should be clearly labelled and separated from the rest of the medication in the PS.6
Depending on storage requirements, the medication can be kept at room temperature (15–25 °C), in refrigerators (2–8 °C), or in freezers (−25 to −15 °C and −80 to −70 °C).
Once the CT samples have been received at the PSs and the receipt has been acknowledged, the medication is labelled with the relevant CT code. The CTPT then relabels the medication and stores it in its final location according to storage conditions and rotation. The medication with the nearest expiry date is placed at the front.
All medication intended for outpatient dispensing or non-numbered parenteral administration is relabelled with a 2D barcode that can be read automatically and is generated when the medication is entered into the CT medication management application. This barcode contains detailed information about the drug, including the corresponding CT number, dose, batch number, and expiry date. It enables the automated recording of the medication used in the preparation of any treatment or outpatient medication dispensing in the chemotherapy application. This automated record, created via 2D barcode scanning, eliminates the need for manual entries and improves the quality and safety of CT documentation.
The relabeling process of CT medication carried out by the CTPT is key to ensuring that CTs function correctly within the PS. For this reason, it is subject to strict quality control to prevent critical errors affecting patient safety. Thus, the label generated automatically by the CT medication management application is reviewed twice by the CTPT before relabeling, verifying the information against the medication pending relabeling. This double-check is recorded in the CT medication management application.
Advanced therapy medicinal product managementAdvanced therapy medicinal products require careful management in CTs, as they are fragile and require special handling and specific storage conditions. Both pharmacists and the CTPT should be integrated into and play an active role within multidisciplinary clinical teams responsible for managing patients treated with cell therapy and providing follow-up.13
Once the medical prescription and pharmaceutical validation of the treatment have been completed, the CTPT prepares the reception area and the thawing equipment for the ATMPin the PS, if applicable. The CTPT also prepares all the necessary documentation for these processes, according to the requirements of each product and each CT.
The ATMP bags arrive at the PS under specific preservation conditions (liquid nitrogen tank for cryopreserved medication; refrigerator for medication preserved at 2–8 °C). The CTPT then receives the medication, and verifies that the correct temperature has been maintained during transport.
Once it has been confirmed that the patient is ready to receive the ATMP, the CTPT, the CT pharmacist, and the hospital blood and tissue bank nurse will perform the following actions:
- •
Review of patient data and labelling of the ATMP bag.
- •
Completion of centre-specific ATMP documentation.
- •
Completion of CT-specific ATMP documentation (as required by protocol).
- •
Confirmation of the thawing procedure, if applicable (recording start/end times).
- •
Updating the expiry date/time of the ATMP bag (according to the specifications of each product).
Coordinating among all those involved in the confirmation, reception, handling, and administration of ATMPs is challenging, but vital, since these drugs involve very complex preparation processes and are commonly tailored to specific patients. They also have very short stability periods (less than 30 minutes in some cases).
Stock control and medication request managementThe stock control procedure for the medication is agreed upon with the monitor during the clinical trial visit. This procedure can be automated in CTs managed through automated systems (IRT or similar), or it can be carried out manually.
The CTPT uses the CT medication management application to generate a weekly list of requirements, detailing manually stock-controlled medication with insufficient stock. This list is based on needs, treatment cycles, and the number of actively enrolled patients. If the stock is insufficient, the CTPT requests the urgent shipment of medication, to prevent incidents and ensure that patients receive their treatments within the CTs.
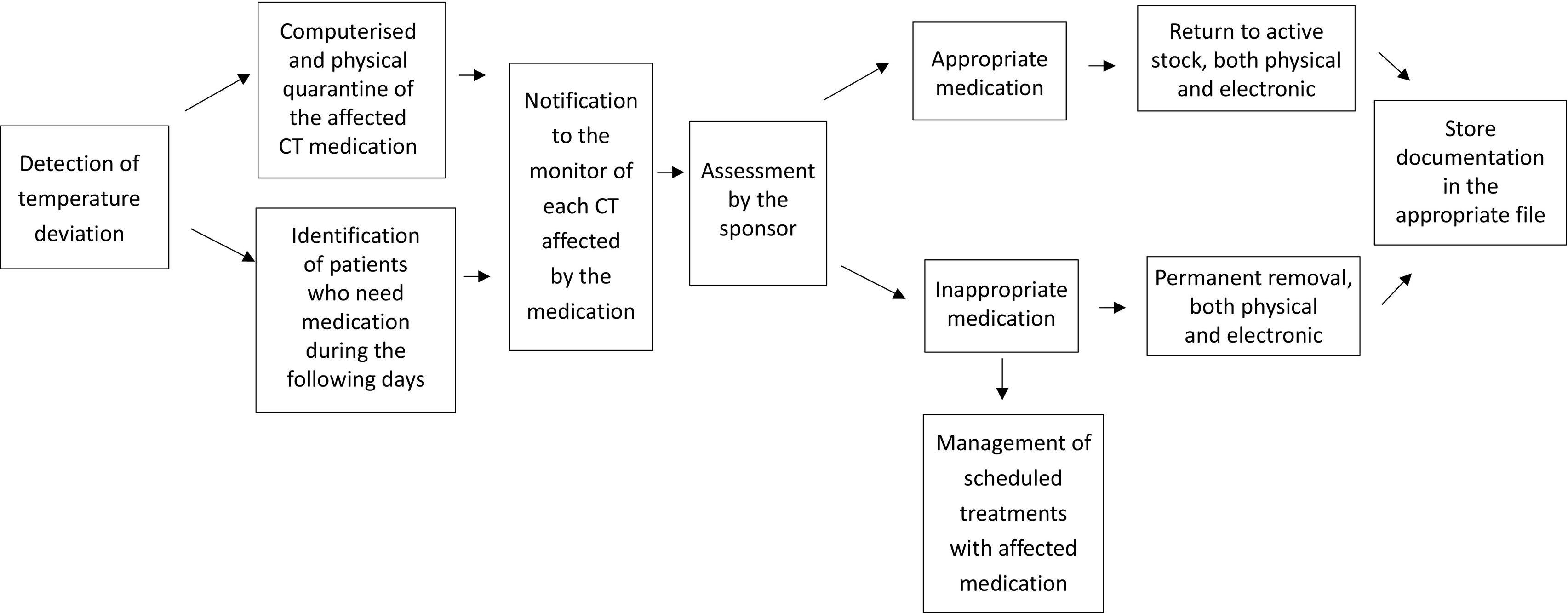
Storage temperature monitoringIt is fundamental to maintain medication within the temperature ranges specified by the pharmacy manual or study protocol. Electronic data logging probes are used to ensure correct temperature control during storage, collecting data every 10 minutes for refrigerator and freezers and every 15 min for ambient temperature. This information is centralised in software that allows temperature readings to be accessed at any time. If two consecutive readings are outside the defined range, this is considered a temperature deviation, and an alarm is triggered and sent by email to the relevant staff for review and management.
The CTPT is responsible for reviewing the temperature records from all medication storage locations each day, checking for any out-of-range readings. To accomplish this, the CTPT examines the records gathered by the software from all locations since the previous working day, documenting this quality control process in the corresponding log.
In the event of a temperature deviation in any CT medication storage area, the CTPT will manage the incident in accordance with the action algorithm outlined in Fig. 3.
Expiration date reviewThe CTPT reviews medication that is close to its expiry date to ensure it is suitable for use in patients.
This quality procedure is carried out in two stages. One week before the end of the month, a check for expiry dates is run in the CT medication management application to identify medications that will expire on a given date. This process takes into account studies in which oral medication is dispensed for more than one month. Based on the resultsof this report, the relevant medication is checked and the monitor is contacted if more medication is required.
On the last day of the month, the medication is removed from the physical stock and this is recorded in the CT medication management application. Parenteral medication is removed from active stock on the last day of its expiry month of expiration, or on itsexpiry date when expressed in DD-MMM-YYYY format. Outpatient medication is removed from active stock one month before its expiry date to prevent it from reaching this date at the patient's home.
Withdrawn medication is stored in clearly labelled boxes classified by CT and kept under the appropriate temperature-controlled storage conditions until it is checked by the CT monitor.
Roles and responsibilities of clinical trial pharmacy technicians associated with monitoring visits, quality process, and continuous improvementSupport for monitors during monitoring visitsThe CTPT is responsible for arranging monitoring visits (MVs), either remotely or in person, requested by the monitors of the CTs running at the centre.
The process of preparing for the MV begins the week before the scheduled visit, when the CTPT sends an email reminder to each monitor. This email includes a link to access the relevant temperature records and includes the following records which are extracted from the CT medication management application. These records include dispensing per patient, medication entries, inventory available at the centre, and records of expired medication withdrawn and pending destruction. This approach enables monitors to review all the documentation prior to the visit. For face-to-face MVs, the monitoring time at the PS can be used to review source documents and resolve any queries, thus optimising time spent at the centre.
Any discrepancies identified by the monitor are brought to the attention of the CTPT on the day of the visit so that can be analysed and resolved.
Hospital stock quality controlTo check the concordance between the actual and theoretical stock, the CTPT counts the available units of certain drugs, and checks the quantity, lot/expiry date, and kit number. This information is then compared the inventory records available on the CT medication management application (theoretical stock). This quality control process is conducted one week before each scheduled MV, based on the available medication stock for each scheduled CT.
Any discrepancy identified is reviewed by the CTPT to establish its origin. It is then resolved within the CT medication management application.
Preparing and documenting the process of returning medication to the sponsorThe growing number of open CTs at the centre has led to an increase in the return of medication to the sponsor (surplus or expired study medication). It is therefore essential to maintain proper control of all the medication pending return and to keep a standardised record of all the medication leaving the CT unit of the PS.
If the CT monitor requests that a medication be returned to the sponsor, the CTPT is responsible for removing the medication from the stock in the CT medication management application, preparing the return, generating the corresponding return certificate, and labelling the box, in accordance with the monitor's guidelines. The CTPT is also responsible for delivering the medication to the carrier, recording its dispatch in the relevant document, and obtaining the signature of the recipient to ensure traceability of this final movement of CT medication within the centre.
Documentary review prior to close-out, pre-close out, and auditsThe quality of the pharmacy documentation archive is essential for the proper functioning of the CT and its subsequent closeout. It is crucial that, when merging the pharmacy and investigator archives at the end of the CT, the pharmacy archive contains all the necessary documentation and is in the correct condition and version. The archive must also be stored for the period specified by current legislation.
Prior to the CT pre-closeout and closeout visits, the CTPT reviews the documentation archive to identify and resolve any potential deficiencies in the study documentation. Any discrepancies or deficiencies are resolved prior to the visit.
The CTPT is also responsible for reviewing documentation prepared in anticipation of CT audits or facility inspections. This includes storage temperature records, medication receipts, and preparation, dispensing, and return records.
Quality control of the transcription of dispensing/preparation records into the CT medication management applicationRecords from the chemotherapy management application are transcribed into the CT application manually. To minimise errors, quality control measures are in place to identify discrepancies between the two applications.
These quality control measures are implemented through a spreadsheet that compares the data recorded in the chemotherapy application with those in the CT medication management application, in order to detect discrepancies in data recording. The following variables, obtained from both applications, are compared: date of dispensing/preparation, CT code, patient, drug, batch number/expiration date, kit number, and quantity.
When discrepancies are identified, the relevant records from both programmes are reviewed in order to resolve the error as appropriate, thereby improving the quality of the CT documentation in the PS.
ConclusionsThe increasing number and complexity of CTs, particularly in oncohaematology, poses a challenge for PSs. As a result, the role of the CTPT has evolved to include new functions, expanded responsibilities, and the development of relevant competencies in the field of CT management. We believe that it is particularly important to highlight the roles and tasks that CTPTs can assume during the development of CTs within PSs, in order to improve their quality, optimise resource availability, and support other developing CT units in integrating them into their workflows.
CRediT authorship contribution statementNúria Farré Cabrerizo: Writing - review & editing, Writing - original draft, Supervision, Project administration, Methodology, Investigation, Data curation, Conceptualization. Olalla Montero Pérez: Writing - review & editing, Writing - original draft, Supervision, Project administration, Methodology, Investigation, Data curation, Conceptualization. Maria Emilia Miquel Zurita: Visualisation, Validation, Project administration, Methodology, Investigation, Conceptualization. Marina Badàs Moreno: Writing - review & editing, Writing - original draft, Supervision, Project administration, Methodology, Investigation, Data curation, Conceptualization. Marta Company Martos: Writing - review & editing, Writing - original draft, Supervision, Project administration, Methodology, Investigation, Data curation, Conceptualization. Gemma Garcia Deu: Writing - review & editing, Writing - original draft, Supervision, Project administration, Methodology, Investigation, Data curation, Conceptualization. Jennifer Rodríguez Rojas: Writing - review & editing, Writing - original draft, Supervision, Project administration, Methodology, Investigation, Data curation, Conceptualization. Mònica González Laguna: Visualisation, Validation, Project administration, Methodology, Investigation, Conceptualization. Sonia Narváez Seixa: Visualisation, Validation, Project administration, Methodology, Investigation, Conceptualization. Sandra Fontanals Martínez: Visualisation, Validation, Project administration, Methodology, Investigation, Conceptualization. Maria Perayre Badia: Visualisation, Validation, Supervision, Project administration, Methodology, Investigation, Conceptualization.
FundingNone declared.
None declared.