Sepsis and septic shock are major global health issues, with significant morbidity and mortality. Early identification and appropriate management during the first few hours are crucial for improving clinical outcomes.
Sepsis treatment focuses on infection control, restoration of perfusion, and the implementation of adjunctive therapies. A thorough understanding of these approaches is essential for the clinical pharmacist in the intensive care unit to provide optimal pharmacotherapeutic validation.
La sepsis, incluida su forma más grave, el shock séptico, representa uno de los problemas de salud más prevalentes a nivel global, con una elevada morbimortalidad asociada. La identificación temprana y el manejo adecuado durante las primeras horas tras el desarrollo de la sepsis son cruciales para mejorar el pronóstico.
El tratamiento de la sepsis se enfoca en 3 grandes pilares: el control de la infección, la restauración de la perfusión y la implementación de terapias adyuvantes. Un conocimiento profundo de estos enfoques es esencial para que el farmacéutico clínico en la unidad de cuidados intensivos pueda realizar una validación farmacoterapéutica óptima.
Sepsis is a potentially fatal organ dysfunction caused by an inadequate host response to infection.1
The severity of the condition can range from sepsis to septic shock. These conditions are significant health problems that affect millions of people worldwide every year. The estimated mortality rate is over 10% for sepsis and over 40% for septic shock. The incidence in Spain is 104 cases for every 100,000 inhabitants/year, with the number of deaths reaching around 17,000 people/year. An association has been found between improved prognosis for patients and early identification and appropriate treatment in the first few hours.1,2
Sepsis is an abnormal inflammatory response of the body to infection, involving components of microorganisms, such as endotoxins, other substances that cause cell damage, as well as mediators of the inflammatory response generated by the host. This response is associated with changes in non-immune pathways, such as the cardiovascular, neuronal, hormonal, metabolic, and coagulation systems, which may lead to potentially fatal organ dysfunction.
The clinical course may involve different stages of severity, potentially progressing to multiple organ dysfunction syndrome and, in severe cases, death.2
EpidemiologySepsis is a global issue, but the causes, frequency, and consequences of the condition vary significantly depending on the geographic region and the age of the population. Low- and middle-income countries account for around 85% of cases, as well as a significant proportion of associated deaths. These regions, which are characterized by higher levels of social vulnerability, have the highest age-adjusted incidence rates. Sub-Saharan Africa accounts for about 40% of global cases, making it one of the regions most affected by this condition.3
In cases of sepsis, the main sites of infection are the lungs (with a prevalence of 40–60% of cases), abdominal organs (15–30%), genitourinary tract (15–30%), bloodstream, skin, and soft tissues. A pathogen is identified in approximately 60–70% of cases. This percentage could increase with the implementation of molecular techniques for nucleic acid detection.
The most common causes are bacterial infections due to both Gram-positive and Gram-negative microorganisms, followed by fungal and viral infections. It is noteworthy that the incidence of viral sepsis can increase substantially during pandemics.3
Definitions and diagnosisThe first modern definition of sepsis was proposed in 1992. It was described as an excessive inflammatory response to infection, characterized by the presence of systemic inflammatory response syndrome (SIRS),4 identified when at least 2 of the following changes are present: body temperature greater than 38 °C or less than 36 °C; heart rate greater than 90 beats/min; respiratory rate greater than 20 breaths/min; PaCO2 less than 32 mmHg; white blood cell count greater than 12,000/μL or less than 4000/μL, or more than 10% immature bands. In 2016, the definitions of the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) were published, redefining sepsis as “organ dysfunction caused by an abnormal host response to infection that threatens survival,” while excluding SIRS from the definition.1
Early recognition of sepsis is challenging due to its heterogeneous clinical manifestations, dynamic evolution, and tendency to present with subtle initial symptoms. In addition, the characteristic signs and symptoms are not unique to sepsis and may be masked by the use of medications such as beta-blockers or antipyretics.
Sepsis should be suspected in any patient with severe infection or acute organ dysfunction for which a non-infectious cause cannot be clearly identified. The most indicative clinical signs include altered mental status, hypotension, and tachypnoea, although their absence does not rule out the diagnosis. Laboratory findings associated with sepsis include leukocytosis or leukopenia, the presence of more than 10% immature granulocytes, hyperglycemia, and elevated serum creatinine and lactate levels. Even if fever or localized signs of infection are absent, a high index of suspicion should be maintained in patients with altered mental status, hypotension, dyspnea, or acute decompensation of chronic diseases, such as diabetic ketoacidosis or decompensated cirrhosis.1
Clinical evaluation should focus on identifying the site and cause of the infection, as well as assessing organ function and tissue perfusion. Depending on the suspected site of infection, commonly used diagnostic tools include imaging studies, microbiological cultures, specific antigen detection tests (e.g. for Streptococcus or Legionella), and multiple polymerase chain reaction pathogen detection panels. In all patients with suspected sepsis, lactate measurement is recommended to identify any underlying hypoperfusion.
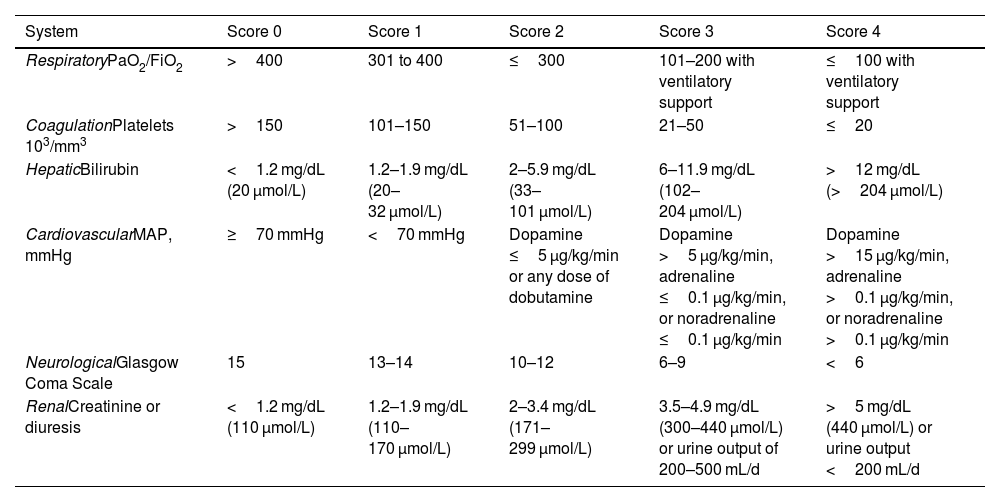
The Sepsis-3 group recommends using a Sequential Organ Failure Assessment (SOFA) score of 2 or more to identify organ dysfunction in these patients, or an increase of 2 or more points if the patient had organ dysfunction prior to the onset of infection. The SOFA scale is the most accurate tool for assessing organ dysfunction in septic patients. It includes an assessment of 6 organs, each scored on a scale ranging from 0 to 4. Scores of 1 or 2 points indicate organ dysfunction and scores of 3 or 4 indicate organ failure (see Table 1). A SOFA score of 2 or more as a result of infection is associated with an overall mortality risk of over 10% in the general population.1
Criteria for organ dysfunction on the SOFA scale.
| System | Score 0 | Score 1 | Score 2 | Score 3 | Score 4 |
|---|---|---|---|---|---|
| RespiratoryPaO2/FiO2 | >400 | 301 to 400 | ≤300 | 101–200 with ventilatory support | ≤100 with ventilatory support |
| CoagulationPlatelets 103/mm3 | >150 | 101–150 | 51–100 | 21–50 | ≤20 |
| HepaticBilirubin | <1.2 mg/dL (20 μmol/L) | 1.2–1.9 mg/dL (20–32 μmol/L) | 2–5.9 mg/dL (33–101 μmol/L) | 6–11.9 mg/dL (102–204 μmol/L) | >12 mg/dL (>204 μmol/L) |
| CardiovascularMAP, mmHg | ≥70 mmHg | <70 mmHg | Dopamine ≤5 μg/kg/min or any dose of dobutamine | Dopamine >5 μg/kg/min, adrenaline ≤0.1 μg/kg/min, or noradrenaline ≤0.1 μg/kg/min | Dopamine >15 μg/kg/min, adrenaline >0.1 μg/kg/min, or noradrenaline >0.1 μg/kg/min |
| NeurologicalGlasgow Coma Scale | 15 | 13–14 | 10–12 | 6–9 | <6 |
| RenalCreatinine or diuresis | <1.2 mg/dL (110 μmol/L) | 1.2–1.9 mg/dL (110–170 μmol/L) | 2–3.4 mg/dL (171–299 μmol/L) | 3.5–4.9 mg/dL (300–440 μmol/L) or urine output of 200–500 mL/d | >5 mg/dL (440 μmol/L) or urine output <200 mL/d |
FiO2, fraction of inspired oxygen; PaO2, arterial oxygen pressure; SOFA, Sequential Organ Failure Assessment; MAP, mean arterial pressure.
The Sepsis-3 group also proposed a simplified version of the SOFA scale, called the quickSOFA (qSOFA) scale for screening patients. Unlike the SOFA scale, qSOFA does not require laboratory parameters and provides simple bedside criteria for identifying adult patients with suspected infection who are likely to have poor outcomes.1 However, the latest update to the Surviving Sepsis Campaign5 guidelines advises against using qSOFA as the sole tool for detecting sepsis or septic shock. Instead, they recommend using the National Early Warning Score6 or the SIRS score, as these are more sensitive than qSOFA in predicting patient prognosis.
The qSOFA scale includes three easily measurable criteria: altered level of consciousness (defined as a Glasgow Coma Scale score of less than 15); systolic blood pressure (SBP) of less than 100 mmHg; and respiratory rate of more than 22 breaths/min.
When 2 or 3 of these variables are present simultaneously, the patient is considered qSOFA-positive, and a complete evaluation using the SOFA scale should be performed to confirm sepsis.
Septic shock is defined as a subgroup of sepsis characterized by severe underlying circulatory and cellular metabolic abnormalities that substantially increase mortality. From a clinical standpoint, septic shock includes patients who meet the criteria for sepsis and who, despite adequate fluid resuscitation, require vasopressors to maintain a mean arterial pressure (MAP) of at least 65 mmHg and have a lactate level at least 2 mmol/L (>18 mg/dL). According to SOFA score predictions, the mortality rate is higher for patients who meet these criteria for septic shock than for those who do not (≥40 vs ≥10%, respectively).1
TreatmentThe treatment of sepsis centers on three key areas: infection control, restoration of perfusion, and adjuvant treatment.
Sepsis is considered a time-dependent disease, with successful outcomes being directly related to the speed with which treatment is initiated.7,8 For this reason, it must be treated as an emergency. The mortality rate increases with every hour of delay in administering antimicrobials and other appropriate measures. Studies show that 80% of patients survive if they receive appropriate treatment within the first hour; however, mortality increases by between 15% and 20% if treatment is delayed beyond the first 12 h.5
We now present a review of the recommendations of the Surviving Sepsis Campaign. Published in 2021, these international guidelines are intended to reflect best practice for managing sepsis and septic shock.5
Infection controlIdentifying and treating both the microorganism causing the sepsis and the site of infection is a priority and should be carried out simultaneously with initial resuscitation. Therefore, blood culture samples and samples from the suspected site of sepsis should be taken for urgent Gram staining.
Early initiation of empirical intravenous antibiotic therapy is a priority therapeutic goal in the treatment of sepsis, and should be implemented as follows:
- •
Initiate immediately and effectively, ideally within the first hour of diagnosis, since mortality increases with each passing hour.
- •
Start with the full intravenous dose, and adjust after 24 to 48 h according to renal and hepatic function.
- •
Be appropriate (i.e. active against the most likely pathogens and able to penetrate the suspected site of infection effectively). The choice of effective empirical antibiotic treatment can be complex and should be based on the following factors: the patient's medical history (e.g. previous antibiotic administration, previous pathogens, colonization), comorbidities, immunosuppression status, clinical context (hospitalized vs community-based), suspected site of infection, presence of invasive devices, and local resistance patterns. Depending on the local epidemiologic situation, patients at risk of infection by multidrug-resistant pathogens should receive treatment that is effective against them.
To meet the above condition, particularly in the most severe cases (i.e. septic shock), broad-spectrum antibiotics containing 1 or more potentially active agents are typically administered initially, with de-escalation based on culture results and clinical improvement. Thus, for most patients, it is recommended to start treatment with 1 or 2 broad-spectrum antibiotics active against Gram-negative and Gram-positive bacteria. The most frequently isolated pathogens are Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, and Streptococcus pneumoniae.9
Combination therapy, involving the use of at least 2 antibiotics with different antimicrobial spectra, is recommended in various situations, such as for neutropenic patients, or those with a high suspicion of difficult-to-treat and multidrug-resistant bacterial pathogens (e.g. Acinetobacter and Pseudomonas spp.).
To optimize antimicrobial dosing strategies, it is recommended that accepted principles of pharmacokinetics/pharmacodynamics are applied, while taking into account the specific properties of each drug. When using a β-lactam antibiotic, prolonged or continuous infusion following an initial bolus (loading dose) should be considered, particularly for patients with increased renal clearance and suspected infection by bacteria with high minimum inhibitory concentrations.
Antifungal treatment is also recommended for patients who are immunocompromised, have central venous catheters, are receiving parenteral nutrition, are experiencing prolonged hospital stays, have recently undergone surgery, have received prolonged broad-spectrum antibiotics, have a history of necrotising pancreatitis, and have previous fungal colonization.10 The need for antiviral or antiparasitic treatment should also be considered.
Restoration of perfusion: Initial resuscitationFluid therapyPatients with severe sepsis presenting septic shock with hypotension or hypoperfusion (as indicated by lactic acidosis) should be resuscitated immediately, wherever they are located, and completed within the first 3 h of the suspected diagnosis. The goal is to improve tissue perfusion, which can be verified by either improved lactate levels or improved capillary refill.
Crystalloids are the fluid therapy of choice for patients with septic shock. The use of colloids is not currently recommended, except for albumin, which may be considered for patients who have received large volumes of crystalloids. Among crystalloids, the administration of normal saline (0.9% sodium chloride) has been common practice for decades. However, the potential for adverse effects, such as hyperchloremic metabolic acidosis, renal vasoconstriction, increased cytokine secretion, and acute renal failure, has led to a growing interest in the use of chloride-restricted solutions, also known as balanced or buffered solutions. This is of special relevance when large volumes are required. Nevertheless, the choice of optimal fluid therapy remains a matter of debate.11,12
It is recommended that an initial loading dose of 30 mL/kg of crystalloids is administered over a period of 30 to 60 min. If no clinical improvement is observed, a second intravenous bolus can be administered. The goal is to achieve an SBP of at least 90 mmHg or a MAP of at least 65 mmHg. The total volume administered should be adjusted according to the characteristics of each patient and hemodynamic variables. Fluid administration should be discontinued if there are signs of volume overload or pulmonary edema, or if additional administration fails to increase perfusion. To avoid excessive or insufficient resuscitation, the administration of fluids beyond the initial resuscitation stage should be guided by careful assessment of intravascular volume status and organ perfusion. Heart rate, central venous pressure, and SBP alone are poor indicators of fluid status. In general, fluid administration should be guided by dynamic preload and fluid response parameters, such as pulse pressure variation or stroke volume variation, or by the response to passive leg raising combined with cardiac output measurement.5
Serum lactate is an important biomarker of both hypoxia and tissue dysfunction. Although it is not a direct measure of tissue perfusion, the guidelines recommend its determination to guide resuscitation in adult patients with sepsis or septic shock and elevated lactate levels. Serum lactate level should be interpreted according to the overall clinical context, bearing in mind that there may be other possible causes of elevated lactate levels. Capillary refill time can also be used alongside other measures of perfusion.5
Vasopressor and inotropic treatmentThe goal of vasoactive therapy is to optimize the perfusion of the vital organs and ensure the supply of oxygen to the cells.13 Vasopressor treatment is recommended if hemodynamic goals are not achieved following adequate fluid replenishment, or even earlier if the patient's condition deteriorates.
The vasopressor of choice is noradrenaline—which is a potent α1- and β1-adrenergic agonist—that produces vasoconstriction and an increase in MAP, with minimal effect on heart rate. If the target MAP is not achieved, it is recommended that a second vasopressor be added, rather than simply increasing the noradrenaline dose. In this case, the current drug of choice is vasopressin. In clinical practice, vasopressin treatment is typically initiated when the noradrenaline dose (base) is between 0.25 and 0.50 μg/kg/min.
As noradrenaline is practically insoluble in water, alcohol, and ether, but soluble in acidic solutions, it must be formulated as a salt, such as noradrenaline tartrate or bitartrate, for intravenous administration. Given that the amount of noradrenaline salt can be up to twice that of noradrenaline base (the active ingredient)—2 mg of noradrenaline bitartrate is equivalent to 1 mg of noradrenaline base—it is clinically relevant to adopt a standardized approach to prescribing, administering, and reporting noradrenaline doses in clinical trials. Recently, a multidisciplinary international working group recommended the adoption of a uniform, standard, noradrenaline-based formulation for global use, as well as a standardized reporting of noradrenaline doses and formulations.14 It also suggested that noradrenaline base should be used instead of noradrenaline salts, such as noradrenaline tartrate or bitartrate. These recommendations should be extended to hospital organizations, clinical care, researchers, and drug manufacturers.
All patients requiring vasopressors should undergo invasive blood pressure monitoring, with the drugs being administered via a central venous line as soon as possible. However, it has been shown that noradrenaline can be safely administered at low doses via a peripheral route (the more proximal, the better), so waiting for central access should not delay its initiation.9
Vasopressin is a non-catecholaminergic vasopressor hormone released by the posterior pituitary in response to hypotension and hypernatremia. Its vasoconstrictor action involves several mechanisms, including binding to V1 receptors in smooth muscle, and is independent of catecholamine activity, which is why vasopressin is used alongside noradrenaline to treat refractory shock. Regarding clinical trials, the VASST study15 found no overall difference in mortality between vasopressin and noradrenaline, except in patients with less severe septic shock (those receiving noradrenaline <15 μg/min), while the VANISH study showed that vasopressin reduced the need for renal replacement therapy.16 As both studies demonstrated a catecholamine-sparing effect, the early use of vasopressin in combination with noradrenaline could help to reduce the adrenergic burden associated with traditional vasoactive agents. Since vasopressin has a half-life of less than 10 min, it should be administered by continuous intravenous infusion at a dose of 0.01 to 0.03 IU/min.
Adrenaline is commonly used as a third vasopressor in very refractory cases, following noradrenaline. Adrenaline is an adrenergic agonist with potent β1 and moderate β2 activity, as well as α1 activity.
Its activity is dose-dependent. At low doses, it exhibits preferential activity on β1 adrenergic receptors, thereby increasing cardiac output and decreasing vascular resistance while having a variable effect on MAP. However, at high doses, it increases both cardiac output and vascular resistance. Potential adverse effects include arrhythmia and splanchnic ischemia.
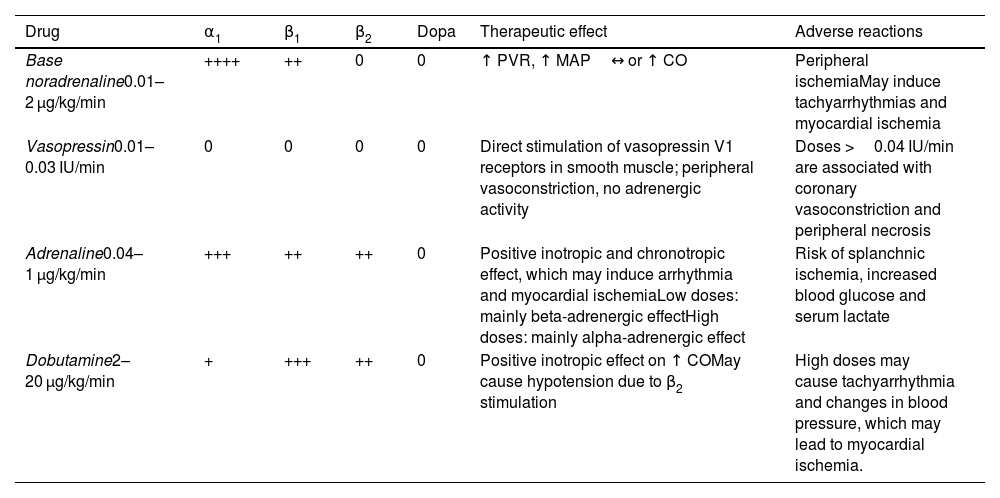
Table 2 shows the doses and effects of common catecholamines.
Doses and effects of common catecholamines.
| Drug | α1 | β1 | β2 | Dopa | Therapeutic effect | Adverse reactions |
|---|---|---|---|---|---|---|
| Base noradrenaline0.01–2 μg/kg/min | ++++ | ++ | 0 | 0 | ↑ PVR, ↑ MAP↔ or ↑ CO | Peripheral ischemiaMay induce tachyarrhythmias and myocardial ischemia |
| Vasopressin0.01–0.03 IU/min | 0 | 0 | 0 | 0 | Direct stimulation of vasopressin V1 receptors in smooth muscle; peripheral vasoconstriction, no adrenergic activity | Doses >0.04 IU/min are associated with coronary vasoconstriction and peripheral necrosis |
| Adrenaline0.04–1 μg/kg/min | +++ | ++ | ++ | 0 | Positive inotropic and chronotropic effect, which may induce arrhythmia and myocardial ischemiaLow doses: mainly beta-adrenergic effectHigh doses: mainly alpha-adrenergic effect | Risk of splanchnic ischemia, increased blood glucose and serum lactate |
| Dobutamine2–20 μg/kg/min | + | +++ | ++ | 0 | Positive inotropic effect on ↑ COMay cause hypotension due to β2 stimulation | High doses may cause tachyarrhythmia and changes in blood pressure, which may lead to myocardial ischemia. |
↑, increase; ↔, no change; CO, cardiac output; MAP, mean arterial pressure; PVR, peripheral vascular resistance.
Preliminary studies suggest that angiotensin II could serve as an alternative vasopressor in cases of septic shock resistant to noradrenaline.17,18 Several clinical trials are currently underway to evaluate the effects of angiotensin II as a vasopressor agent. The main concern regarding the administration of angiotensin II in cases of septic shock is its potent vasoconstrictive properties, which could compromise regional blood flow and aggravate tissue perfusion.
Methylene blue is another treatment that has been evaluated as an alternative method for achieving hemodynamic goals.19 It acts by inhibiting the enzyme guanylate cyclase. This reduces the production of excessive nitric oxide and lessens its vasorelaxant effect on vascular smooth muscle. The result is a restoration of vascular tone and an increase in blood pressure. The lack of randomized clinical trials makes it difficult to accurately assess the effectiveness of methylene blue in patients with sepsis. However, a recent meta-analysis indicated that its use significantly reduces the time taken to withdraw vasopressors, the duration of mechanical ventilation, and the length of stay in intensive care.19
It is recommended that dobutamine be added to noradrenaline or that adrenaline be used alone in patients with septic shock and cardiac dysfunction—which occurs in 20% to 70% of cases, depending on the case series20—who show signs of persistent hypoperfusion despite adequate control of blood volume and blood pressure. Studies show that dobutamine increases the transport of carbon dioxide and oxygen, improves splanchnic perfusion and tissue oxygenation, and alleviates acidosis and hyperlactacidemia.15 However, the effects may be unpredictable, potentially leading to severe vasodilation and reduced MAP. Levosimendan is an inotropic that increases the sensitivity of contractile proteins to calcium. Although it has been evaluated in cases of septic shock, no clear benefit has been demonstrated, and therefore, its use is not currently recommended.
Adjuvant treatmentsAdditional therapeutic strategies have been shown to improve the prognosis of septic patients and are therefore recommended.
GlucocorticoidsIntravenous corticosteroids are recommended for patients with septic shock requiring high doses of vasopressors (e.g. noradrenaline ≥0.25 μg/kg/min for at least 4 h after initiation). Three randomized clinical trials and a subsequent meta-analysis16,21–23 have demonstrated reductions in the time taken for shock to resolve, as well as increases in the number of days without the need for vasopressors. Increases in complications inherent to the use of corticosteroids were observed, including hyperglycaemia, hypernatraemia, gastrointestinal bleeding, muscle weakness, and superinfection. However, statistically significant evidence was only found for hyperglycaemia and hypernatraemia, neither of which was associated with worse clinical outcomes.24
The corticosteroid of choice, optimal dose, and duration of treatment are not well established. The most commonly used corticosteroid in studies is intravenous hydrocortisone, administered at a dose of 200 mg/day in 50-mg boluses every 6 h, or by continuous infusion on a tapering schedule according to clinical response. The total treatment duration is 5 to 7 days.
Vitamin C and thiamineAlthough 1 study found that administering a combination of high doses of vitamin C, hydrocortisone, and thiamine led to shorter vasopressor therapy durations and lower mortality rates,25 there are currently no sufficiently high-quality studies to recommend its use in cases of sepsis or septic shock.24
Blood productsThe transfusion of packed red blood cells is recommended when the hemoglobin concentration is less than 7 g/dL, unless there is active bleeding, lactic acidosis, or coronary artery disease, in which cases higher thresholds may be chosen. The administration of erythropoietin or antithrombin is not indicated.
The administration of platelet concentrates or fresh frozen plasma is indicated for thrombocytopenia or prolonged coagulation times, respectively. This is particularly important when there is active bleeding or when invasive procedures are to be performed.
The use of intravenous immunoglobulins is not recommended.
Stress ulcer prophylaxisProphylaxis for stress ulcers is recommended for patients at risk of gastrointestinal bleeding, since stress ulcers in critically ill patients can lead to significant morbidity and mortality. Proton pump inhibitors and H2-antagonists are both indicated, with no preference for either.
ThromboprophylaxisCritically ill patients are at an increased risk of developing deep vein thrombosis and pulmonary embolism. In the intensive care unit, the incidence of these conditions can reach 10%26 and 4%, respectively. Pharmacological prophylaxis is recommended for the prevention of thromboembolic disease. Low molecular weight heparin is the drug of choice rather than unfractionated heparin, unless there is a contraindication to such treatment. In this case, mechanical methods such as pneumatic compression stockings may be used instead.
Blood glucose controlHyperglycaemia (>180 mg/dL), hypoglycaemia, and glycaemic variability in general are all associated with an increased risk of death in critically ill patients.27,28 It is recommended that blood glucose levels be kept as close to normal as possible (140–180 mg/dL) through the initial use of insulin and frequent monitoring. The onset of hypoglycaemia should be avoided.
Renal supportIn adults with sepsis or septic shock and acute renal failure requiring renal replacement therapy, both continuous and intermittent renal replacement techniques have been shown to be effective. The former is more common in patients with severe septic shock due to better tolerance in unstable patients.
BicarbonateSodium bicarbonate should only be used to improve hemodynamics or reduce vasopressor requirements in patients with severe metabolic acidosis (pH ≤7.20) and an Acute Kidney Injury Network score of 2 or 3.
NutritionWhen possible, early enteral nutrition (within the first 72 h) is recommended due to its potential advantages in maintaining intestinal integrity, preventing intestinal hyperpermeability, reducing the inflammatory response, and modulating metabolic responses.
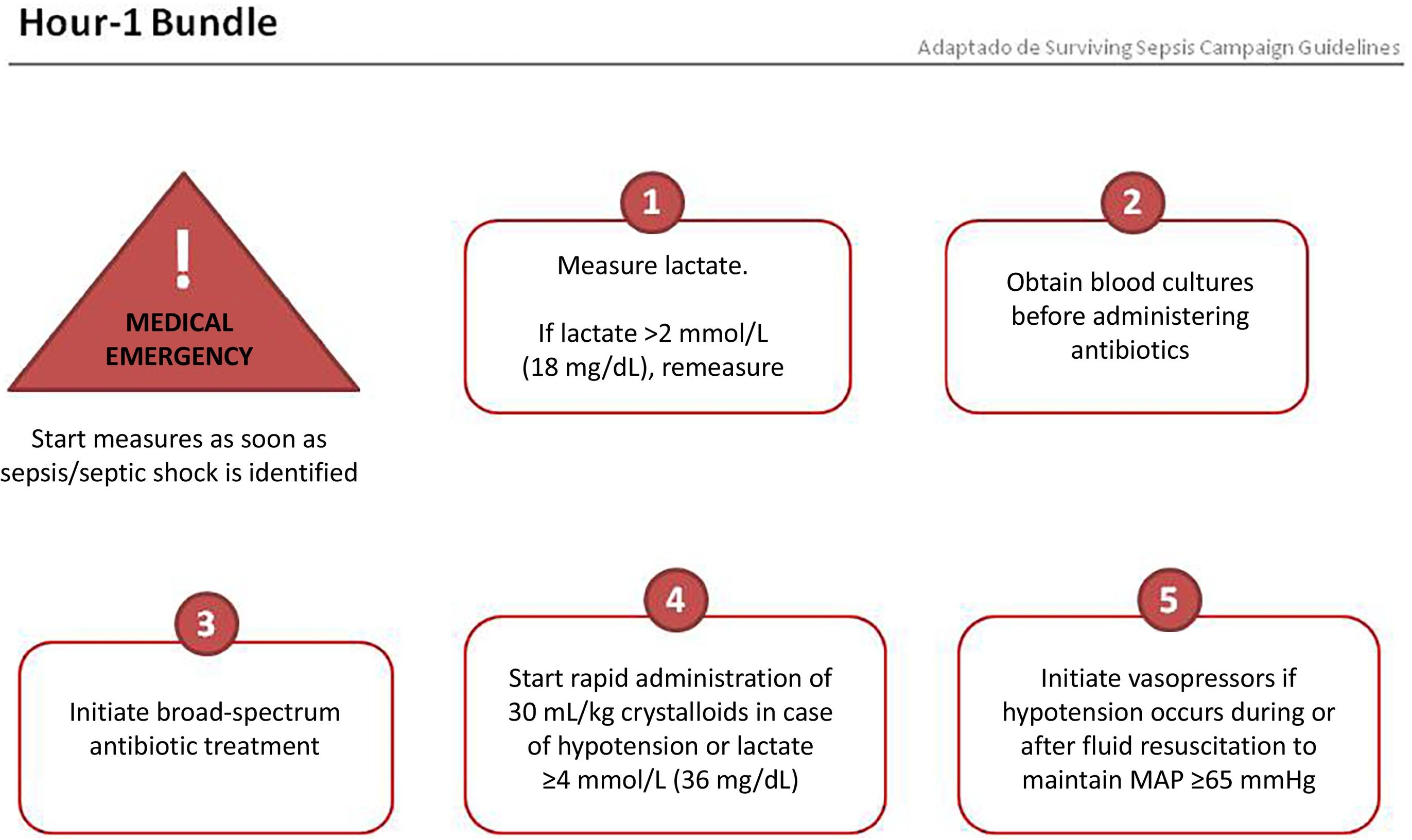
Sets of measuresThe Surviving Sepsis Campaign has recently proposed a set of measures to be implemented within the first hour of identifying an episode of sepsis, which could help reduce the high mortality rate.26 These measures are shown in Fig. 1 and include the following: measuring serum lactate; obtaining blood cultures before starting antibiotic treatment; early initiation of antibiotic treatment (ideally within the first hour after diagnosis and no later than 3 h); and in the presence of hypotension or lactate ≥36 mg/dL, initiating resuscitation with 30 mL/kg of crystalloids and using vasopressors to treat hypotension during and after fluid resuscitation.
The second set of measures to be implemented in the first 24 h includes the following: consider administering high-dose corticosteroids in refractory septic shock; maintain blood glucose between 140 and 180 mg/dL; provide prophylaxis for stress ulcers; perform prophylaxis for deep vein thrombosis; and in patients with invasive mechanical ventilation, implement protective ventilation strategies (maintain plateau pressure below 30 cmH2O).
ConclusionsSepsis is recognized as an abnormal inflammatory response of the body to infection, which can progress to potentially fatal organ dysfunction. Early identification and appropriate treatment are crucial to improving patient prognosis. Definitions and diagnostic criteria, such as those established by the International Consensus on Sepsis and Septic Shock, provide a basis for clinical evaluation. It is essential to implement risk stratification and escalated therapeutic measures, such as early antimicrobial treatment and initial resuscitation. The treatment of sepsis and septic shock includes the use of vasopressor and inotropic drugs, alongside additional therapies such as glucocorticoids and various supportive measures. These recommendations should be adapted according to each patient's clinical progress and the resources available in each healthcare setting.
Declaration of authorshipAll authors are members of the FARMIC Working Group. All authors participated in the concept, design, definition of intellectual content, preparation, and review of the project. Carla Bastida and Sara Cobo were responsible for writing the manuscript. All authors critically reviewed and approved the final version of the manuscript for publication.
CRediT authorship contribution statementCarla Bastida: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Data curation, Conceptualization. Amaia Egües Lugea: Writing – review & editing. Aurora Fernández Polo: Writing – review & editing. Fernando Becerril Moreno: Writing – review & editing. Maria Martín Cerezuela: Writing – review & editing. Esther Domingo Chiva: Writing – review & editing. Tatiana Betancor García: Writing – review & editing. Miguel Angel Amor García: Writing – review & editing. Irene Aquerreta González: Writing – review & editing. Marta Albanell-Fernández: Writing – review & editing. Laura Doménech Moral: Writing – review & editing. Sara Ortiz Pérez: Writing – review & editing. Sara Cobo Sacristán: Writing – review & editing, Writing – original draft.
FundingNone declared.
None declared.