The cardiovascular disease is the first cause of deaths in patients with diabetes mellitus 2. The objective is to evaluate and compare the weight loss in patients with diabetes treated with the different GLP-1 receptor agonists for the first time. Secondary endpoints are glycosylated hemoglobin reduction, changes in quality of life and physical activity and the safety of these drugs.
MethodIt is a postauthorization, multicenter, non-randomized and prospective study. 360 Patients that will start treatment for the first time with GLP-1 receptor agonists will be recruited in 10 centers in the National Health System for a period of 6 months and 44 weeks of follow-up. The primary endpoint will be weight loss achieved with the different GLP-1 receptor agonists and the secondary endpoint will be glycosylated hemoglobin reduction, changes in the quality of life through the EuroQol-5D and changes physical activity through the SF-12 questionnaire, and also the safety of these drugs. The estimate recruitment period will be 6 months, from 1 December 2021 to 1 May 2022. The follow up will finish in December 2022.
DiscussionThe SEVERAL study will try to provide information about weight loss efficacy, changes in quality of life, physical activity and safety of the GLP-1 receptor agonists in patients with diabetes that start treatment with these drugs in the real life. This study try to compare different GLP-1 receptor agonists in terms of effectiveness and safety for a better posterior election when these drugs are used in patients with diabetes mellitus 2 an d obesity.
La enfermedad cardiovascular es la causa principal de muerte en pacientes con diabetes mellitus 2. El objetivo principal es evaluar y comparar prospectivamente la pérdida de peso en pacientes con diabetes mellitus 2 tratados por primera vez con los diferentes anál ogos de la GLP-1. Como variables secundarias se estudiará reducción de la hemoglobina glicosilada, cambios en calidad de vida y actividad física y la seguridad de estos fármacos.
MétodoSe trata de un estudio postautorización, multicéntrico, no aleatorizado de seguimiento prospectivo. Se reclutarán 360 pacientes que inicien tratamiento por primera vez con análogos de la GLP1 en 10 centros del sistema público durante un período de 6 meses y un seguimiento de 44 semanas. La variable principal será la pérdida de peso con los diferentes análogos de la GLP1 y como variable secundaria se valorarán: reducción de hemoglobina glicosilada, cambios en la calidad de vida y actividad física a través del EuroQol-5D y SF-12 y seguridad. Se ha estimado un período de reclutamiento de 6 meses, desde el 1 de Diciembre 2021 al 1 de Mayo 2022. El seguimiento finalizará en Diciembre de 2022.
DiscusiónEl estudio intentará aportar información sobre la efectividad en pérdida de peso, cambios en calidad de vida, actividad física y seguridad de los análogos de la GLP1 en pacientes con diabetes mellitus 2 que inician tratamiento con estos fármacos en la vida real. Este trabajo pretende comparar los diferentes análogos de la GLP1 en términos de eficacia y seguridad para una posterior mejor elección en la prescripción de estos fármacos en pacientes con diabetes mellitus 2 y obesidad.
Diabetes mellitus 2 (DM2) is a metabolic disease characterised by poor glycaemic control caused by increased insulin resistance; thus, there is a need for simple, appropriate, safe, and effective therapies1. Furthermore, the leading causes of death in patients with DM2 are cardiovascular (CV) and cerebrovascular complications1, which highlights the need for new drugs to reduce the risk of CV events in patients with DM2.
Glucagon-like peptide-1 (GLP-1) is an incretin hormone that is synthesised in intestinal L-cells and secreted in response to meals. It acts by increasing pancreatic insulin secretion in response to glucose, reducing glucagon secretion, and suppressing appetite by acting centrally2.
In general, in order to maintain adequate glycaemic control, it is essential to implement lifestyle modifications such as diet and exercise, weight loss, and the use of blood-glucose-lowering drugs. In addition to glycaemic control, several pharmacological therapies, such as GLP-1 agonists (aGLP-1) and renal glucose inhibitors (SGLT-2i), have been shown to provide macrovascular protection, reduce major cardiovascular events (MACE), and reduce hospitalisation due to heart failure (HF)3,4,5.
Semaglutide (oral and subcutaneous) is a new generation aGLP-1 that is very similar to human GLP-11. Clinical studies have shown that oral semaglutide is safe, well tolerated, and provides a dose-dependent reduction in HbA1c and body weight according to its use in specific indications6–10.
It is difficult to compare the results of different studies on GLP-1 analogues because of differences in design, duration, and variability in the definition of the primary endpoint. Based on the hypothesis that greater weight reductions are achieved with semaglutide than with other types of aGLP-1, this study assesses weight reductions with the different aGLP-1 analogues in patients initiating aGLP-1 treatment in real-life settings. However, due to the high rate of gastrointestinal events and possible treatment abandonment, a further aim is to determine whether weight reduction or possible changes in quality of life and physical activity could lower the rate of treatment failure.
The primary objective of this study is to assess changes in weight loss as measured in kilograms and by percentage, as well as changes in Body Mass Index (BMI) in patients with DM2 treated with semaglutide vs patients treated with other GLP-1 agonists.
Secondary objectives:
- •
Reductions in HbA1c.
- •
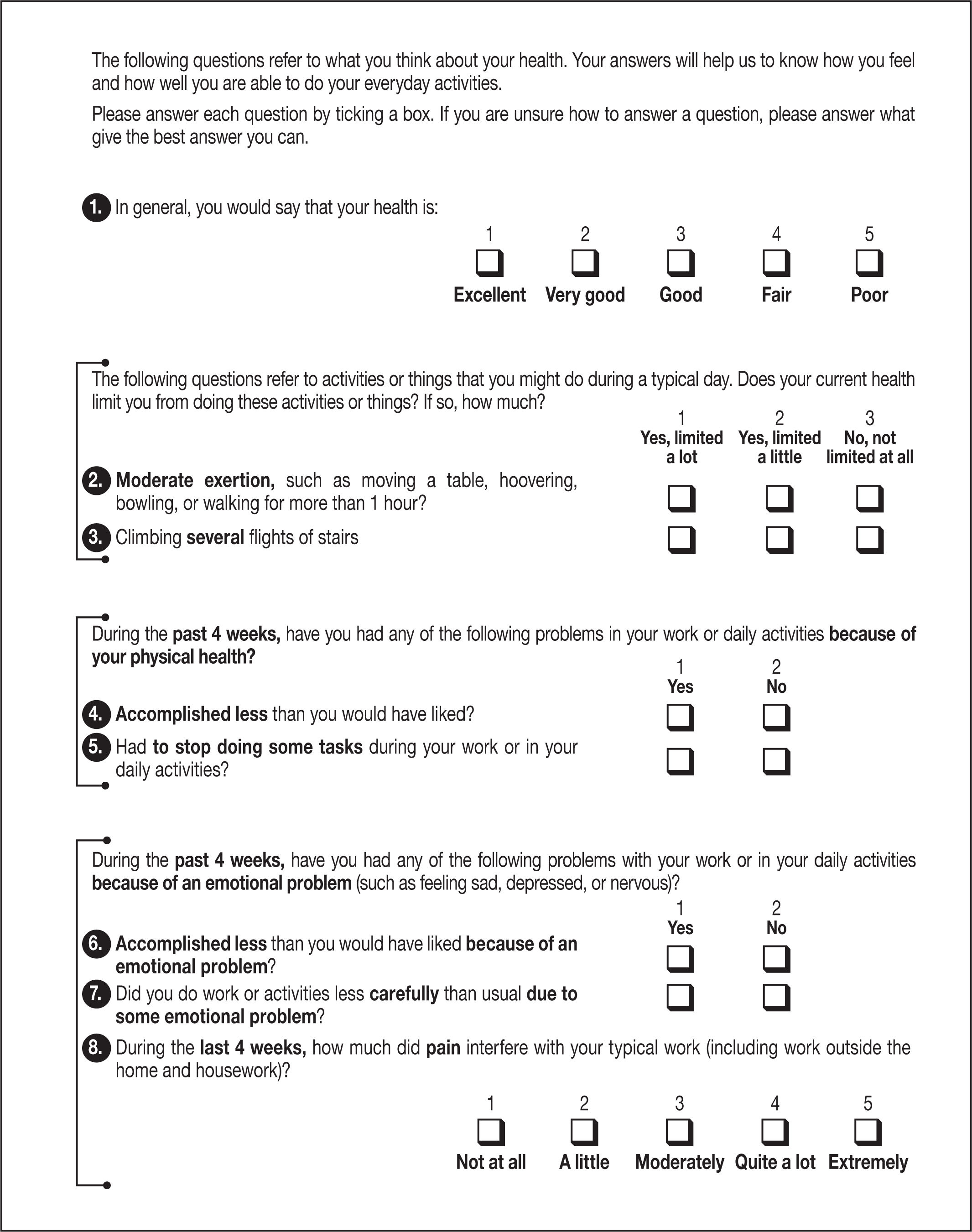
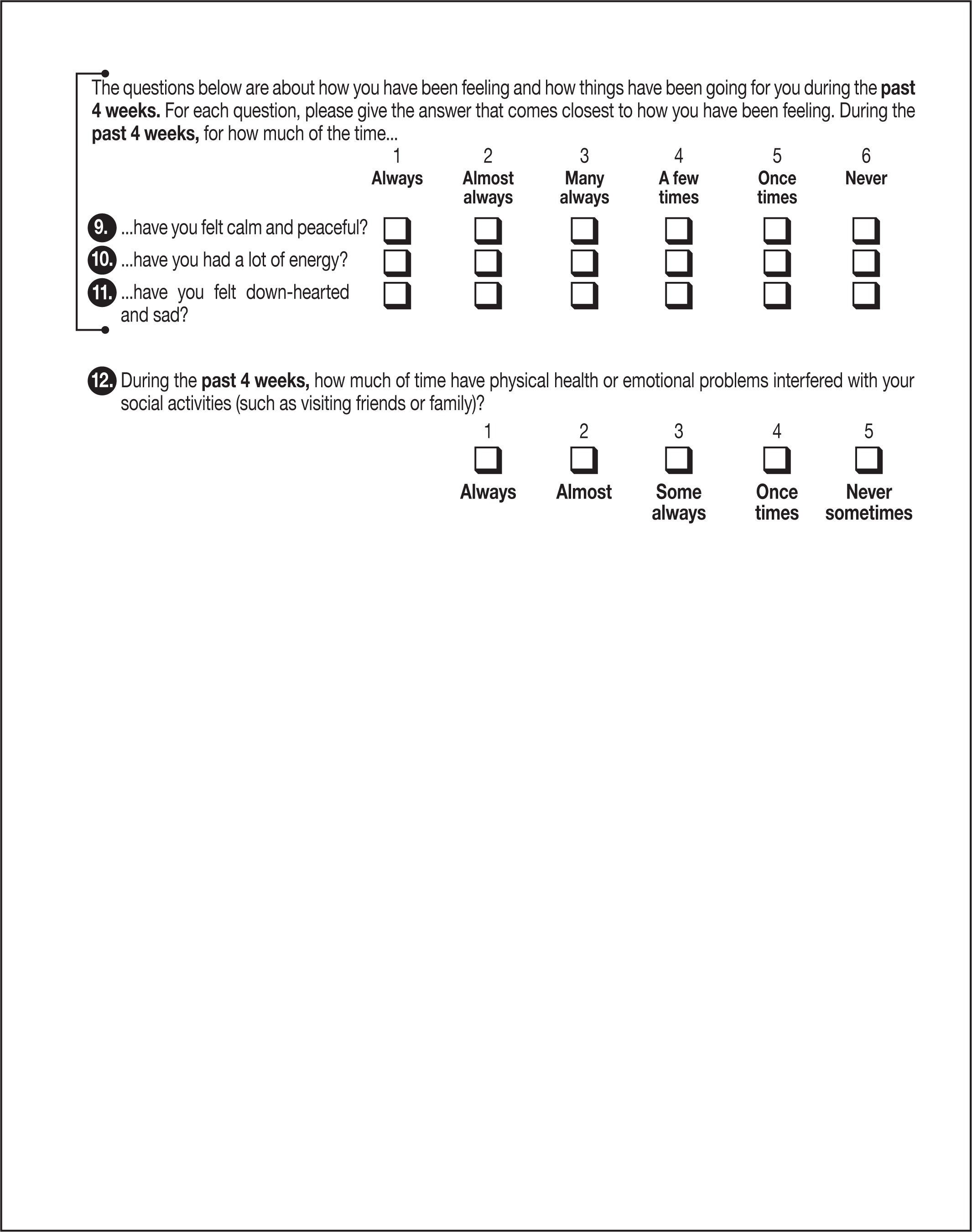
Changes in quality of life and physical activity (as measured on the EQ-5D-5L and SF-12 questionnaires).
- •
Safety of these drugs.
The SEVERAL study is a multicentre prospective follow-up study with a epidemiological cohort design that includes patients treated with semaglutide (oral and subcutaneous) and patients treated with other GLP-1 analogues (subcutaneous). It is a multidisciplinary study across different levels of health care. The study is promoted and coordinated by a Spanish National Health System (NHS) Cardiology Service. Patients will be recruited from 10 NHS centres. A total of 360 patients will be included in the study (approximately 36 patients per centre). Recruitment will be competitive and will be closed once the total is reached. Based on previous prescription statistics, it is estimated that the two groups will be approximately equal (semaglutide is financed for approximately half of the patients who are currently prescribed the drug).
The target population is the DM2 population in the Autonomous Community in which the study will be conducted.
Selection criteriaInclusion criteria- •
Being of legal age and more than 18 years of age without an upper limit.
- •
Being able to understand the study aims and to give consent to participate in the study.
- •
Prescribed and started treatment with a financed and approved aGLP-1. Exclusion criteria
- •
Diagnosis of any disease, such as proliferative diabetic retinopathy or a family history of thyroid cancer (contraindicated in the Summary of Product Characteristics).
- •
Gestational diabetes.
- •
Pregnancy.
The approved pharmacists at each centre will be responsible for enrolling patients after they have signed the informed consent form. The assignment of patients to particular therapeutic strategies is not decided in advance by the study protocol, but is determined by standard medical practice. No diagnostic or follow-up intervention will be applied to the patients that is not standard clinical practice.
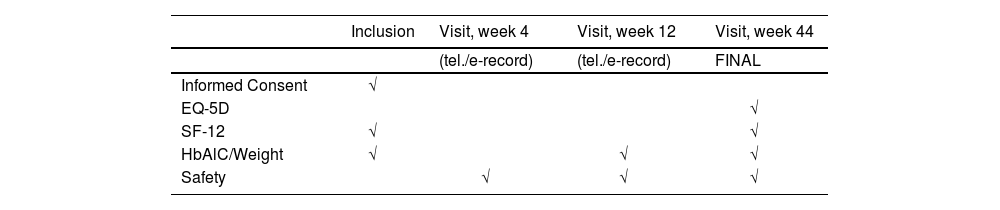
Follow-upPatients will be followed up at the visits listed below, with a window of +/- 2 weeks. Visits 4 and 12 will be by telephone and/or data will be obtained from electronic medical record (when available). If the weight variable is not available for the week 12 visit, patients will be seen in consultations. The week 44 visit will be conducted in the consultation room. Data on the HbA1c variables will be collected according to usual clinical practice and always using the value closest to the consultation (see Table 1, Schedule of Visits).
Data collectionThe pharmacists in each centre will be responsible for including patients and collecting data on possible adverse events and different variables (including medication and medical history) through clinical histories and/or telephone interviews. The database will be electronic and anonymised. The study sponsor will be responsible for data validity and custody.
Statistical analysisSample size. To achieve the main objective of this study (i.e. to assess weight reduction in patients with DM2 on semaglutide vs patients on other GLP-1 agonists), it is expected that 360 patients will be included during the study period. It has been estimated that approximately 36 patients per centre (10 centres) will be enrolled over a 6-month period. This estimate is based on previous data on the number of monthly approvals at participating centres. Previous studies11–15 have assessed weight loss in patients with DM2 treated with semaglutide and other GLP-1 agonists, and have described mean weight losses of 3.6 to 4.9 kg in patients on semaglutide vs 0.86 to 2.96 kg in patients on other GLP-1 agonists. Based on these data, and assuming a P-value of 5% as a cutoff for statistical significance, a mean weight loss of 2.5 kg in the GLP-1 agonist group, a mean weight loss of 4.2 kg in the semaglutide group, and a pooled deviation of 3.0 kg, the inclusion of 360 patients will achieve a statistical power of more than 90% to detect differences using the Student t-test for independent samples. On the other hand, assuming a possible loss to follow-up of up to 20%, the sample could be reduced by up to 72 patients. In this situation, the statistical power to detect differences in weight loss between the study groups would still be more than 90%. The SPSS 3.0 (Chicago, USA) statistical package was used to estimate statistical power based on the sample size.
Qualitative variables will be expressed as frequency and percentage and quantitative variables will be expressed as mean and standard deviation or as median and interquartile range if they do not conform to normal distribution. Normal distributions will be determined using the Kolmogorov-Smirnov test.
The overall objective will be assessed according to weight loss in each group. Other variables associated with weight loss will be analysed using the Student t-test for quantitative variables and the chi-square test for qualitative variables. Multivariate linear regression analyses will be used to adjust for weight loss in relation to the variables shown to be statistically significantly associated in the univariate analysis. Similarly, the specific objectives will be investigated using univariate and multivariate analyses. The coding variables will be categorical, the Student t-test or ANOVA will be applied to continuous variables, and the chi-square test will be applied to categorical variables. Multivariate logistic regression will be conducted using significant variables.
Questionnaires (see Annexes 1 and 2)- •
During the visits indicated in the schedule, the EQ-5D-5L and SF-12 questionnaires will be given out and completed by the participants in consultations.
This study has been approved by the Regional Clinical Research Ethics Committee (CEICm) on December 22, 2021 (No. 2021/471). It has been registered in the Spanish Clinical Trials Register (GESTO) with identifier 0065–2021-OBS (No. AEMPS 21–0022) and it has also been registered at www.clinicaltrials.gov. The study has been approved by the health authorities.
DiscussionThe SEVERAL study aims to assess potential differences between available GLP-1 agonists using real-life data related to the following aspects: 1) effectiveness in weight reduction; and 2) changes in quality of life, physical activity, and safety and tolerability.
The initial hypothesis is that there are differences between classes regarding weight reduction and tolerability, although an acceptable level of safety as reported in pivotal studies is to be expected. Such results are believed to be of clinical interest to professionals who prescribe and dispense these promising molecules for the prevention and treatment of patients with diabetes and cardiovascular disease. In addition to semaglutide (oral and subcutaneous), four drugs of the aGLP-1 family are currently available: dulaglutide, exenatide, liraglutide, lixisenatide (subcutaneous). However, no evidence is available on their differences regarding real-life weight reduction and, in particular, to what extent patients adhere to treatment as a result of the high rate of gastrointestinal events outside the setting of clinical trials.
Clinical trials with GLP-1 analoguesSeveral clinical trials have been conducted on GLP-1 agonists. All these trials have demonstrated their effectiveness in reducing HbA1c in patients on monotherapy or on combinations with other oral antidiabetic drugs and/or insulin. Dulaglutide was associated with a weight reduction of –0.35 kg to –2.90 kg8. In the case of exenatide, 3% of patients experienced at least one period of rapid weight loss (greater than 1.5 kg/wk)8. In the case of liraglutide, weight reduction was more significant when the baseline BMI was higher8. Lixisenatide was associated with a weight reduction of 1.76 kg to 2.96 kg8. Finally, semaglutide was associated with a weight losses of at least 5% and at least 10% in more patients than in those on the active comparators dulaglutide and exenatide, respectively (SUSTAIN 7 and SUSTAIN 3)12–13. In the SUSTAIN 69,10 trial, weight reduction was –3.6 kg to –4.9 kg. Semaglutide was approved by the FDA for the treatment of obesity in diabetic and non-diabetic patients following the publication of the results of the STEP1 study, which found a weight reduction of –14.9% and a change in baseline weight of –15.3 kg10. Subsequently, the STEP 8 trial (weekly subcutaneous semaglutide vs liraglutide) found a weight reduction of –15.8% with semaglutide vs –6.4% with liraglutide11.
After assessing these data, it can be concluded that all GLP-1 agonists reduce body weight to varying extents: however, the clinical trials that led to their approval have some limitations that could affect the external validity of the results and their extrapolation to real clinical practice. Each of these trials had different study populations, followed different methodological designs, and differed in relation to the primary endpoint. With the exception of the STEP 1 study10, most of these trials were designed to assess HbA1c reduction and cardiovascular events as endpoints, but not to assess weight reductions as endpoints. In contrast to the SEVERAL study, no other study has compared the effectiveness of all GLP-1 agonists.
Safety of GLP-1 agonistsThese weight losses have been accompanied by specific adverse events, the most common being gastrointestinal adverse events, such as nausea, vomiting, diarrhoea, dyspepsia, and constipation8. The most recent study on this issue, STEP 811, found that treatment discontinuation with semaglutide was 13.5% and 27.6% with liraglutide. Gastrointestinal events were reported in 84.1% of patients on semaglutide and 82.7% of patients on liraglutide. Most events were mild to moderate, did not lead to permanent discontinuation, were mainly of short duration, and occurred during dose escalation. These results may indicate a lack of adherence in real life: that is, outside the setting and control to which patients included in clinical trials are exposed. The foregoing suggestion is one of the main motivations for this study (i.e. the assessment of the safety of these drugs in real life).
The SEVERAL study has been designed to collect data on all possible adverse events during the dose escalation and follow-up periods. Data on quality of life and physical activity will also be collected and correlated with potential therapeutic failure and clinical effectiveness.
LimitationsThe present study has several limitations that must be taken into account when interpreting the data. The first limitation is the lack of randomisation, which means that comparative conclusions cannot be drawn: thus, the study merely provides guidance on the current situation, prescription rates, results, and tolerability. If marked differences between classes were to emerge from the present study, a randomised controlled trial could be considered.
Secondly, there is a potentially relevant bias arising from possible loss to follow-up, such that patients with poorer drug tolerability may drop out of the study, thus distorting the results. To avoid this bias, possible loss to follow-up was limited to < 20% to calculate sample size. All patients in our Autonomous Community have electronic records, and so the electronic prescription can be checked for medication collection, which will be of great help in validating adherence (as is done in daily clinical practice).
It is worth mentioning that the present working group, which is exclusively made up of professionals from pharmacy units, will minimise inclusion losses as they represent the largest nucleus of dispensing control. Thus, possible bias can be avoided had this study been initiated, for example, by cardiology or endocrinology units, where it would have been more difficult to ensure the correct inclusion of all patients who are prescribed GLP-1 agonists in our Autonomous Community.
In conclusion, this study will attempt to provide information on the effectiveness of GLP-1 agonists on weight reduction and on changes in quality of life, physical activity, and safety in patients with DM2 starting on treatment with these drugs in real life. This study attempts to compare different GLP-1 agonists in terms of effectiveness and safety in order to make better choices in the prescription of these drugs in patients with cardiovascular disease.
FundingNo funding.
AcknowledgementsWe would like to acknowledge all the main and sub-investigators of the centres participating in the study belonging to the Pharmacy Consultations of SERGAS public centres.
Conflict of interestNo conflict of interest.
José Seijas-Amigo1,5,7, Ángel Salgado-Barreira2, Rosana Castelo-Domínguez3, Mercedes Pereira-Pía4, Moisés Rodríguez-Mañero1-5, Alberto Cordero5,6, Begoña Cardeso-Paredes1,7, Diego Rodríguez-Penas1,7, José Ramón González-Juanatey1,5, Agustina Fernández Pérez8, Montserrat Fernández-Montenegro9, Ana Belén Ponce-Piñón10, Marlen Fernández-Silva11, María Teresa Pérez-Álvarez12, María del Mar Gago-García13, José Manuel Iglesias-Moreno14, Marta Rodríguez-Barreiro15, María Moure-González1, Ana Seoane-Blanco1, Rita Soler-Martín1, Adrián Paz-Couce’
1Cardiology Department. Complexo Hospitalario Universitario de Santiago de Compostela, A Coruña. Spain. 2Unidad de Epidemiología e Investigación Clínica, Universidad de Santiago de Compostela, A Coruña. Spain. 3Pharmacy Department, Centro de Salud de Ribeira, A Coruña. Spain. 4Servicio de Farmacia, Centro de Salud de San Roque, Lugo. Spain. 5Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV). 6Hospital de la Fe de Valencia, Santiago de Compostela, A Coruña. Spain. 7Fundación IDIS de Santiago de Compostela, A Coruña. Spain. 8Pharmacy Department, Centro de Salud de Vilalba, Lugo. Spain. 9Pharmacy Department, Centro de Salud de O Carballiño, Ourense. Spain. 10Pharmacy Department, Centro de Salud de Fene, A Coruña. Spain. 11Pharmacy Department, Centro de Salud O Ventorrillo, A Coruña. Spain. 12Pharmacy Department, Centro de Salud de Culleredo, A Coruña. Spain. 13Pharmacy Department, Centro de Salud de Ribadeo, Lugo, Spain. 14Pharmacy Department, Centro de Salud de Valmiñor, Pontevedra. Spain. 15Pharmacy Department, Centro de Salud Virxe Peregrina, Pontevedra. Spain.