To develop a safety working procedure for the employees in the Intermutual Hospital de Levante (HIL) in those areas of activity that deal with the handling of hazardous drugs (MP).
MethodsThe procedure was developed in six phases: 1) hazard definition; 2) definition and identification of processes and development of general correct work practices about hazardous drugs’ selection and special handling; 3) detection, selection and set of specific recommendations to handle with hazardous drugs during the processes of preparation and administration included in the hospital GFT; 4) categorization of risk during the preparation/administration and development of an identification system; 5) information and training of professionals; 6) implementation of the identification measures and prevention guidelines.
ResultsSix processes were detected handling HD. During those processes, thirty HD were identified included in the hospital GFT and a safer alternative was found for 6 of them. The HD were classified into 4 risk categories based on those measures to be taken during the preparation and administration of each of them.
ConclusionsThe development and implementation of specific safety-work processes dealing with medication handling, allows hospital managers to accomplish effectively with their legal obligations about the area of prevention and provides healthcare professional staff with the adequate techniques and safety equipment to avoid possible dangers and risks of some drugs.
Desarrollar un procedimiento de trabajo seguro para los trabajadores del Hospital Intermutual de Levante (HIL) en las distintas áreas de actuación relacionadas con la manipulación de medicamentos peligrosos (MP).
MétodosEl procedimiento se desarrolló en seis fases: 1) definición de medicamento peligroso; 2) definición e identificación de procesos y elaboración de recomendaciones generales sobre selección y manejo de MP; 3) detección, selección y establecimiento de recomendaciones específicas para el manejo durante la preparación y la administración de los MP incluidos en la GFT del hospital; 4) categorización del riesgo durante la preparación/administración y desarrollo de un sistema de identificación; 5) información y formación a los profesionales; 6) implantación de las medidas de identificación y las pautas de actuación.
ResultadosSe detectaron seis procesos implicados en el manejo de MP. Se identificaron 30 MP incluidos en la GFT del hospital y se encontró una alternativa más segura para seis de ellos. Los MP se clasificaron en cuatro categorías de riesgo en función de las medidas a adoptar durante la preparación y administración de cada uno de ellos.
ConclusionesEl desarrollo e implementación de procedimientos de trabajo específicos para el manejo seguro de medicamentos permite a los responsables de un hospital cumplir de forma efectiva con las obligaciones legales en materia preventiva, así como proporcionar a los trabajadores un medio adecuado para evitar la posible peligrosidad de algunos medicamentos.
In 2004, the National Institute for Occupational Safety and Health (NIOSH) defined the term Hazardous Drug (HD)1 as those medications presenting one or more of the following risk criteria in humans: carcinogenicity, tetarogenicity or other toxicity for development, reproductive toxicity, organ toxicity at low doses, genotoxicity; and new medications with structure and toxicity profiles similar to existing medications already classified as hazardous according to the previous criteria.
At the same time as the HD definition from 20041, the NIOSH published a list of HDs, which was updated in 20102, 20123, 20144 and the draft for the 2016 update was being prepared at the time of writing this document5, differentiating the risk of those drugs included in three groups (Table 1).
Level of risk posed by HDs.
| Group 1 | Antineoplastic drugs. |
|---|---|
| Group 2 | Non-antineoplastic drugs that meet at least one criterion for hazardous drugs. |
| Group 3 | Drugs that pose a reproductive risk to men and women who are actively trying to conceive and women who are pregnant or breast feeding, but that present no risk for the rest of the staff. |
The effects of HDs on health, both therapeutic and side effects, are justified in patients because there is a favourable benefit / risk balance; however, exposure in healthcare staff must be reduced as much as possible6. The safety and health of the staff is a key matter in any healthcare centre; therefore, it has been necessary to determine some recommendations for handling those HDs that pose a risk for the healthcare staff handling said medications.
Faced with the lack of specific regulations in Spain for handling HDs, and the existence of publications warning about the health risks associated with certain medications, there has been a social alarm that justifies the fast preparation and dissemination of a working procedure for handling hazardous drugs, in order to minimize risks for the healthcare staff. Following the criteria scientifically established by organizations with acknowledged prestige, we have selected the documents published by the NIOSH in 20144, and its 2016 update5, and by the National Institute of Occupational Safety and Hygiene (INSHT): “Hazardous Drugs: Prevention measures for their preparation and administration”, as well as the consensus document “Safety for patient and healthcare staff in the preparation and administration of hazardous drugs”7, as the basis for the development of a working procedure for handling HDs in our healthcare centre, with the aim to facilitate a method of work that will observe the safety and health of the staff within a safe setting.
ObjectivesTo develop a safe working procedure for the staff in the Hospital Intermutual de Levante (HIL) in the different areas associated with handling HDs, including any protection equipment required, with the aim to reduce risk and minimize exposure as much as possible.
To disseminate the current information about the risk for workers derived by handling HDs and their waste material in all work areas and positions that might be affected by said handling.
To ensure safety for the staff, the working environment, and the medication.
Material and methodsThe working procedure was developed in six stages:
- a)
Definition of Hazardous Drug (HD).
- b)
Definition and identification of procedures and preparation of general recommendations for the selection and handling of HDs. The procedures involved in HD procedures were defined and identified, and a bibliographic review was conducted about the protection measures for the handler at each one of these procedures, based on the recommendations by the main international associations dealing with HD handling.
- c)
Detection, selection and implementation of specific handling recommendations during the preparation and administration of those HDs included in the hospital formulary8. The information about the molecules included in the “NIOSH list of antineoplastic and other hazardous drugs in healthcare settings, 2014”4 and “Proposed additions to the NIOSH 2016 hazardous drugs list”5 was compared with the list of medications included in our hospital formulary8. In order to determine the specific handling recommendations (infrastructures and individual protection equipment (IPE) for each HD, the following were taken into account: their category within the NIOSH lists (1, 2 or 3), their formulation, way of administration, and place and conditions of preparation and administration. Besides, therapeutic alternatives with lower handling risks were assessed for each HD.
- d)
Classification of risk during preparation / administration and development of a system for identification. In order to facilitate the identification and homogeneity of HD handling, those HDs that shared handling measures during their preparation and administration were grouped into categories.
- e)
Information and training for professionals.
- f)
Implementation of measures for identification and guidelines for action.
In terms of occupational exposure, Hazardous Drugs were defined as: those agents that, due to their inherent toxicity, represent a risk for the healthcare staff handling them. The risk posed by these medications is understood in terms of chemical risk, specifically, associated with the carcinogenic, teratogenic, genotoxic, toxic for the reproductive process or for a specific organ at low doses, or by being a new drug similar to those with this type of risks. In this sense, the following rules apply to HDs: protection rules for workers associated with the exposure to chemical agents (RD 374/2001)9, carcinogenic agents (RD 665/1997)10 and their subsequent modification (RD 349/2003)11, and the 2004/37/CE directive12 about protection against the risks of exposure to carcinogenic or mutagenic agents during work.
Stage b) Definition and identification of procedures and preparation of general recommendations for the selection and handling of HDsThe “potentially hazardous” procedures, activities, operations, equipment or products (article 4.5 of the Law for Prevention of Occupational Risks)13 will be defined as those that, in the absence of specific preventive measures, will pose risks for the safety and health of the staff developing or using them.
Six procedures involved in HD handling were identified: selection, reception, transportation and distribution, preparation, administration, and waste management.
The following HD handling recommendations were reviewed: by the NIOSH4, INSHT6, Oncology Nursing Society (ONS)14, American Society of Health-System Pharmacists (ASHP)15, National School of Occupational Medicine in the Instituto de Salud Carlos III (ISC)16, U.S. Pharmacopoeia (USP)17 and International Society of Oncology Pharmacy Practitioners (ISOPP)18; and based on these, general recommendations for HD handling were prepared for each of the procedures detected (Appendix 1)19,20.
Stage c) Detection, selection and implementation of specific handling recommendations for those HDs included in the hospital formularyTwenty-nine (29) medications included in the hospital formulary8 were identified as molecules classified as hazardous by the “NIOSH list of antineoplastic and other hazardous drugs in healthcare settings, 2014”4 and “Proposed additions to the NIOSH 2016 hazardous drugs list”5. Besides, even though acenocoumarol was not included in the NIOSH lists, it was considered a HD given its similarity with warfarin (NIOSH List 3). Therefore, 30 medications included in the hospital formulary were finally identified as HDs. According to their level of risk, none was included in List 1 (antineoplastic drugs), 12 were from List 2 (non-antineoplastic drugs that meet at least one criterion for hazardous drugs), and 18 were from List 3 (drugs that pose a reproductive risk to men and women who are actively trying to conceive and women who are pregnant or breast feeding, but that present no risk for the rest of the staff).
A pharmaceutical alternative with lower risk in handling was found for six of the 30 HDs included in the hospital formulary. Five of these six HD were oral solid formulations that could be split or crushed for their administration to patients with swallowing problems; therefore, instead of splitting or crushing, it was decided to prepare oral liquid formulations as magistral formula in a biological safety cabinet (BSC), to simplify preparation and administration and reduce handler exposure to the drug. The remaining HD was marketed in vials, so it was decided to purchase it as magistral formula in vials in order to minimize exposure. Thus, the final list included 36 HDs.
Finally, based on the classification of each HD in the NIOSH lists4,5, their formulation, and the Technical Document by the INSHT: “Hazardous Drugs: Prevention measures for their preparation and administration”6, a list was prepared with all HDs and the specific handling recommendations for each one of them (Appendix 2).
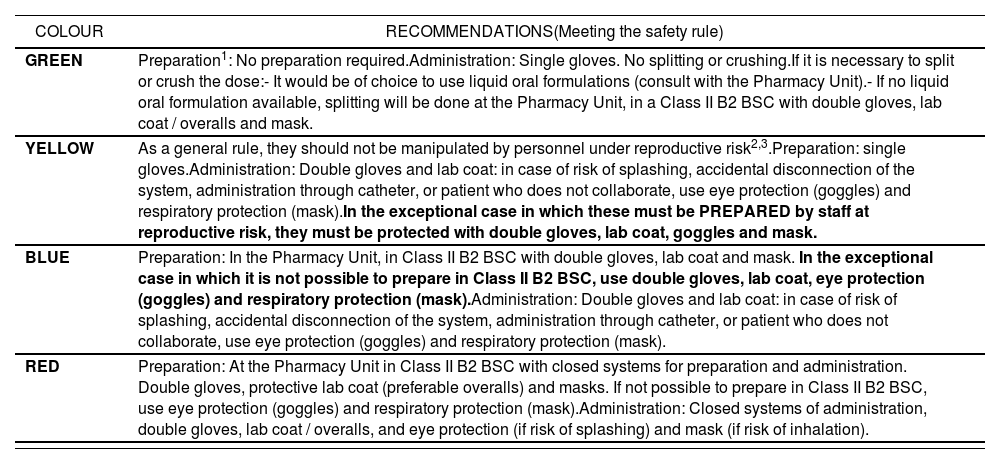
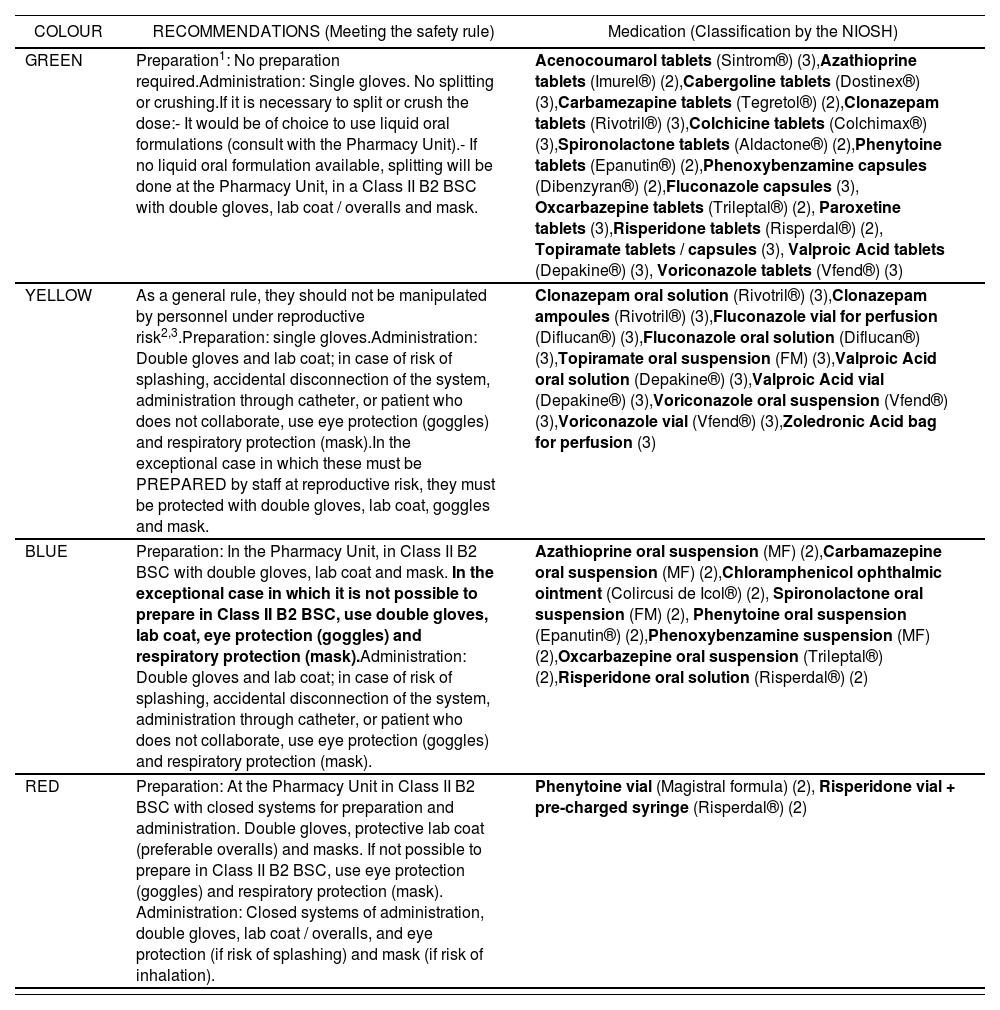
Stage d) Classification of risk during preparation / administration, and development of a system for identificationAccording to the specific measures for handling each HD included in the list of specific recommendations (Appendix 2), these were classified into four categories from lower to higher risk at handling during preparation and administration. In order to simplify HD handling, common measures were established for each category. In the case of those medications with different protection measures according to the reproductive risk for the staff, it was decided to implement the protection measures for the group of professionals with the highest level of protection. In order to differentiate each category, an identification system was implemented, based on a colour code from lower to higher level of protection during preparation and administration (green, yellow, blue and red) (Table 2). The number of HDs included in each category was: 16 in Green, 10 in Yellow, 8 in Blue and 2 in Red (Appendix 3).
Color code based on the specific management recommendations during the preparation and administration of the MP
| COLOUR | RECOMMENDATIONS(Meeting the safety rule) |
|---|---|
| GREEN | Preparation1: No preparation required.Administration: Single gloves. No splitting or crushing.If it is necessary to split or crush the dose:- It would be of choice to use liquid oral formulations (consult with the Pharmacy Unit).- If no liquid oral formulation available, splitting will be done at the Pharmacy Unit, in a Class II B2 BSC with double gloves, lab coat / overalls and mask. |
| YELLOW | As a general rule, they should not be manipulated by personnel under reproductive risk2,3.Preparation: single gloves.Administration: Double gloves and lab coat: in case of risk of splashing, accidental disconnection of the system, administration through catheter, or patient who does not collaborate, use eye protection (goggles) and respiratory protection (mask).In the exceptional case in which these must be PREPARED by staff at reproductive risk, they must be protected with double gloves, lab coat, goggles and mask. |
| BLUE | Preparation: In the Pharmacy Unit, in Class II B2 BSC with double gloves, lab coat and mask. In the exceptional case in which it is not possible to prepare in Class II B2 BSC, use double gloves, lab coat, eye protection (goggles) and respiratory protection (mask).Administration: Double gloves and lab coat: in case of risk of splashing, accidental disconnection of the system, administration through catheter, or patient who does not collaborate, use eye protection (goggles) and respiratory protection (mask). |
| RED | Preparation: At the Pharmacy Unit in Class II B2 BSC with closed systems for preparation and administration. Double gloves, protective lab coat (preferable overalls) and masks. If not possible to prepare in Class II B2 BSC, use eye protection (goggles) and respiratory protection (mask).Administration: Closed systems of administration, double gloves, lab coat / overalls, and eye protection (if risk of splashing) and mask (if risk of inhalation). |
List 1 : Antineoplastic or cytostatic agents.
List 2: Non-antineoplastic medications that meet one or more NIOSH criteria to be considered of risk.
List 3: Non-antineoplastic medications with effects on reproduction, and that can affect men and women who are trying actively to conceive, and pregnant or breastfeeding women, but that don’t represent a risk for the rest of the staff.
1Whenever possible, the original blister will be kept at re-packaging.
2Staff at reproductive risk: men and women who are actively trying to conceive, and pregnant or breastfeeding women.
3For treatments prescribed continuously and that need to be prepared, this will be done at the Pharmacy Unit (PU) in CSBIIB2. Exceptional treatmetns will be prepared according to the protection measures already mentioned and, in case of persistence, their preparation will be similarly assumed by the PU.
MG: Magistral Formula: Class II B2 BSC: Class II Type B2 Biological Safety Cabinet: CLDT: Closed System for Drug Transfer.
With general application, and according to Articles 18 and 19 of the Law for Prevention of Occupational Risks13, workers must receive adequate information and training about the risks derived of the presence of any hazardous chemical agent present in their working place, as well as about the prevention and protection measures to be adopted. In particular, the training of the staff working with hazardous drugs is a key aspect to prevent occupational risks.
Therefore, all professionals involved in handling medications were informed through a Conference for the Safe Use of Medications that was publicized externally and given by the Pharmacy Unit and the Occupational Risk Prevention Unit, with the participation of the Occupational Risk Prevention Unit from the Conselleria de Sanitat Universal y Salut Pública of the Community of Valencia.
The number between brackets corresponds to the number of the NIOSH list in which this medication is included. Acenocoumarol by similarity with warfarin (not included in NIOSH).
Moreover, fortnightly internal sessions were given to the entire healthcare staff, in order to present the code of identification by colours; all the required materials for fast reference were provided, and there was training on the Procedure for “Safe Drug Handling”.
In the same manner, the PPEs required were provided in all grounds and departments in which MPs could be handled.
Stage f) Implementation of measures for identification and guidelines for actionReception and storage: HD labelling by colours based on their category in the classification, both in their packaging (for those HDs distributed in their original package) and in their primary preparation.
Distribution: HDs in the Green and Yellow categories will be normally distributed with the label assigned at reception. The drugs in red and blue categories will be prepared at the Pharmacy Unit and will be identified in the secondary conditioning with red and blue labels, respectively.
Preparation and Administration: Based on the colour code recommendations (Table 2).
DiscussionThe potential exposure to HDs in each procedure where these are present will depend on various factors19:
- •
Intrinsic risk posed by the medication due to its carcinogenic, teratogenic, or genotoxic potential, as well as reproductive toxicity and organ toxicity at low doses.
- •
Sensitivity of the handler: allergies, pregnancy, breastfeeding, reproductive age.
- •
Level of exposure: Penetration or absorption ability of the medication, concentration, amount, handling duration and frequency, type of activity, place, and associated risk of exposure (formulation).
- •
Structure: human resources (education and training, number of handlers), premises (design and technical specifications, availability and type of BSC), use of closed system drug transfer devices (CSTDs) for preparation and administration, and availability of automatic systems.
- •
Use of prevention measures: technical measures (BSC), CSTDs, automatic systems, organization measures (cleaning procedures, actions for spillage and maintenance, waste management and handling techniques) and secondary prevention measures (SPMs), that should feature some minimum characteristics19 (Table 3).
Table 3.Minimum characteristics of SPMs19
Guantes Two pairs of surgical gloves or one pair of gloves that meets the safety characteristics required for handling cytostatic agents. Double use, to protect both the patient and the professional. In case of contact with the HD, these should be immediately replaced. Lab coat / overalls With back opening / central zipper, elastic cuffs, and waterproof front area and sleeves. In case of contact with the HD, it must be replaced. Respiratory protection Self-filtering masks type FFP3. Surgical masks don’t protect against cytostatic sprays. Eye protection Integral-frame panoramic goggles.
Therefore, staff protection must be adapted to each one of these activities and drug formulations, because the precautions to be taken are different in each case. For example, as stated in the NIOSH document from 20144, “in situations such as capsule opening, or tablet splitting or crushing, when there are no extraction cabinets, safety cabinets or isolators, at least double gloves should be used, as well as mask, lab coat, and working surface protection”. Besides, as this list included a great variability of medications, from high-risk cytotoxic agents to medications that could only affect fertile-age staff to a lower degree, the actions to be determined should not be general; instead, there should be a case-by-case analysis, and therefore the following procedure should only be used as a reference.
This means that the list of medications stated in the publication by the NIOSH4,5 can be used as a reference in order to determine specific measures for managing each HD, and each centre should adapt it to its own situation and needs, as well as keep it updated with the new medications, taking into account the recommendations by their manufacturers.
However, given the large number of HDs included in the list, it will be impossible to implement a procedure to ensure the correct and safe manipulation of each HD in the different procedures where it appears.
Therefore, a simplification is necessary in order to ensure an adequate identification. As a general rule, HDs should be identified during the process of their use, including the whole chain, from selection, reception, re-packaging, storage, distribution, preparation, administration, and waste management; and medications should be classified in order to implement common measures of handling in each procedure, to ensure a correct and safe handling of each HD.
ConclusionsIt has been impossible to determine clearly he toxic effects at long-term of exposure to these drugs, but there is evidence of their danger, the potential occupational risk represented by their handling, and the consequences derived. Therefore, it is imperative to adopt measures that will help to reduce this exposure, and guarantee optimal working conditions. In this sense, the most adequate activity will be prevention.
Different incidents associated with HDs have forced hospitals to prepare working procedures with higher or lower complexity or difficult to understand and follow by the staff. The classification and categorization designed for our working procedure has simplified to a great extent the identification and handling of HDS included in the hospital formulary, so that any healthcare professional will know how to act adequately and safely in any procedure involving HDs.
Moreover, the Hospital Administration should regulate the hazard level of medications through visual codes or symbols, in a way similar to the one stated in this procedure. This will equally become a useful tool within the policies for occupational risk prevention in any healthcare centre.
In conclusion, the development and implementation of specific work procedures for the safe handling of drugs will allow hospital authorities to meet effectively all legal duties in prevention matters, as well as to provide staff with an adequate setting to avoid the potential risk posed by some medications.
Conflict of interestsThe authors declare not having any conflict of interests.
Tables 4, 5 and 6 detail the recommendations for the SPMs to be used in different activities.
Among other criteria, the following should be studied:
- •
To choose those marketed formulations with contents better adapted to usual dosing, with the aim to minimize handling.
- •
To choose the most adequate and easy-to-use strength (1, 2, 5, 10, 25 mg/ml).
- •
Not mixing / preparing at the same time formulations of the same medication with different strengths.
- •
To choose, if possible, and in case of hazardous drug, what appears in Appendix Ia and Ib, which are basically formulations with a design that guarantee their low external contamination. Thus, it is better to select:
- –
Vials vs. ampoules.
- –
Formulations as solution for immediate use vs. lyophilized formulations.
- –
Drugs packaged in breakage-proof polypropylene vs. glass.
- –
Certain compounds already pre-charged in syringes ready for administration.
- –
- •
To select among marketed formulations those with a more efficient sealing of the vial after puncture, because if there is an incomplete sealing when the needle is removed, there will be an increased risk of environmental contamination.
- •
The presence or not of preservatives will affect the use-by date of the solution from its first use; and therefore it will determine, together with its physicochemical stability, the validity of the remaining fractions after preparing one treatment.
All medications are received at the Pharmacy Unit in the Hospital Intermutual de Levante.
It is recommended to wear synthetic globes (nitrile, polyurethane, neoprene) for the manipulation and distribution of drugs in storage.
When opening the packaging of these drugs, attention should be paid to the presence of any broken packaging; in this case, the person must get adequate protection and follow the rules of action established against spillage.
Hazardous drugs must be stored with caution, in order to avoid any packaging breakage.
The fact that some drugs require low temperature for their storage must be taken into account (there is cold storage for these medications), and/or protection from the light (these must be kept in their original packaging).
Tables 4, 5 and 6 show the recommendations for SPMs to be used in different activities.
- •
HDs must be prepared by authorized staff, and as far as possible this preparation should be centralized in the Pharmacy Unit.
- •
In the working areas where medications are administered:
- –
The staff must not eat, drink, chew gum or store food.
- –
The staff must not use makeup or other cosmetic products that could lead to prolonged exposure in case of contamination.
- –
Measures for adequate hand washing will be implemented, as well as working in a sterile area (if necessary), and the adequate SPMs will be used if necessary.
- –
- •
Any members of staff of reproductive age, and actively trying to conceive, who are handling drugs from List 3, must adopt all prevention measures recommended in this procedure.
- •
This type of drugs might have restrictions for those professionals in the following situations:
- –
Pregnant or breastfeeding women.
- –
Staff considered at high-risk (with previous history of miscarriages or congenital malformations, previous treatments with cytostatic agents or ionizing radiations) and cutaneous allergies.
- –
Fertile men and women actively trying to conceive.
- –
Any of these situations should be reported to the Unit for Occupational Risk Prevention.
- –
- •
The number of persons handling HDs should be reduced as much as possible, through organization measures and the use of preparations that require the lowest level of handling.
Transportation must be conducted in such a way as to avoid breakages or spillages.
No mechanical transportation systems will be used, such as pneumatic tubes.
In case any drug is not administered, it will be returned to the Pharmacy Unit through the same procedure and in the same packaging as it was delivered to the place of administration.
- •
Administration will include all techniques required for administering the treatment, regardless of its way of administration; the most frequently used are: intravenous, subcutaneous (SC), topical, intramuscular (IM) and oral.
- •
Administration will be conducted following the recommendations in the product specifications for each drug, and always according to the Standard Operating Procedures (SOPs) or HIL Guidelines.
Oral Formulations
- •
The selection of formulation will be conducted by prioritizing whole units (tablets, pills and/or capsules) and oral suspensions. If fragmented formulations are required, these must be prepared at the Pharmacy Unit.
- •
When oral formulations are handled, direct contact with the drug should be avoided (see Section on SPMs).
- •
When the medication is supplied in a package, or even packaged in single doses, and it is self-administered by the patient, it will ONLY be handled with single gloves, without any other measure required.
Topical Application
- •
Creams or other topical formulations must be applied with the SPMs stated. During this technique, handling must be restricted to the lower extent, using a spatula or other application products in order to avoid contact with the product.
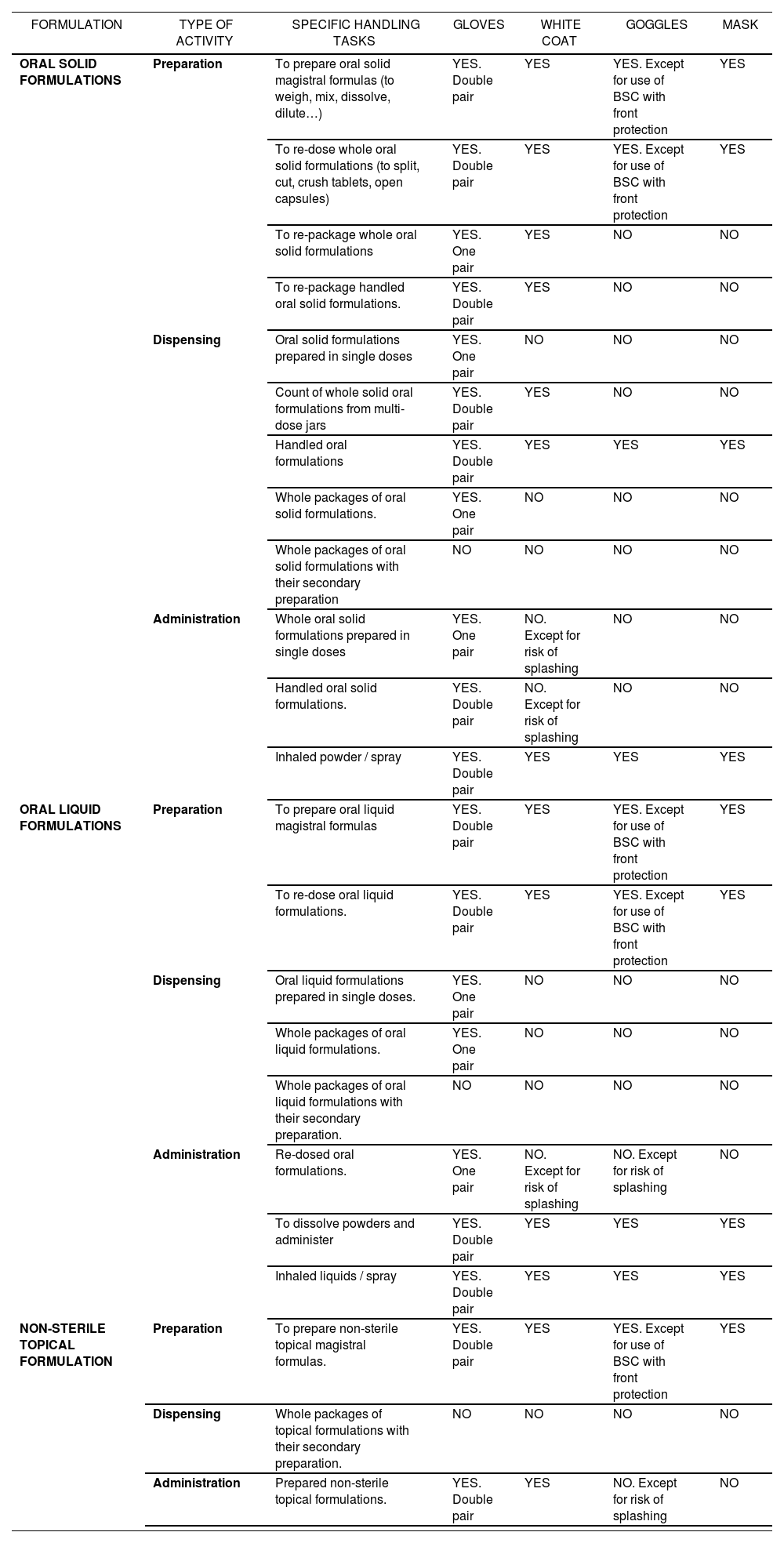
Recommendations about the equipment to be used according to specific tasks in handling non-sterile hazardous drugs
| FORMULATION | TYPE OF ACTIVITY | SPECIFIC HANDLING TASKS | GLOVES | WHITE COAT | GOGGLES | MASK |
|---|---|---|---|---|---|---|
| ORAL SOLID FORMULATIONS | Preparation | To prepare oral solid magistral formulas (to weigh, mix, dissolve, dilute…) | YES. Double pair | YES | YES. Except for use of BSC with front protection | YES |
| To re-dose whole oral solid formulations (to split, cut, crush tablets, open capsules) | YES. Double pair | YES | YES. Except for use of BSC with front protection | YES | ||
| To re-package whole oral solid formulations | YES. One pair | YES | NO | NO | ||
| To re-package handled oral solid formulations. | YES. Double pair | YES | NO | NO | ||
| Dispensing | Oral solid formulations prepared in single doses | YES. One pair | NO | NO | NO | |
| Count of whole solid oral formulations from multi-dose jars | YES. Double pair | YES | NO | NO | ||
| Handled oral formulations | YES. Double pair | YES | YES | YES | ||
| Whole packages of oral solid formulations. | YES. One pair | NO | NO | NO | ||
| Whole packages of oral solid formulations with their secondary preparation | NO | NO | NO | NO | ||
| Administration | Whole oral solid formulations prepared in single doses | YES. One pair | NO. Except for risk of splashing | NO | NO | |
| Handled oral solid formulations. | YES. Double pair | NO. Except for risk of splashing | NO | NO | ||
| Inhaled powder / spray | YES. Double pair | YES | YES | YES | ||
| ORAL LIQUID FORMULATIONS | Preparation | To prepare oral liquid magistral formulas | YES. Double pair | YES | YES. Except for use of BSC with front protection | YES |
| To re-dose oral liquid formulations. | YES. Double pair | YES | YES. Except for use of BSC with front protection | YES | ||
| Dispensing | Oral liquid formulations prepared in single doses. | YES. One pair | NO | NO | NO | |
| Whole packages of oral liquid formulations. | YES. One pair | NO | NO | NO | ||
| Whole packages of oral liquid formulations with their secondary preparation. | NO | NO | NO | NO | ||
| Administration | Re-dosed oral formulations. | YES. One pair | NO. Except for risk of splashing | NO. Except for risk of splashing | NO | |
| To dissolve powders and administer | YES. Double pair | YES | YES | YES | ||
| Inhaled liquids / spray | YES. Double pair | YES | YES | YES | ||
| NON-STERILE TOPICAL FORMULATION | Preparation | To prepare non-sterile topical magistral formulas. | YES. Double pair | YES | YES. Except for use of BSC with front protection | YES |
| Dispensing | Whole packages of topical formulations with their secondary preparation. | NO | NO | NO | NO | |
| Administration | Prepared non-sterile topical formulations. | YES. Double pair | YES | NO. Except for risk of splashing | NO |
(*) It is considered that there is Risk of Splashing in the following situations: there is risk of resistance by the patient, administration diluted in liquid, and administration through feeding tube.
Subcutaneous (SC) and Intramuscular (IM) Administration
- •
It will be preferred to prescribe drugs which are pre-charged and purged with closed drug transfer devices systems. In those cases where purge is required, sterile gauze will be used, soaked in 70° alcohol, to prevent sprays and surface contamination.
- •
A cloth with an absorbent upper side and a waterproof lower side should be placed under the administration line, with the aim to avoid contamination of bedclothes or the administration chair, in case of spillage.
- •
Both in SC and IM administration, the needle won’t be unattached to the syringe at any point; the connections must be luer-lock to prevent any accidental disconnection, and discarded as one single piece in the adequate container.
- •
After the injection, extraction will be conducted with gauze soaked in 70° alcohol, to prevent any drug reflux or dripping.
- •
During administration, individual protection equipment must be used (see Section on SPMs). Eye and respiratory protection will only be necessary if there is a reasonable risk of splashing, or if the patient’s conditions could cause a potential accidental disconnection.
Intravenous Administration
- •
It is recommended to implement closed drug transfer devices systems. This task must be conducted at preparation, so that no connection or disconnection are required during drug administration.
- •
The system will be disposed of as one single piece in the specific container, the drugs used must not be disconnected.
- •
The staff involved in this technique should be trained to ensure an adequate use of closed systems, in order to achieve an optimal performance and reduce to the lowest extent any risk of dripping, spillage, or spray.
- •
During administration, individual protection equipment must be used (see Section on SPMs). Eye and respiratory protection will only be necessary if there is a reasonable risk of splashing, or if the patient’s conditions could cause a potential accidental disconnection.
Spillage
- •
The worker must be protected with a waterproof lab coat, goggles or screen with lateral protection, shoe covers, and 2 pairs of gloves, one made of thick rubber and another one of non-powdered latex. In case the spillage has occurred outside the BSC, a FFP3-type mask for respiratory protection will also be used. These will be worn in the following order: shoe covers, lab coat, mask, first pair of gloves over the coat, second pair of gloves, and goggles or screen.
- •
The spillage will be absorbed with cellulose or an absorbing cloth (dry if liquids and wet if dry powder) before proceeding to cleaning it. If there are rests of glass, these will never be collected with the hand, but with tweezers (or a collecting brush), and they will be placed in a small rigid container for sharp / cutting objects.
- •
The dry surface must be cleaned afterwards with cellulose soaked in 70° alcohol. The area will be cleaned 3 times with soap and water or detergent cleaner / bleach, finally rinsing it with plenty of water, always going from the less contaminated to the most contaminated areas.
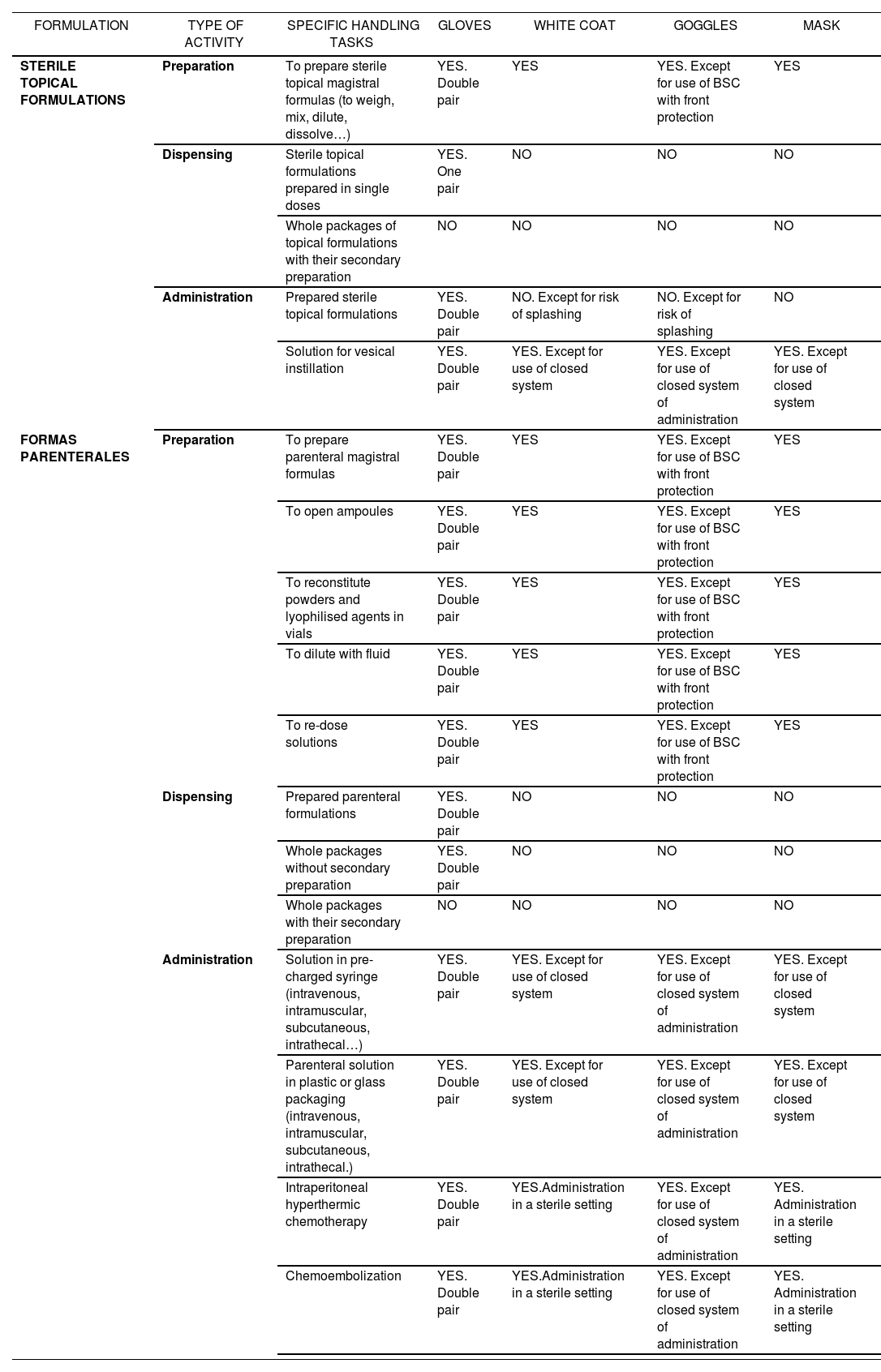
Recommendations about the equipment to be used according to specific tasks in handling sterile hazardous drugs
| FORMULATION | TYPE OF ACTIVITY | SPECIFIC HANDLING TASKS | GLOVES | WHITE COAT | GOGGLES | MASK |
|---|---|---|---|---|---|---|
| STERILE TOPICAL FORMULATIONS | Preparation | To prepare sterile topical magistral formulas (to weigh, mix, dilute, dissolve…) | YES. Double pair | YES | YES. Except for use of BSC with front protection | YES |
| Dispensing | Sterile topical formulations prepared in single doses | YES. One pair | NO | NO | NO | |
| Whole packages of topical formulations with their secondary preparation | NO | NO | NO | NO | ||
| Administration | Prepared sterile topical formulations | YES. Double pair | NO. Except for risk of splashing | NO. Except for risk of splashing | NO | |
| Solution for vesical instillation | YES. Double pair | YES. Except for use of closed system | YES. Except for use of closed system of administration | YES. Except for use of closed system | ||
| FORMAS PARENTERALES | Preparation | To prepare parenteral magistral formulas | YES. Double pair | YES | YES. Except for use of BSC with front protection | YES |
| To open ampoules | YES. Double pair | YES | YES. Except for use of BSC with front protection | YES | ||
| To reconstitute powders and lyophilised agents in vials | YES. Double pair | YES | YES. Except for use of BSC with front protection | YES | ||
| To dilute with fluid | YES. Double pair | YES | YES. Except for use of BSC with front protection | YES | ||
| To re-dose solutions | YES. Double pair | YES | YES. Except for use of BSC with front protection | YES | ||
| Dispensing | Prepared parenteral formulations | YES. Double pair | NO | NO | NO | |
| Whole packages without secondary preparation | YES. Double pair | NO | NO | NO | ||
| Whole packages with their secondary preparation | NO | NO | NO | NO | ||
| Administration | Solution in pre-charged syringe (intravenous, intramuscular, subcutaneous, intrathecal…) | YES. Double pair | YES. Except for use of closed system | YES. Except for use of closed system of administration | YES. Except for use of closed system | |
| Parenteral solution in plastic or glass packaging (intravenous, intramuscular, subcutaneous, intrathecal.) | YES. Double pair | YES. Except for use of closed system | YES. Except for use of closed system of administration | YES. Except for use of closed system | ||
| Intraperitoneal hyperthermic chemotherapy | YES. Double pair | YES.Administration in a sterile setting | YES. Except for use of closed system of administration | YES. Administration in a sterile setting | ||
| Chemoembolization | YES. Double pair | YES.Administration in a sterile setting | YES. Except for use of closed system of administration | YES. Administration in a sterile setting |
(*) It is considered that there is Risk of Splashing in the following situations: there is risk of resistance by the patient, administration diluted in liquid, and administration through feeding tube.
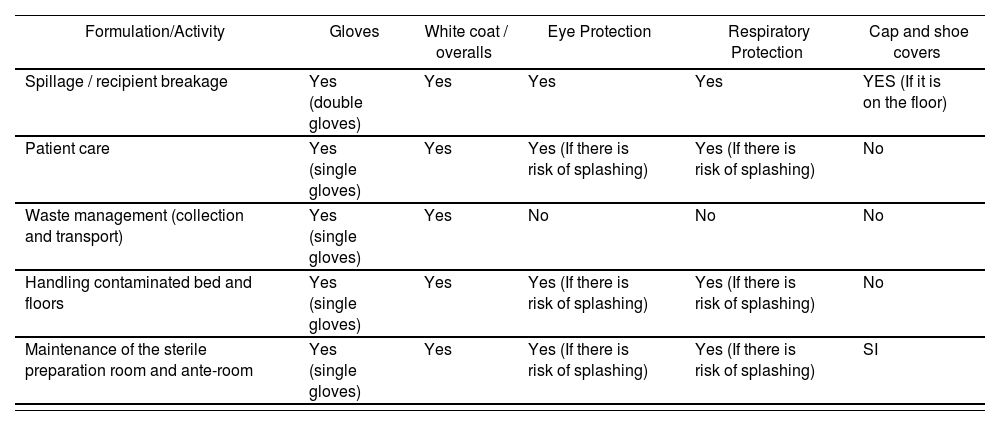
Individual Protection Equipment. Recommended SPMs for different handling of hazardous drugs
| Formulation/Activity | Gloves | White coat / overalls | Eye Protection | Respiratory Protection | Cap and shoe covers |
|---|---|---|---|---|---|
| Spillage / recipient breakage | Yes (double gloves) | Yes | Yes | Yes | YES (If it is on the floor) |
| Patient care | Yes (single gloves) | Yes | Yes (If there is risk of splashing) | Yes (If there is risk of splashing) | No |
| Waste management (collection and transport) | Yes (single gloves) | Yes | No | No | No |
| Handling contaminated bed and floors | Yes (single gloves) | Yes | Yes (If there is risk of splashing) | Yes (If there is risk of splashing) | No |
| Maintenance of the sterile preparation room and ante-room | Yes (single gloves) | Yes | Yes (If there is risk of splashing) | Yes (If there is risk of splashing) | SI |
Adapted from Source “Guía de Buenas Prácticas para Trabajadores Profesionalmente Expuestos a Agentes Citostáticos” (“Good Practice Guidelines for Workers Professionally Exposed to Cytostatic Agents”) (Escuela Nacional de Medicina del Trabajo. Instituto de Salud Carlos III. Ministerio de Economía y Competitividad. Asociación Madrileña de Medicina del Trabajo en el Ámbito Sanitario (AMMTAS) 2014).
Source: “Monografías de Farmacia Hospitalaria y Atención Primaria. Medicamentos Peligrosos.” Issue 6. Year 2016.
Individual Protection Equipment. Recommended SPMs for different handling of hazardous drugs
| Way of entry | Type of protection | Equipment to be used |
|---|---|---|
| Spray inhalation | Respiratory protection | Mask |
| Direct or indirect contact with the skin | Dermal protection | Gloves, clothes, shoes |
| Direct or indirect eye contact | Eye protection | Goggles |
Source: Monografías de Farmacia Hospitalaria y Atención Primaria. Medicamentos Peligrosos.” Issue 6. Year 2016.
- •
The protection equipment must be removed in this order: first pair of gloves, shoe covers, second pair of gloves, mask, lab coat and goggles. Everything should be discarded, except if eye-glasses are used; tweezers can be re-used after being carefully washed with soap and water (hands should be protected with a clean pair of gloves in order to conduct this procedure).
- •
All waste generated, as well as the materials used, will be treated as contaminated materials at the time of disposal.
Accidental exposure
- •
If there is direct contact of the drug with the skin: The affected area will be immediately washed with soap and water, during approximately 10 minutes. If the skin appears irritated, it should be examined by a specialist.
- •
If the drug splashes the eyes: rinse the affected eye with water or isotonic solution during at least 15 minutes, and then consult the specialist.
- •
In case of accidental ingestion, it is necessary to see a doctor immediately.
- •
In any of these cases, it will be reported to the Occupational Health area of the Prevention Unit to enable the specific pharmacovigilance.
Drug waste must be treated in the Group III or Group IV containers, according to the current procedure for waste management.
Potential exposure to HDs in each of the activities described will depend on the set of preventive measures adopted; therefore, the potential exposure and its level must be established according to the risk evaluation conducted in each specific case.
The extent of the risk will depend on:
- •
The toxicity inherent to each medication.
- •
The level of exposure, associated with:
- –
Workload.
- –
Handling conditions.
- –
- •
Environmental protection.
- •
Protection materials.
- •
Handling technique, involving procedures, training and periodical assessment.
- –
Time of exposure.
- –
Stage of the process. There is higher risk in preparation and accidental spillage, though protection measures should include all stages of the process.
- •
Characteristics of the handler (reproductive age, simultaneous exposure to other agents, etc.).
- –
Another important aspect to be taken into account is the potential ways of penetration of these substances into the body, which are:
- a)
Inhalation of sprays and micro-drops emitted during the preparation of the hazardous drug solutions for administration, when vials are broken, while purging the system, etc.
- b)
Through direct contact, by penetration of the medication through the skin or mucosa.
- c)
Orally: ingestion of food and drinks, contaminated cigarettes.
- d)
Parenterally: through direct introduction of the medication by accidental needle sticks or cuts caused by vial breakage.
The worker exposed should be qualified, aware of the risks taken if exposed to these drugs without an adequate protection, as well as of the conditions required for patient safety.
Thee requirements about the use of Individual Protection Equipment according to each medication have been stated in previous sections of this protocol (Appendix II and III).
The SPM specifications for each activity are not the object of this document.
With general application, and according to Articles 18 and 19 of the Law for Prevention of Occupational Risks13, workers must receive adequate information and training about the risks derived of the presence of any hazardous chemical agent present in their working place, as well as about the prevention and protection measures to be adopted. In particular, the training of staff working with bio-hazardous drugs is a key aspect to prevent occupational risks.
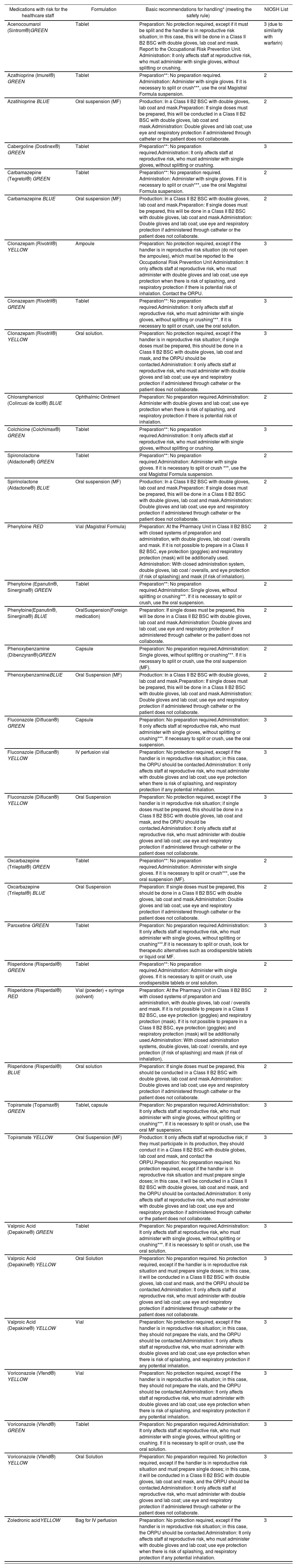
| Medications with risk for the healthcare staff | Formulation | Basic recommendations for handling* (meeting the safety rule) | NIOSH List |
|---|---|---|---|
| Acenocoumarol (Sintrom®)GREEN | Tablet | Preparation: No protection required, except if it must be split and the handler is in reproductive risk situation; in this case, this will be done in a Class II B2 BSC with double gloves, lab coat and mask. Report to the Occupational Risk Prevention Unit. Administration: It only affects staff at reproductive risk, who must administer with single gloves, without splitting or crushing. | 3 (due to similarity with warfarin) |
| Azathioprine (Imurel®) GREEN | Tablet | Preparation**: No preparation required. Administration: Administer with single gloves. If it is necessary to split or crush***, use the oral Magistral Formula suspension. | 2 |
| Azatihioprine BLUE | Oral suspension (MF) | Production: In a Class II B2 BSC with double gloves, lab coat and mask.Preparation: If single doses must be prepared, this will be conducted in a Class II B2 BSC with double gloves, lab coat and mask.Administration: Double gloves and lab coat; use eye and respiratory protection if administered through catheter or the patient does not collaborate. | 2 |
| Cabergoline (Dostinex®) GREEN | Tablet | Preparation**: No preparation required.Administration: It only affects staff at reproductive risk, who must administer with single gloves, without splitting or crushing. | 3 |
| Carbamazepine (Tegretol®) GREEN | Tablet | Preparation**: No preparation required. Administration: Administer with single gloves. If it is necessary to split or crush***, use the oral Magistral Formula suspension. | 2 |
| Carbamazepine BLUE | Oral suspension (MF) | Production: In a Class II B2 BSC with double gloves, lab coat and mask.Preparation: If single doses must be prepared, this will be done in a Class II B2 BSC with double gloves, lab coat and mask.Administration: Double gloves and lab coat; use eye and respiratory protection if administered through catheter or the patient does not collaborate. | 2 |
| Clonazepam (Rivotril®) YELLOW | Ampoule | Preparation: No protection required, except if the handler is in reproductive risk situation (do not open the ampoules), which must be reported to the Occupational Risk Prevention Unit Administration: It only affects staff at reproductive risk, who must administer with double gloves and lab coat; use eye protection when there is risk of splashing, and respiratory protection if there is potential risk of inhalation. Contact the ORPU. | 3 |
| Clonazepam (Rivotril®) GREEN | Tablet | Preparation**: No preparation required.Administration: It only affects staff at reproductive risk, who must administer with single gloves, without splitting or crushing***. If it is necessary to split or crush, use the oral solution. | 3 |
| Clonazepam (Rivotril®) YELLOW | Oral solution. | Preparation: No protection required, except if the handler is in reproductive risk situation; if single doses must be prepared, this should be done in a Class II B2 BSC with double gloves, lab coat and mask, and the ORPU should be contacted.Administration: It only affects staff at reproductive risk, who must administer with double gloves and lab coat; use eye and respiratory protection if administered through catheter or the patient does not collaborate. | 3 |
| Chloramphenicol (Colircusi de Icol®) BLUE | Ophthalmic Ointment | Preparation: No preparation required.Administration: Administer with double gloves and lab coat; use eye protection when there is risk of splashing, and respiratory protection if there is potential risk of inhalation. | 2 |
| Colchicine (Colchimax®) GREEN | Tablet | Preparation**: No preparation required.Administration: It only affects staff at reproductive risk, who must administer with single gloves, without splitting or crushing. | 3 |
| Spironolactone (Aldactone®) GREEN | Tablet | Preparation**: No preparation required.Administration: Administer with single gloves. If it is necessary to split or crush ***, use the oral Magistral Formula suspension. | 2 |
| Spirinolactone (Aldactone®) BLUE | Oral suspension (MF) | Production: In a Class II B2 BSC with double gloves, lab coat and mask.Preparation: If single doses must be prepared, this will be done in a Class II B2 BSC with double gloves, lab coat and mask.Administration: Double gloves and lab coat; use eye and respiratory protection if administered through catheter or the patient does not collaborate. | 2 |
| Phenytoine RED | Vial (Magistral Formula) | Preparation: At the Pharmacy Unit in Class II B2 BSC with closed systems of preparation and administration, with double gloves, lab coat / overalls and mask. If it is not possible to prepare in a Class II B2 BSC, eye protection (goggles) and respiratory protection (mask) will be additionally used. Administration: With closed administration system, double gloves, lab coat / overalls, and eye protection (if risk of splashing) and mask (if risk of inhalation). | 2 |
| Phenytoine (Epanutin®, Sinergina®) GREEN | Tablet | Preparation**: No preparation required.Administration: Single gloves, without splitting or crushing***. If it is necessary to split or crush, use the oral suspension. | 2 |
| Phenytoine(Epanutin®, Sinergina®) BLUE | OralSuspension(Foreign medication) | Preparation: If single doses must be prepared, this will be done in a Class II B2 BSC with double gloves, lab coat and mask.Administration: Double gloves and lab coat; use eye and respiratory protection if administered through catheter or the patient does not collaborate. | 2 |
| Phenoxybenzamine (Dibenzyran®)GREEN | Capsule | Preparation: No preparation required.Administration: Single gloves, without splitting or crushing***. If it is necessary to split or crush, use the oral suspension (MF). | 2 |
| PhenoxybenzamineBLUE | Oral Suspension (MF) | Production: In a Class II B2 BSC with double gloves, lab coat and mask.Preparation: If single doses must be prepared, this will be done in a Class II B2 BSC with double gloves, lab coat and mask.Administration: Double gloves and lab coat; use eye and respiratory protection if administered through catheter or the patient does not collaborate. | 2 |
| Fluconazole (Diflucan®) GREEN | Capsule | Preparation: No preparation required.Administration: It only affects staff at reproductive risk, who must administer with single gloves, without splitting or crushing***. If necessary to split or crush, use the oral suspension. | 3 |
| Fluconazole (Diflucan®) YELLOW | IV perfusion vial | Preparation: No protection required, except if the handler is in reproductive risk situation; in this case, the ORPU should be contacted.Administration: It only affects staff at reproductive risk, who must administer with double gloves and lab coat; use eye protection when there is risk of splashing, and respiratory protection if any potential inhalation. | 3 |
| Fluconazole (Diflucan®) YELLOW | Oral Suspension | Preparation: No protection required, except if the handler is in reproductive risk situation; if single doses must be prepared, this should be done in a Class II B2 BSC with double gloves, lab coat and mask, and the ORPU should be contacted.Administration: It only affects staff at reproductive risk, who must administer with double gloves and lab coat; use eye and respiratory protection if administered through catheter or the patient does not collaborate. | 3 |
| Oxcarbazepine (Trileptal®) GREEN | Tablet | Preparation**: No preparation required.Administration: Administer with single gloves. If it is necessary to split or crush***, use the oral suspension (MF). | 2 |
| Oxcarbazepine (Trileptal®) BLUE | Oral Suspension | Preparation: If single doses must be prepared, this should be done in a Class II B2 BSC with double gloves, lab coat and mask.Administration: Double gloves and lab coat; use eye and respiratory protection if administered through catheter or the patient does not collaborate. | 2 |
| Paroxetine GREEN | Tablet | Preparation: No preparation required.Administration: It only affects staff at reproductive risk, who must administer with single gloves, without splitting or crushing***.If it is necessary to split or crush, look for therapeutic alternatives such as orodispersible tablets or liquid oral MF. | 3 |
| Risperidone (Risperdal®) GREEN | Tablet | Preparation**: No preparation required.Administration: Administer with single gloves. If it is necessary to split or crush, use orodispersible tablets or oral solution. | 2 |
| Risperidone (Risperdal®) RED | Vial (powder) + syringe (solvent) | Preparation: At the Pharmacy Unit in Class II B2 BSC with closed systems of preparation and administration, with double gloves, lab coat / overalls and mask. If it is not possible to prepare in a Class II B2 BSC, use eye protection (goggles) and respiratory protection (mask). If it is not possible to prepare in a Class II B2 BSC, eye protection (goggles) and respiratory protection (mask) will be additionally used.Administration: With closed administration systems, double gloves, lab coat / overalls, and eye protection (if risk of splashing) and mask (if risk of inhalation). | 2 |
| Risperidone (Risperdal®) BLUE | Oral solution | Preparation: If single doses must be prepared, this should be conducted in a Class II B2 BSC with double gloves, lab coat and mask.Administration: Double gloves and lab coat; use eye and respiratory protection if administered through catheter or the patient does not collaborate. | 2 |
| Topiramate (Topamax®) GREEN | Tablet, capsule | Preparation: No preparation required.Administration: It only affects staff at reproductive risk, who must administer with single gloves, without splitting or crushing***. If it is necessary to split or crush, use the oral MF suspension. | 3 |
| Topiramate YELLOW | Oral Suspension (MF) | Production: It only affects staff at reproductive risk; if they must participate in its production, they should conduct it in a Class II B2 BSC with double globes, lab coat and mask, and contact the ORPU.Preparation: No preparation required. No protection required, except if the handler is in reproductive risk situation and must prepare single doses; in this case, it will be conducted in a Class II B2 BSC with double gloves, lab coat and mask, and the ORPU should be contacted.Administration: It only affects staff at reproductive risk, who must administer with double gloves and lab coat; use eye and respiratory protection if administered through catheter or the patient does not collaborate. | 3 |
| Valproic Acid (Depakine®) GREEN | Tablet | Preparation: No preparation required.Administration: It only affects staff at reproductive risk, who must administer with single gloves, without splitting or crushing***. If it is necessary to split or crush, use the oral solution. | 3 |
| Valproic Acid (Depakine®) YELLOW | Oral Solution | Preparation: No preparation required. No protection required, except if the handler is in reproductive risk situation and must prepare single doses; in this case, it will be conducted in a Class II B2 BSC with double gloves, lab coat and mask, and the ORPU should be contacted.Administration: It only affects staff at reproductive risk, who must administer with double gloves and lab coat; use eye and respiratory protection if administered through catheter or the patient does not collaborate. | 3 |
| Valproic Acid (Depakine®) YELLOW | Vial | Preparation: No protection required, except if the handler is in reproductive risk situation; in this case, they should not prepare the vials, and the ORPU should be contacted.Administration: It only affects staff at reproductive risk, who must administer with double gloves and lab coat; use eye protection when there is risk of splashing, and respiratory protection if any potential inhalation. | 3 |
| Voriconazole (Vfend®) YELLOW | Vial | Preparation: No protection required, except if the handler is in reproductive risk situation; in this case, they should not prepare the vials, and the ORPU should be contacted.Administration: It only affects staff at reproductive risk, who must administer with double gloves and lab coat; use eye protection when there is risk of splashing, and respiratory protection if any potential inhalation. | 3 |
| Voriconazole (Vfend®) GREEN | Tablet | Preparation: No preparation required.Administration: It only affects staff at reproductive risk, who must administer with single gloves, without splitting or crushing. If it is necessary to split or crush, use the oral solution. | 3 |
| Voriconazole (Vfend®) YELLOW | Oral Solution | Preparation: No preparation required. No protection required, except if the handler is in reproductive risk situation and must prepare single doses; in this case, it will be conducted in a Class II B2 BSC with double gloves, lab coat and mask, and the ORPU should be contacted.Administration: It only affects staff at reproductive risk, who must administer with double gloves and lab coat; use eye and respiratory protection if administered through catheter or the patient does not collaborate. | 3 |
| Zoledronic acidYELLOW | Bag for IV perfusion | Preparation: No protection required, except if the handler is in reproductive risk situation; in this case, the ORPU should be contacted.Administration: It only affects staff at reproductive risk, who must administer with double gloves and lab coat; use eye protection when there is risk of splashing, and respiratory protection if any potential inhalation. | 3 |
MF: Magistral Formula; ORPU: Occupational Risk Prevention Unit; Class II B2 BSC: Class II Type B2 Biological Safety Cabinet.
Since the display of this article is not in color, the words green, yellow, blue and red have been recorded in the tables below each medication. This color indicates the category of protection of that drug according to the color code from the lowest to the highest degree of protection during the preparation and administration (green, yellow, blue and red) as explained in the procedure described”.
There is a summary below of the Hazardous Drugs (HDs) included in the formulary of the HIL.
| COLOUR | RECOMMENDATIONS (Meeting the safety rule) | Medication (Classification by the NIOSH) |
|---|---|---|
| GREEN | Preparation1: No preparation required.Administration: Single gloves. No splitting or crushing.If it is necessary to split or crush the dose:- It would be of choice to use liquid oral formulations (consult with the Pharmacy Unit).- If no liquid oral formulation available, splitting will be done at the Pharmacy Unit, in a Class II B2 BSC with double gloves, lab coat / overalls and mask. | Acenocoumarol tablets (Sintrom®) (3),Azathioprine tablets (Imurel®) (2),Cabergoline tablets (Dostinex®) (3),Carbamezapine tablets (Tegretol®) (2),Clonazepam tablets (Rivotril®) (3),Colchicine tablets (Colchimax®) (3),Spironolactone tablets (Aldactone®) (2),Phenytoine tablets (Epanutin®) (2),Phenoxybenzamine capsules (Dibenzyran®) (2),Fluconazole capsules (3), Oxcarbazepine tablets (Trileptal®) (2), Paroxetine tablets (3),Risperidone tablets (Risperdal®) (2), Topiramate tablets / capsules (3), Valproic Acid tablets (Depakine®) (3), Voriconazole tablets (Vfend®) (3) |
| YELLOW | As a general rule, they should not be manipulated by personnel under reproductive risk2,3.Preparation: single gloves.Administration: Double gloves and lab coat; in case of risk of splashing, accidental disconnection of the system, administration through catheter, or patient who does not collaborate, use eye protection (goggles) and respiratory protection (mask).In the exceptional case in which these must be PREPARED by staff at reproductive risk, they must be protected with double gloves, lab coat, goggles and mask. | Clonazepam oral solution (Rivotril®) (3),Clonazepam ampoules (Rivotril®) (3),Fluconazole vial for perfusion (Diflucan®) (3),Fluconazole oral solution (Diflucan®) (3),Topiramate oral suspension (FM) (3),Valproic Acid oral solution (Depakine®) (3),Valproic Acid vial (Depakine®) (3),Voriconazole oral suspension (Vfend®) (3),Voriconazole vial (Vfend®) (3),Zoledronic Acid bag for perfusion (3) |
| BLUE | Preparation: In the Pharmacy Unit, in Class II B2 BSC with double gloves, lab coat and mask. In the exceptional case in which it is not possible to prepare in Class II B2 BSC, use double gloves, lab coat, eye protection (goggles) and respiratory protection (mask).Administration: Double gloves and lab coat; in case of risk of splashing, accidental disconnection of the system, administration through catheter, or patient who does not collaborate, use eye protection (goggles) and respiratory protection (mask). | Azathioprine oral suspension (MF) (2),Carbamazepine oral suspension (MF) (2),Chloramphenicol ophthalmic ointment (Colircusi de Icol®) (2), Spironolactone oral suspension (FM) (2), Phenytoine oral suspension (Epanutin®) (2),Phenoxybenzamine suspension (MF) (2),Oxcarbazepine oral suspension (Trileptal®) (2),Risperidone oral solution (Risperdal®) (2) |
| RED | Preparation: At the Pharmacy Unit in Class II B2 BSC with closed systems for preparation and administration. Double gloves, protective lab coat (preferable overalls) and masks. If not possible to prepare in Class II B2 BSC, use eye protection (goggles) and respiratory protection (mask). Administration: Closed systems of administration, double gloves, lab coat / overalls, and eye protection (if risk of splashing) and mask (if risk of inhalation). | Phenytoine vial (Magistral formula) (2), Risperidone vial + pre-charged syringe (Risperdal®) (2) |
The number between brackets corresponds to the number of the NIOSH list in which this medication is included. Acenocoumarol by similarity with warfarin (not included in NIOSH)
List 1: Antineoplastic or cytostatic agents.
List 2: Non-antineoplastic medications that meet one or more NIOSH criteria to be considered of risk.
List 3: Non-antineoplastic medications with effects on reproduction, and that can affect men and women who are trying actively to conceive, and pregnant or breastfeeding women, but that don’t represent a risk for the rest of the staff.
Whenever possible, the original blister will be kept at re-packaging
2Staff at reproductive risk: men and women who are actively trying to conceive, and pregnant or breastfeeding women.
3For treatments prescribed continuously and that need to be prepared, this will be done at the Pharmacy Unit (PU) in CSBIIB2. Exceptional treatmetns will be prepared according to the protection measures already mentioned and, in case of persistence, their preparation will be similarly assumed by the PU.
MG: Magistral Formula; Class II B2 BSC: Class II Type B2 Biological Safety Cabinet; CLDT: Closed System for Drug Transfer. Since the display of this article is not in color, the words green, yellow, blue and red have been recorded in the tables below each medication. This color indicates the category of protection of that drug according to the color code from the lowest to the highest degree of protection during the preparation and administration (green, yellow, blue and red) as explained in the procedure described”.