To standardize the drug dilutions administered intravenously in a Pediatric Intensive Care Unit and to characterize these dilutions based on their pH, osmolarity, and vesicant nature. This aims to guide the selection of the most appropriate vascular access device, minimizing associated complications, and preserving the patient's venous capital.
MethodsThrough a consensus between Pharmacy and Pediatric Services, the most frequently administered intravenous drugs in the Pediatric Intensive Care Unit were selected. Two different dilutions were established for each drug, followed by the determination of their respective osmolarity and pH values. The vesicant nature of each drug was assessed according to the classification proposed by Clark et al. Additionally, vascular access device selection was guided by the algorithm proposed by Manrique et al., which considers the drug’s properties, the duration of intravenous therapy, and the patient's venous capital status.
ResultsA total of 60 dilutions corresponding to 30 drugs from the following therapeutic groups were analyzed: antimicrobials (56%), antiepileptics (13%), sedatives (7%), diuretics (7%), anti-inflammatory and analgesics (7%), and others (10%). Twenty-five percent of the dilutions exhibited at least one high-risk factor for phlebitis (osmolarity >600 mOsm/L or pH < 4 or > 9), while 35% were classified as intermediate risk (osmolarity 450–600 mOsm/L or pH 4–5 or > 7.5–9). Only 10% of the analyzed drugs were classified as vesicants (acyclovir, phenytoin, and vancomycin). Seventeen dilutions of nine different drugs were identified that should not be administered through a peripheral venous catheter, even in short-term treatments. Of these, 15 had a high risk of causing phlebitis, while 2 had an intermediate risk.
ConclusionsThe physicochemical properties (osmolarity and pH) and vesicant nature of drugs are key factors contributing to the development of phlebitis in critically ill pediatric patients. Standardizing and characterizing drug dilutions will facilitate the selection of the most appropriate vascular access device, improving the safety and effectiveness of intravenous therapy.
Estandarizar las diluciones de los fármacos administrados por vía intravenosa en una unidad de cuidados intensivos pediátricos y caracterizar dichas diluciones en función de su pH, osmolaridad y poder vesicante, con la finalidad de guiar la elección del dispositivo de acceso vascular más adecuado, minimizando el riesgo de complicaciones asociadas y preservando el capital venoso del paciente.
MétodosMediante consenso entre los servicios de farmacia y pediatría, se seleccionaron los fármacos de administración intravenosa empleados con más frecuencia en la unidad de cuidados intensivos pediátricos. Se establecieron 2 diluciones diferentes para cada fármaco, con la posterior determinación de los valores de osmolaridad y pH correspondientes a cada una. El poder vesicante del fármaco se estableció según la clasificación de Clark et al. Asimismo, la selección del dispositivo de acceso vascular se realizó siguiendo el algoritmo propuesto por Manrique et al., considerando las propiedades del fármaco, la duración de la terapia intravenosa y el estado del capital venoso del paciente.
ResultadosSe analizaron 60 diluciones asociadas a 30 fármacos correspondientes a los siguientes grupos terapéuticos: antiinfecciosos (56%), antiepilépticos (13%), sedantes (7%), diuréticos (7%), antiinflamatorios y analgésicos (7%), y otros (10%). El 25% de las diluciones mostraron al menos un factor de riesgo elevado de producir flebitis (osmolaridad >600 mOsm/l o pH < 4 o > 9), mientras que el 35% presentaron un riesgo intermedio (osmolaridad 450–600 mOsm/l o pH 4–5 o > 7,5–9). Solo el 10% de los fármacos analizados fueron clasificados como vesicantes (aciclovir, fenitoína y vancomicina). Se identificaron 17 diluciones asociadas a 9 fármacos distintos que no deberían administrarse a través de un catéter venoso periférico, incluso en tratamientos de corta duración. De estas diluciones, 15 presentaron un riesgo elevado de producir flebitis y 2 un riesgo intermedio.
ConclusionesLas propiedades fisicoquímicas (osmolaridad y pH) y el poder vesicante de los fármacos administrados por vía intravenosa son factores que contribuyen a la aparición de flebitis en el paciente crítico pediátrico. Estandarizar y caracterizar las diluciones de estos fármacos facilitará la selección del dispositivo de acceso vascular más adecuado, incrementando la seguridad y efectividad del tratamiento intravenoso.
Peripheral intravenous catheters (PIVCs) are widely used in hospital settings for the delivery of drugs and fluids in pediatric patients. Their use, however, may cause adverse events including phlebitis, extravasation, infiltration, occlusion and infections, to name a few.1
The incidence of PIVC-related complications in pediatric patients ranges from 34 to 56%,2 with a notable increase observed from the second to third day post-insertion.3 These complications are associated with increased morbidity and mortality, as well as delays or interruptions in intravenous therapy and prolonged hospital stays.2,4
One of the most common catheter-related complications in pediatric patients is phlebitis,1,5 defined as the inflammation of a vein secondary to endothelial damage. Manifestations of these complications include pain, erythema, inflammation, hardening, or the presence of a palpable venous cord.6 Numerous factors are involved in the occurrence of catheter-related complications such as the patient's venous patrimony and the therapy administered.
The appearance of complications is also influenced by the characteristics of the catheter, including diameter, size, insertion site and duration of the therapy.1
The most relevant patient-related risk factor is venous patrimony status. Pediatric patients are at a higher risk of developing phlebitis,7 as they exhibit thinner and more fragile veins and a higher proportion of adipose tissue.1,5,8
Risk factors associated with the type of therapy include pH and osmolarity of the infusate. When these properties are not aligned with those of blood (pH 7.35–7.45 and osmolarity 285–310 mOsm/l), the risk for phlebitis increases.9,10 Other risk factors include the type of diluents; route of administration; infusion rate; vesicant potential of the drug; and duration of intravenous therapy.6
The physico-chemical properties of drugs are not consistently reported in the summaries of product characteristics; when available, the information generally refers to the undiluted form of the drug. The information provided in the scientific literature is not necessarily applicable to our context due to differences in the concentrations considered and the intravenous devices used, among other factors.
The objective of this study was to standardize and characterize dilutions of the intravenous drugs most commonly used in pediatric intensive care units (PICUs) based on their physico-chemical properties (pH, osmolarity) and vesicant nature to guide the selection of the most suitable vascular access device (VAD) and estimated duration of intravenous therapy.
MethodsA multidisciplinary study was conducted involving members of different departments of a tertiary hospital, including the hospital pharmacy, PICU, and Department of Clinical Biochemistry.
The Units of Pharmacy and Pediatry selected the drugs most commonly used in the PICU. Each drug was evaluated at two concentrations, selected based on their known stability range11–14 and in accordance with the local protocol. These concentrations corresponded to the upper and intermediate limits of the stability range, respectively, as infusates in the pediatric intensive care unit (PICU) are typically administered at higher concentrations to minimize fluid overload.
The section of Pharmacological Technology of the Department of Pharmacy prepared the dilutions and measured their pH and density. Osmolality was measured by the Department of Clinical Biochemistry of the same hospital.
Reconstitutions and dilutions were performed according to the specifications provided in the respective summaries of product characteristics.11 Whenever possible, water for injection (WFI) –having a osmolarity of 0 mOsm/l– was used as the reconstituent. In most cases, 0.9% physiological saline (0.9% NaCl) was used as the diluent, as it is the preferred vehicle in the PICU. For drugs incompatible with 0.9% sodium chloride solution, dilution was performed using 5% dextrose in water (D5W).
Each preparation was subsequently characterized according to its pH, osmolarity, and vesicant potential.
Determination of osmolarity and pHOsmolarity is defined as the number of milliosmoles of solute per liter of solution. In turn, osmolality refers to the number of milliosmoles of solute per kilogram of solvent. Both parameters are interrelated by a conversion factor based on the density of the solution:10
Osmolarity (mOsm ∕ l) = osmolality (mOsm ∕ kg) × dilution density (g ∕ ml).
Osmolality was measured using the microsmometer Osmo1® (Advanced Instruments Inc.) Results, expressed as means ± standard deviation (SD) of three different measurements, were used to calculate osmolarity using the equation provided.
Density was determined as a function of the solution weight and volume based on the following formula:
Density = solution weight (g)∕solution volume (ml)
Solution weight was measured using the analytical balance Mettler-Toledo, S.A.E.
Levels of pH were measured, with pH indicating the level of acidity or alkalinity of a solution as a function of the concentration of hydrogen ions. Measurements were performed using the Basic 20 Crison® pH meter (Hach Lange Spain, S.L.U). Results were also expressed as means ± SD of three different measurements.
Each dilution was prepared to a final volume of 50 ml, of which 30 μl were used to measure osmolality; 6 ml were used to determine density; and the remaining volume was used to measure pH.
Determination of vesicant natureVesicant agents are substances that cause blistering, tissue detachment or even necrosis upon extravasation into surrounding tissue. In turn, irritant agents induce burning, stinging or pain as a result of irritation to the inner lumen of the vein, with or without the immediate presence of external signs of inflammation. Despite these differences, damage to the vascular endothelium may also result in phlebitis, infiltration or extravasation.15 These events are more frequent in pediatric patients due to their smaller vein diameter and immobilization difficulties.16,17
The severity of extravasation is proportional to drug leakage volume; however, factors such as pH, osmolarity, vasoactive and cytotoxic effects may also influence its vesicant potential.16,17
Although extravasation can occur with any type of vascular access device, the risk is notably higher with peripheral intravenous catheters (PIVCs). Consequently, the use of a central venous catheter (CVC) is recommended for the administration of drugs associated with a high risk of tissue injury.17
The vesicant potential of drugs was assessed according to the Clark et al. classification.17 Most of the vesicant agents considered in this classification have extreme pH values (e.g. acyclovir, phenobarbital, phenytoin), or high osmolarity (e.g., sodium chloride ≥3%, glucose solutions ≥12.5%). However, other drugs not featuring any of these properties are considered vesicant agents due to their direct mechanism of cytotoxicity.15
Selection of the vascular access deviceThe risk for phlebitis increases when the pH and osmolarity of a drug differ from those of blood (pH 7.35–7.45 and osmolarity 285–310 mOsm/l).9,10 Based on this premise, Manrique et al. established a phlebitis risk classification based on the characteristics of the dilutions considered:18
Hight risk: osmolarity >600 mOsm/l o pH < 4 or pH > 9 or vesicant.
Intermediate risk: osmolarity 600–450 mOsm/l or pH 4–5 o pH 7,.–9 or non-vesicant.
Low risk: osmolarity <450 mOsm/l or pH 5–7.5 or non-vesicant.
The estimated duration of therapy is also an important factor to be considered when deciding about the type of VAD.19
Solutions administered through catheters placed in larger-diameter vessels generally achieve higher flow rates and greater dilution, thereby minimizing venous irritation. This method facilitates the delivery of hypersomolar solutions with extreme pH values, vesicant drugs, and long-term therapies.10,20
ResultsA total of 30 drugs of the following therapeutic groups were analyzed: anti-infectives (56%); antiepileptics (13%); sedatives (7%); diuretics (7%); anti-inflammatory and analgesic drugs (7%); and others (10%). All drugs were analyzed at two concentrations, with the exception of rasburicase –which was analyzed at a single dilution–, and ampicillin, analyzed at three dilutions. In total, 60 different dilutions were performed.
Of the 60 dilutions tested, 15 had at least a high risk factor for phlebitis, whereas 21 had an intermediate risk.
Regarding osmolarity, 5/60 dilutions of three different drugs exhibited an osmolarity >600 mOsm/l, whereas four different medications had an osmolarity ranging from 600 to 450 mOsm/l.4/60.
In relation to pH, 13/60 dilutions were characterized by extreme pH values. Of them, 8 dilutions corresponding to four different drugs showed a pH > 9, whereas 5 dilutions of three different drugs had a pH < 4. Additionally, pH values ranged from 4 to 5 in 17 of the 60 dilutions, and from 7.5 to 9 in 14 dilutions involving nine different drugs. Specifically, three dilutions of three distinct drugs had a pH of 4–5, while 14 dilutions corresponding to nine drugs had a pH of 7.5–9.
Of the 30 drugs considered, only three (10%) were considered to be vesicant according to Clark et al. classification,17 including acyclovir, phenytoin and vancomycin. Vesicant drugs at the concentrations considered were also a risk factor for phlebitis as a function of their pH values and osmilarity.
Selection of the most suitable VAD was performed on the basis of the algorithm developed by Manrique et al. This tool considers the properties of the drug (osmolarity, pH and vesicant potential), estimated duration of treatment and status of the venous patrimony of the patient to determine the most suitable VAD.18
According to this algorithm, if the venous patrimony of the patient is poor or the infusate to be administered contains at least a high-risk factor for phlebitis (>600 mOsm/l, pH < 4 or >9, or if the drug is a vesicant agent), a central catheter is recommended. The type of catheter to be used will depend on the estimated duration of treatment:
- •
<1 month: Non-tunneled CVC or peripheral intravenous central catheter (PICC)
- •
1 month-1 year: PICC
- •
>1 year: Tunneled or implanted CVC
On another note, if the venous patrimony of the patient is in good condition and the dilution is not associated with a high risk for phlebitis, the VAD will be selected according to the following criteria:
- •
Osmolarity < 450 mOsm/l and pH within low (5–7,5) or intermediate (4–5o7,5–9) risk limits:
- o
<7 days: CVP
- p
7 days - 1 month: midline catheter (MC)
- q
>1 month: Non-tunneled CVC or PICC
- •
Osmolarity 450–600 mOsm/l and pH 5–7.5:
- o
<7 days: CVP
- p
7 days - 1 month: MC
- q
<1 month: Non-tunneled CVC or PICC
- •
Osmolarity ≤ 600 mOsm/l and pH 4–5 or 7.5–9:
- o
<7 days: MC
- p
7 days - 1 month: MC
- q
>1 month: Non-tunneled CVC or PICC
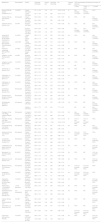
Physico-chemical properties and vesicant potential of the dilutions considered and the most appropriate vascular access device according to the duration of intravenous treatment.
| Medications | Reconstitution | Dilution | Osmolality (mOsm/kg) | Density g/ml | Osmolarity (mOsm/kg) | pH | Vesicant agent | VAD recommended according to the duration of treatment | ||
|---|---|---|---|---|---|---|---|---|---|---|
| <7 days | 7 days – <1 month | >1 montha | ||||||||
| Acyclovir 250 mg (Accord Healthcare S.L.U.) | 10 ml WFI | 5 mg/ml 0.9% NaCl | 271 ± 1.53 | 1.00 | 271 | 10.53 ± 0.08 | Si | Non-tunneled CVC/PICC | Non-tunneled CVC/PICC | PICC |
| 7 mg/ml 0.9% NaCl | 261 ± 1.53 | 1.01 | 263 | 10.57 ± 0.10 | ||||||
| Amikacin 500 mg 2 ml (Normon S.A.) | Not required | 5 mg/ml 0.9% NaCl | 293 ± 0.58 | 1.00 | 293 | 6.67 ± 0.05 | No | PIVC | MC | Non-tunneled CVC/PICC |
| 10 mg/ml 0.9% NaCl | 304 ± 2.89 | 1.1 | 334 | 5.92 ± 0.09 | ||||||
| Ampicillin 500 mg (Normon S.A.) | 4 ml WFI | 30 mg/ml 0.9% NaCl | 408 ± 0.58 | 1.01 | 412 | 9.00 ± 0.02 | No | PIVC | MC | Non-tunneled CVC/PICC |
| 50 mg/ml 0.9% NaCl | 469 ± 0.58 | 1.02 | 479 | 9.05 ± 0.08 | Non-tunneled CVC/PICC | Non-tunneled CVC/PICC | PICC | |||
| 100 mg/ml 0.9% NaCl | 697 ± 4.58 | 1.00 | 697 | 9.33 ± 0.03 | ||||||
| Liposomal B amphotericin 50 mg (Gilead Sciences S.L.) | 12 ml WFI | 1 mg/ml D5W | 295 ± 1.73 | 1.03 | 305 | 5.47 ± 0.06 | No | PIVC | MC | Non-tunneled CVC/PICC |
| 2 mg/ml D5W | 294 ± 1.15 | 1.04 | 305 | 5.43 ± 0.01 | ||||||
| Azithromycin 500 mg (Altan Pharmaceuticals S.A.) | 4.8 ml WFI | 1 mg/ml 0.9% NaCl | 309 ± 0.58 | 1.00 | 310 | 6.43 ± 0.12 | No | PIVC | MC | Non-tunneled CVC/PICC |
| 2 mg/ml 0.9% NaCl | 332 ± 0.58 | 1.00 | 333 | 6.31 ± 0.06 | ||||||
| Potassium canreonate 200 mg (Pfizer S.L.) | 2 ml WFI | 0.4 mg/ml 0.9% NaCl | 284 ± 0.00 | 1.00 | 284 | 8.72 ± 0.02 | No | PIVC | MC | Non-tunneled CVC/PICC |
| 2 mg/ml D5W | 295 ± 2.52 | 1.00 | 295 | 7.96 ± 0.05 | ||||||
| Cefazolin 1 g (Qilu Pharma Spain S.L.) | 4 ml WFI | 10 mg/ml 0.9% NaCl | 288 ± 1.73 | 1.03 | 296 | 6.15 ± 0.12 | No | PIVC | MC | Non-tunneled CVC/PICC |
| 20 mg/ml 0.9% NaCl | 310 ± 0.58 | 1.02 | 315 | 5.34 ± 0.16 | ||||||
| Cefepime 1 g (Qilu Pharma Spain S.L.) | 10 ml WFI | 20 mg/ml 0.9% NaCl | 397 ± 0.58 | 1.01 | 400 | 5.16 ± 0.18 | No | PIVC | MC | Non-tunneled CVC/PICC |
| 40 mg/ml 0.9% NaCl | 479 ± 1.53 | 1.06 | 506 | 4.94 ± 0.09 | MC | |||||
| Cefotaxime 1 g (Normon S.A.) | 4 ml WFI | 30 mg/ml 0.9% NaCl | 346 ± 2.65 | 1.02 | 352 | 5.48 ± 0.03 | No | PIVC | MC | Non-tunneled CVC/PICC |
| 60 mg/ml 0.9% NaCl | 435 ± 1.53 | 1.06 | 460 | 5.63 ± 0.05 | ||||||
| Ceftazidime 1 g (Qilu Pharma Spain S.L.) | 10 ml WFI | 10 mg/ml 0.9% NaCl | 307 ± 1.15 | 1.03 | 315 | 6.76 ± 0.04 | No | PIVC | MC | Non-tunneled CVC/PICC |
| 40 mg/ml 0.9% NaCl | 321 ± 1.15 | 1.03 | 332 | 6.59 ± 0.18 | ||||||
| Ceftriaxone 1 g (Qilu Pharma Spain S.L.) | 10 ml WFI | 20 mg/ml 0.9% NaCl | 307 ± 2.08 | 1.03 | 316 | 6.27 ± 0.06 | No | PIVC | MC | Non-tunneled CVC/PICC |
| 40 mg/ml 0.9% NaCl | 328 ± 1.00 | 1.02 | 335 | 6.31 ± 0.04 | ||||||
| Clindamycin 600 mg 4 ml (Normon S.A.) | Not required | 6 mg/ml in 0.9% NaCl | 295 ± 0.58 | 1.03 | 302 | 6.57 ± 0.04 | No | PIVC | MC | Non-tunneled CVC/PICC |
| 12 mg/ml in 0.9% NaCl | 314 ± 1.73 | 1.03 | 323 | 6.61 ± 0.05 | ||||||
| Chlorpromazine 25 mg 5 ml (Sanofi Aventis S.A.) | Not required | 0.5 mg/ml in 0.9% NaCl | 285 ± 1.53 | 1.00 | 285 | 5.59 ± 0.03 | No | PIVC | MC | Non-tunneled CVC/PICC |
| 10 mg/ml in 0.9% NaCl | 284 ± 0.58 | 1.00 | 283 | 5.90 ± 0.03 | ||||||
| Dexketoprofen 50 mg 2 ml (Menarini) | Not required | 1 mg/ml in 0.9% NaCl | 375 ± 1.53 | 1.00 | 375 | 7.48 ± 0.19 | No | PIVC | MC | Non-tunneled CVC/PICC |
| 2 mg/ml in 0.9% NaCl | 461 ± 1.73 | 1.00 | 461 | 7.71 ± 0.01 | MC | MC | Non-tunneled CVC/PICC | |||
| Diazepam 10 mg 2 ml (Alloga Logística España S.L.U) | Not required | 0.2 mg/ml in 0.9% NaCl | 632 ± 2.08 | 1.00 | 633 | 5.43 ± 0.04 | No | Non-tunneled CVC/PICC | Non-tunneled CVC/PICC | PICC |
| 0.4 mg/ml in 0.9% NaCl | 985 ± 2.65 | 1.01 | 998 | 5.47 ± 0.02 | ||||||
| Phenytoin 100 mg 2 ml (Altan Pharmaceuticals S.A.) | Not required | 3 mg/ml 0.9% NaCl | 1048 ± 2.31 | 1.02 | 1.069 | 10.31 ± 0.02 | Sí | CVC no non-tunneled/ PICC | CVC no non-tunneled/ PICC | PICC |
| 6 mg/ml 0.9% NaCl | 1344 ± 6.51 | 1.00 | 1.344 | 10.25 ± 0.03 | ||||||
| Furosemide 20 mg 2 ml (Sanofi Aventis S.A.) | Not required | 5 mg/ml in 0.9% NaCl | 286 ± 2.00 | 1.02 | 291 | 8.42 ± 0.12 | No | PIVC | MC | Non-tunneled CVC/PICC |
| 10 mg/ml in 0.9% NaCl | 283 ± 1.53 | 1.02 | 288 | 8.90 ± 0.04 | ||||||
| Gentamicin 80 mg 2 ml (Normon S.A.) | Not required | 5 mg/ml 0.9% NaCl | 263 ± 0.58 | 1.00 | 264 | 3.63 ± 0.09 | No | CVC non-tunneled/ PICC | CVC tunneled/ PICC | PICC |
| 10 mg/ml 0.9% NaCl | 250 ± 1.15 | 1.00 | 248 | 3.86 ± 0.13 | ||||||
| Lacosamide 10 mg/ml (UCB Pharma S.A.) | Not required | 5 mg/ml 0.9% NaCl | 286 ± 2.08 | 1.01 | 288 | 5.36 ± 0.11 | No | PIVC | MC | CVC no tunelizado/ PICC |
| 10 mg/ml 0.9% NaCl | 288 ± 0.58 | 1.00 | 288 | 4.80 ± 0.10 | ||||||
| Levetiracetam 100 mg/ml (Aurovitas Spain S.A.U.) | Not required | 2 mg/ml 0.9% NaCl | 295 ± 1.00 | 1.01 | 297 | 5.61 ± 0.15 | No | PIVC | MC | CVC non-tunneled/ PICC |
| 5 mg/ml 0.9% NaCl | 315 ± 2.65 | 1.01 | 317 | 5.77 ± 0.12 | ||||||
| Meropenem 1 g (Aurovitas Spain S.A.U.) | 20 ml WFI | 25 mg/ml 0.9% NaCl | 300 ± 0.57 | 1.00 | 300 | 8.13 ± 0.06 | No | PIVC | MC | CVC non-tunneled/ PICC |
| 50 mg/ml 0.9% NaCl | 315 ± 1.15 | 1.01 | 318 | 8.08 ± 0.06 | ||||||
| Metamizol 2 g 5 ml (Normon S.A.) | Not required | 20 mg/ml 0.9% NaCl | 362 ± 1.00 | 1.00 | 362 | 7.26 ± 0.26 | No | PIVC | MC | CVC non-tunneled/ PIC |
| 40 mg/ml 0.9% NaCl | 439 ± 1.00 | 1.02 | 449 | 7.56 ± 0.10 | ||||||
| Midazolam 50 mg 10 ml (Normon S.A.) | Not required | 2.5 mg/ml 0.9% NaCl | 259 ± 2.08 | 1.02 | 263 | 3.40 ± 0.08 | No | CVC non-tunneled/ PICC | CVC non-tunneled/ PICC | PICC |
| 5 mg/ml 0.9% NaCl | 235 ± 0.58 | 1.00 | 235 | 3.27 ± 0.12 | ||||||
| Pantoprazol 40 mg (Normon S.A.) | 10 ml WFI | 0.8 mg/ml 0.9% NaCl | 289 ± 3.51 | 1.00 | 289 | 9.31 ± 0.11 | No | CVC non-tunneled/ PICC | CVC non-tunneled/ PICC | PICC |
| 4 mg/ml 0.9% NaCl | 308 ± 1.15 | 1.01 | 311 | 9.99 ± 0.08 | ||||||
| Piperacillin/ Tazobactam 2/0,25 g (Augia Pharma) | 10 ml WFI | 20 mg/ml 0.9% NaCl | 332 ± 0.58 | 1.02 | 340 | 5.72 ± 0.20 | No | PIVC | MC | CVC non-tunneled/ PICC |
| 80 mg/ml 0.9% NaCl | 456 ± 0.58 | 1.01 | 462 | 5.58 ± 0.02 | ||||||
| Rasburicasa 1.5 mg (Sanofi Aventis S.A.) | 1 ml de disolvente | 0.2 mg/ml 0.9% NaCl | 283 ± 1.53 | 1.00 | 284 | 8.24 ± 0.01 | No | PIVC | MC | CVC non-tunneled/ PICC |
| Sulfamethoxazole/ Trimetoprim 800/160 mg (Admitall S.A.) | 5 ml (trimetoprim) | Dilution1:30 D5W | 423 ± 4.73 | 1.00 | 423 | 8.57 ± 0.09 | No | PIVC | MC | CVC non-tunneled/ PICC |
| Dilution 1:50 0.9% NaCl | 365 ± 1.53 | 1.01 | 369 | 8.84 ± 0.02 | ||||||
| Valproic 400 mg (Altan Pharmaceuticals S.A.) | 4 ml WFI | 1 mg/ml 0.9% NaCl | 284 ± 2.08 | 1.00 | 285 | 8.37 ± 0.06 | No | PIVC | MC | CVC non-tunneled/ PICC |
| 2 mg/ml 0.9% NaCl | 283 ± 0.58 | 1.00 | 283 | 8.12 ± 0.25 | ||||||
| Vancomycin 1 g (Reig Jofré S.A.) | 20 ml WFI | 2.5 mg/ml 0.9% NaCl | 268 ± 0.58 | 1.03 | 277 | 4.15 ± 0.06 | Sí | PIVC | MC | CVC non-tunneled/ PICC |
| 5 mg/ml 0.9% NaCl | 257 ± 0.58 | 1.02 | 263 | 3.96 ± 0.03 | CVC non-tunneled/ PICC | CVC non-tunneled/ PICC | PICC | |||
| Voriconazol 200 mg (Teva Pharma) | 10 ml WFI | 5 mg/ml 0.9% NaCl | 208 ± 1.00 | 1.02 | 211 | 6.31 ± 0.06 | No | PIVC | MC | CVC non-tunneled/ PICC |
| 10 mg/ml SF 0,9% | 129 ± 0,58 | 1,02 | 132 | 6,60 ± 0,11 | ||||||
WFI: water for injection; CVC: central venous catheter; PIVC: peripheral intravenous catheter; VAD: vascular access device; MC: midline catheter; PICC: peripherally inserted central catheter; 0.9% NaCl: 0.9% sodium chloride solution; D5W: dextrose 5% in water. If the venous patrimony of the patient is in poor condition, VAD will be selected as established for dilutions containing at least a high-risk factor for phlebitis.
We identified 17 dilutions associated with nine different drugs that should not be administered through a PVC, even in short-term treatments. Of these dilutions, 15 had a high risk for phlebitis, and 2 had an intermediate risk.
DiscussionTo the best of our knowledge, this is the first study to investigate the physico-chemical properties of infusates administered intravenously in critically ill pediatric patients.
Previous studies have been conducted to characterize intravenous therapies,18,21 with substantial differences regarding the selection of infusates, concentrations and vehicles used in each dilution. Differences were noted in the reconstituting agent employed: while water for injection (WFI) was used in our study, Ballesteros-Peña et al utilized 0.9% sodium chloride, whereas the agent employed was not reported in the study by Manrique et al. These discrepancies may influence the physico-chemical properties of dilutions, which hinders the comparability of results across studies.
ReconstitutionIn most cases, dilutions were reconstituted using WFI, as it is a hypotonic diluent (0 mOsm/l). Should a compatible solution other than WFI be used, it would be necessary to extrapolate the osmolarity contributed by the alternative diluent 0.9% NaCl: 280 mOsm/l; D5W: 289 mOsm/l) to the value obtained.21
DilutionIn relation to the diluent used, 0.9% NaCl was employed in most cases, as it is the vehicle of choice in the PICU. When a diluent other than 0.9% NaCl was used, the osmolarity value contributed by this diluent should not be considered. These effects are a consequence of the non-ideal behavior of solutions, whereby solvation and ionic interactions occur due to deviations from ideal mixing.22 The study by Manrique et al. unveiled that osmolarity was slightly higher in the solutions containing D5W, as compared to those prepared with 0.9% NaCl; notably, differences in pH were minimal.18 One of the strategies adopted to reduce the osmotic load of hyperosmolar solutions is using 0.5% sodium chloride (137 mOsm/l)21 as a diluent, provided that it is compatible with the drug to be administered.10,18
ConcentrationOsmolarity, defined as the number of osmotically active particles per liter of solution, is considered a measure of concentration. Hence, osmolarity increases as concentration rises. However, this premise is not always true, since the concentration-osmolarity relationship is not consistently linear.22 As an example, the osmolarity of a 5 mg/mL voriconazole solution is 211 mOsm/L versus 132 mOsm/L in the 10 mg/mL dilution. Given that the more concentrated solution contains a greater amount of drug and a smaller volume of diluent, it can be assumed that the diluent contributes less to the overall osmolarity than the drug itself, in this context.
In turn, increased concentrations of ampicillin, dexketoprofen and piperacillin/tazobactam resulted in higher osmolarity, thereby leading to changes in phlebitis risk classification.
Although differences in pH across the distinct dilutions of the same drug were minimal, increased concentrations –resulting either from increased or decreased pH levels– led to changes in phlebitis risk classification. This finding was observed in dexketoprofen, metamizol, levetiracetam, lacosamide and vancomycin.
DensityPhlebitis risk classification was established as a function of osmolarity; however, since density of the dilutions considered was close to 1 g/ml, the terms “osmolality” and “osmolarity” can be considered interchangeable.
ManufacturerFor the purposes of this study, drug manufacturers were taken into account, as the physicochemical properties of drugs may vary depending on the excipients used in their formulations. Manrique et al. reported slight variations in pH and osmolarity between manufacturers; however, these differences did not affect the classification of phlebitis risk.18
Vesicant potentialVancomycin has been associated with higher rates of complications, as compared to other antimicrobials with an acidic pH.7 This demonstrates that extreme pH cannot cause endothelial damage by itself.
Measures to reduce endothelial damageApart from physico-chemical properties and vesicant effects, other drug-related factors may influence a drug's potential to induce endothelial damage.
Method of administration: as compared to continuous infusion, intermittent infusion may reduce venous irritation and the incidence of phlebitis. Hence, local toxicity is not only concentration-dependent, but also time-dependent.23
Administration rate: Reducing infusion rate and increasing solution volume have been considered useful strategies to minimize endothelial damage.7 However, adopting this strategy is not always feasible, due to fluid restrictions and urgency of treatment,7 as it is the case in PICUs. Other studies reveal that increasing the infusion rate of irritating solutions reduces the risk for phlebitis.10,24
This study has some limitations that should be considered when interpreting results. Firstly, the study was conducted in the specific context of a PICU; therefore, the results obtained may not be generalizable to other clinical settings where different protocols are used for the preparation and administration of intravenous dilutions. Additionally, the VAD selection algorithm developed by Manrique et al. establishes different periods of treatment duration. Thus, the therapies with a duration <7 days are administered following the same recommendations as those used for <1-month therapies. This could determine decision-making in certain clinical settings.
Apart from the physico-chemical properties of dilutions, other factors not addressed in this study should be considered when selecting a VAD. These include age, comorbidities and clinical status of the patient, added to personnel's skills, catheter insertion site, and concomitant intravenous administration of several therapies.25
In conclusion, the standardization and characterization of infusates for critically ill pediatric patients assist in the selection of the most appropriate VAD. Given that 25% and 35% of dilutions are associated with a high and an intermediate risk for phlebitis, respectively, an appropriate selection of VAD will contribute to minimizing endothelial damage; this approach will also help preserve the integrity of venous walls, thereby optimizing treatment safety and efficacy.
Contribution to the scientific literatureStandardizing and characterizing intravenous drug dilutions commonly used in critically ill pediatric patients will support evidence-based selection of the most appropriate vascular access device, thereby reducing the risk of associated complications.
FundingThis study did not receive any funding.
CRediT authorship statementLaura Torralba-Fernández: Writing – original draft, Supervision, Methodology, Formal analysis, Conceptualization. Marta García-Palomo: Writing – original draft, Supervision, Methodology, Formal analysis, Conceptualization. Miguel López de Abechuco-Ruíz: Writing – original draft, Supervision, Methodology, Formal analysis, Conceptualization. Natalia Ramos-Sánchez: Writing – original draft, Supervision, Methodology, Formal analysis, Conceptualization. Clara Jiménez-Méndez: Writing – original draft, Supervision, Methodology, Formal analysis, Conceptualization. Rocío Prieto-Galindo: Writing – original draft, Supervision, Methodology, Formal analysis, Conceptualization. María Carmen Lorenzo-Lozano: Writing – original draft, Supervision, Methodology, Formal analysis, Conceptualization. Pablo Aguado-Barroso: Writing – original draft, Supervision, Methodology, Formal analysis, Conceptualization.
The authors declare no conflicts of interest associated with this publication.