The off-label use in clinical practice of non-approved syringes for intravitreal drug administration has resulted in the detection of silicone oil drops in the vitreous of some patients. This situation derives from the lack of approved syringes for intraocular use in the Spanish market.
The aim of this work is to review the use of syringes for intraocular administration, as well as to search for alternatives that meet the legal requirements for these unmet needs.
MethodA systematic review was performed following the PRISMA 2020 guidelines by searching PubMed with the descriptors: (silicone) AND (syringes) AND ((intraocular) OR (intravitreal)) and filtering all existing publications from January 2006 to December 2023, including all those articles dealing with silicone oil release in intravitreal injections and analysing the possible consequences.
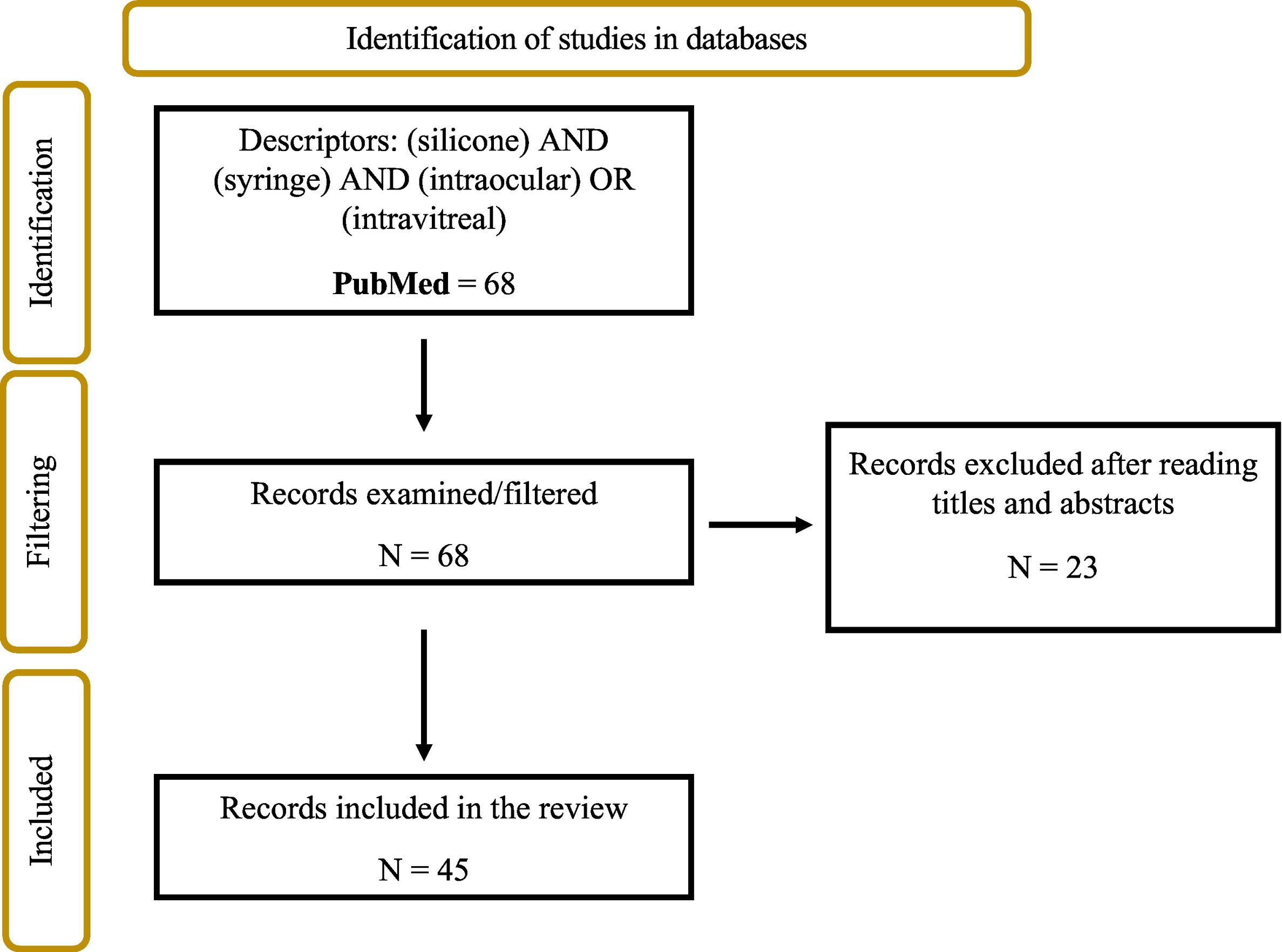
ResultsSixty-eight results were found, 23 of which were excluded because they did not deal with the subject under study, leaving a total of 45 articles for the systematic review. These were classified according to the conclusions obtained in 4 groups: the adverse reactions produced by silicone; the administration technique; the physicochemical aspects of silicone release; and the characteristics of the medical device. After reviewing the current manufacturers and technical data sheets of commercialised syringes, the existing syringes for this use have been collected, finding 2 that will probably be commercialised in Spain at the beginning of 2024: Zero Residual™ 0.2 ml SiO-free and VitreJect® Ophthalmic.
ConclusionsFrom the results obtained, it can be interpreted that the use of syringes and needles with silicone for intravitreal use is a concern for health professionals due to the implications and consequences that may arise in patients, the most important being adverse reactions, so it is necessary to have silicone-free syringes on the market that are specific for intraocular use.
Safety and legality in the use of intraocular syringes and needles is essential to guarantee ocular integrity and patient health.
El uso off-label en práctica clínica de jeringas no homologadas para la administración de fármacos por vía intravítrea ha tenido como consecuencia la detección de gotas de aceite de silicona en el vítreo de algunos pacientes. Esta situación deriva de la falta de jeringas aprobadas para la indicación de uso intraocular en el mercado español. El objetivo de este trabajo es realizar una revisión sobre el uso de las jeringas para administración intraocular, además de la búsqueda de alternativas comercializadas que cumplan la legalidad para estas necesidades no cubiertas.
MétodoSe realizó una revisión sistemática siguiendo la Guía PRISMA 2020 mediante la búsqueda en PubMed con los descriptores: (silicone) AND (syringes) AND ((intraocular) OR (intravitreal)) y se filtraron todas las publicaciones existentes desde enero de 2006 hasta diciembre de 2023, incluyendo todos aquellos artículos que tratasen sobre la liberación de aceite de silicona en inyecciones intravítreas y que analizasen las posibles consecuencias.
ResultadosSe encontraron 68 resultados de los que se excluyeron 23 por no tratar sobre el tema objeto de estudio, quedando para la revisión sistemática un total de 45 artículos. Estos se clasificaron en función de las conclusiones obtenidas en 4 grupos; las reacciones adversas producidas por la silicona; la técnica de administración; los aspectos físico-químicos de liberación de silicona; y las características del producto sanitario. Tras la revisión de los actuales fabricantes y fichas técnicas de jeringas comercializadas se han recogido las jeringas existentes para este uso encontrando dos que serán probablemente comercializadas es España a principios de 2024: Zero Residual™ 0,2 ml SiO-free y la VitreJect® Ophthalmic.
ConclusionesDe los resultados obtenidos, se puede interpretar que el uso de jeringas y agujas con silicona para uso intravítreo es una preocupación para los profesionales sanitarios por las implicaciones y consecuencias que pueden derivar en los pacientes, siendo la más importante las reacciones adversas, por lo que es necesario que existan en el mercado jeringas libres de silicona y específicas para uso intraocular. La seguridad y legalidad en el uso de jeringas y agujas intraoculares es fundamental para garantizar la integridad ocular y la salud del paciente.
Intraocular drug delivery has a relevant role in the treatment of common eye diseases such as glaucoma, macular degeneration, diabetic retinopathy, infections (e.g., conjunctivitis, keratitis, endophthalmitis), and autoimmune disorders (e.g., Sjögren's syndrome, uveitis).1
Over the last 20 years, the use of intravitreal drugs has become widespread as the leading treatment option for many intraocular diseases. The intravitreal route is a more targeted form of intraocular administration in which various pharmacological agents are directly delivered into the eye through the sclera and into the region behind the crystalline lens, known as the vitreous cavity. In Spain, this technique is performed by ophthalmologists due to the risk involved and the critical nature of the ocular area.2
Some intravitreally administered drugs, such as anti-VEGF, corticosteroids, or antifungals, are available in pre-filled syringes ready for administration,3 but others are marketed as multidose vials/ampoules due to the small volume to be administered, requiring safe handling to transfer their contents into syringes prior to intravitreal administration.4
The focus of concern arises from the off-label use of non-approved syringes in clinical practice, as they fail to meet the indications for intraocular administration,5 given the absence of syringes approved for this route on the Spanish market.
As a result of their off-label use, the Becton Dickinson company ([BD]; New Jersey, USA) issued a safety warning in January 2021, stating that it does not validate or take responsibility for the use of its syringes and needles for intraocular use. Specifically, “When using syringes and needles that are not intended for intraocular injections, there is a potential for ‘floating bodies’ to appear in patients' eyes. These are thought to be caused by the silicone oil used as a lubricant inside the syringe barrel allowing the plunger (piston) to slide more easily, thus reducing the force required to manipulate the syringes. These ‘floating bodies’ are annoying, sometimes even impairing vision, and may require removal by vitrectomy”.6
The Working Groups on Medical Devices (GPS) and Pharmacotechnics of the Spanish Society of Hospital Pharmacy (SEFH) took this notification into account, and issued an information note in February 2021, acknowledging that there was no syringe available on the market in Spain that met the characteristics required for intravitreal drug delivery. The note recommended syringes that either did not contain silicone oil or similar lubricants, or those with a low concentration of silicone oil, despite lacking an indication for ophthalmic use.7
In 2006, the first cases of floating bodies in the vitreous cavity of patients treated with pegaptanib (brand name: Macugen) were reported, and the silicone oil coating the inside of the syringes (plungers) was pinpointed as the cause.8
Currently, this issue remains a recurring theme in the literature and has yet to be resolved, because siliconised syringes not approved for intravitreal injections continue to be used in many cases.9,10
In addition to the clinical consequences, this issue may also have legal implications. According to RD 1/2015 of 24 July, which approves the revised text of the Law on Guarantees and Rational Use of Medicines and Medical Devices, under Article 112, “Infringements in the field of medical devices” (point 26), “it is a serious offence to use a professional product under conditions and for purposes other than those indicated by the manufacturer, or by unqualified or duly trained personnel”.11
For this reason, syringes and needles used for intraocular drug administration must be legally approved for this use by the manufacturer.
Given the importance of the issue, the aim of this study was to review current knowledge on the use of siliconised syringes for intraocular drug administration, and to search for commercially available legally compliant alternatives to improve the safety and efficacy of treatment for patients.
MethodsA systematic review was conducted following the PRISMA 202012 guidelines through a literature search of scientific publications in PubMed. The descriptors used in the search were: “silicone” AND “syringe” AND (“intraocular” OR “intravitreal”). All existing publications from January 2006 up to and including December 2023 were filtered.
Other sources of information included documents published by the SEFH7 and the journal of the Spanish Macula-Retina Club.13
The main aim of the study was to identify the factors influencing the use of silicone oil as a lubricant in syringes used for intravitreal administration and its consequences.
The inclusion criteria were as follows: all articles on the release of silicone oil in intravitreal injections through the syringes used, and those analysing the potential consequences, whether in terms of adverse reactions, the administration technique used by ophthalmologists, drug stability, or the characteristics of the medical device. The exclusion criterion was as follows: articles that did not have as their main objective the study or analysis of the use of siliconised syringes for intravitreal administration.
Four reviewers independently assessed all titles and abstracts against the defined eligibility criteria, and disagreements between reviewers were resolved by discussion and consensus.
General and detailed information was recorded and tabulated using Microsoft Excel:
- •
General information: title, authors, and year of publication.
- •
Specific information: results of each article and grouping according to the conclusions of each study.
The team also reviewed the technical specifications provided by the manufacturers of both approved and non-approved syringes on the market.14–23
ResultsA total of 68 results were found in PubMed, 23 of which were excluded because they did not address the study topic, leaving a total of 45 articles for the systematic review (Fig. 1).
Flowchart (PRISMA 2020 guidelines12) of the systematic search of the reviewed articles.
The 45 articles were classified into 4 groups according aims and conclusions of the studies.
The most studied topic and the one that raised the most concern among researchers was silicone-related adverse reactions (20 articles; Table 1), followed by studies on silicone release such as administration technique (7 articles; Table 2), physicochemical aspects of silicone release such as stability and preservation of drugs in prefilled syringes (10 articles; Table 3), and medical device characteristics related to silicone release (8 articles; Table 4).
Articles on adverse reactions associated with siliconised syringes.
| Article | Year | Conclusions |
|---|---|---|
| Intravitreal silicone oil droplets after intravitreal drug injections. Bakri et al.24 | 2008 | Syringes with silicone oil as a lubricant may leak silicone particles into the eye and may be responsible for adverse reactions.The greater the number of intravitreal injections with siliconised syringes, the greater the likelihood of finding silicone oil in the vitreous. and the greater the likelihood of adverse reactions.The most common adverse reactions caused by silicone include visual disturbances (floaters), inflammatory reactions, endophthalmitis, and increased intraocular pressure.Silicone oil-related symptoms are usually transient and reversible, although some patients require pharmacological treatment or vitrectomy.The use of non-siliconised syringes is recommended to avoid adverse reactions. |
| Toxic vitreitis outbreak after intravitreal injection. Ness et al25 | 2010 | |
| Silicone oil droplets following intravitreal bevacizumab injections. Yu et al26 | 2017 | |
| Incidence of presumed silicone oil droplets in the vitreous cavity after intravitreal bevacizumab injection with insulin syringes. Khurana et al.27 | 2017 | |
| Can anti-VEGF injections cause glaucoma or ocular hypertension? Lanzl et al.28 | 2017 | |
| Silicone oil droplets following intravitreal injection. Yu et al.29 | 2017 | |
| Prevalence of silicone oil droplets in eyes treated with intravitreal injection. Melo et al.30 | 2019 | |
| Large silicone droplets after intravitreal bevacizumab (avastin). Avery et al.31 | 2019 | |
| Inflammatory reaction after aflibercept intravitreal injections associated with silicone oil droplets released from syringes: a case–control study. Melo et al.32 | 2019 | |
| Silicone oil droplets after intravitreal injections: An uncomfortable adverse effect in our consultations. Gómez-Mariscal et al.33 | 2020 | |
| Silicone oil droplets in repackaged anti-vascular endothelial growth factors for intravitreal injections: In search of the main source of contamination. Olea et al.34 | 2020 | |
| Silicone oil droplets after intravitreal bevacizumab injection in two patients. Caroca et al.35 | 2020 | |
| Survey among retina specialists in Brazil about inflammatory reactions after intravitreal antiangiogenic therapy. Melo et al.36 | 2020 | |
| Mechanisms of sterile inflammation after intravitreal injection of antiangiogenic drugs: a narrative review. Anderson et al.37 | 2021 | |
| Cluster of symptomatic silicone oil droplets following intravitreal injections: a one-year observational study. Sivertsen et al.38 | 2021 | |
| Prospective study of silicone oil microdroplets in eyes receiving intravitreal anti-vascular endothelial growth factor therapy in 3 different syringes. Thompson39 | 2021 | |
| Frequency and symptoms of intravitreal silicone oil droplets following Becton Dickinson and norm-ject bevacizumab injections. Miller et al.40 | 2022 | |
| Differences in the incidence of aflibercept-related sterile endophthalmitis according to types of disposable syringes used. Kim et al.41 | 2022 | |
| Presumed silicone oil droplets after intravitreal pegcetacoplan injections. Dessouki et al.42 | 2023 | |
| Incidence of ocular hypertension after anti-VEGF injections: examining the effect of drug filtration and silicone-free syringes. Bae et al.43 | 2023 |
Articles on the effect of administration technique on silicone release.
| Article | Year | Conclusions |
|---|---|---|
| Release of silicone oil droplets from syringes. Melo et al.44 | 2019 | Off-label use of siliconised syringes and needles in ophthalmology may release silicone oil droplets into the vitreous of patients if syringes are shaken during handling prior to intravitreal injection.The greater the shaking, the greater the release of silicone into the eye.It is recommended to use silicone-free syringes approved for use in ophthalmology and to avoid sudden movements and shaking during handling. |
| Agitation of the syringe and release of silicone oil. Dias Júnior et al.45 | 2020 | |
| Release of silicone oil and the off-label use of syringes in ophthalmology. Melo et al.46 | 2020 | |
| Silicone oil-free syringes, siliconized syringes and needles: quantitative assessment of silicone oil release with drugs used for intravitreal injection. Melo et al.47 | 2021 | |
| The risks behind the widespread use of siliconized syringes in the healthcare practice. Melo et al.48 | 2021 | |
| Quantitative assessment of silicone oil release with siliconized and silicone oil-free syringes by microflow imaging microscopy. Agra et al.49 | 2022 | |
| Ocular inflammation after agitation of siliconized and silicone oil-free syringes: a randomised, double-blind, controlled clinical trial. da Cruz et al.50 | 2022 |
Articles on the physicochemical aspects of silicone release, stability, and preservation of drugs in prefilled syringes.
| Article | Year | Conclusions |
|---|---|---|
| Long-term stability of bevacizumab repackaged in 1 ml polypropylene syringes for intravitreal administration. Paul et al.51 | 2012 | No association between needle gauge and amount of silicone oil. High variability in silicone oil content between syringes from the same batch.Different factors are involved in adverse reactions after intravitreal injections: the drug, storage, temperature variations, and syringe design.Fractionated anti-VEGF drugs can be stored in silicone oil-free plastic syringes without compromising the stability, molecular integrity, and functional properties of the drugs provided they are prepared in biosafety cabinets, use sterile primary and secondary packaging, are stored between 4 °C and 8 °C, and are protected from light for up to 7 days.52There are no differences in silicone particle burden between commercially available pre-filled anti-VEGF glass syringes and siliconised plastic syringes pre-filled in biosafety cabinets.53There is great variability in the amount of silicone particles from syringes and needles in the vitreous of patients. |
| A new method for pharmaceutical compounding and storage of anti-VEGF biologics for intravitreal use in silicone oil-free prefilled plastic syringes. Lode et al.52 | 2019 | |
| Contamination of anti-VEGF drugs for intravitreal injection: How do repackaging and newly developed syringes affect the amount of silicone oil droplets and protein aggregates? Schargus et al.53 | 2018 | |
| Silicone oil found in syringes commonly used for intravitreal injections. Agra et al.54 | 2019 | |
| Issues with intravitreal administration of anti-VEGF drugs. Schargus et al.55 | 2020 | |
| In-vitro assessment of release of silicone oil droplets with the use of variety of syringes and needles used in intravitreal injections. da Cruz et al.56 | 2021 | |
| Comparison of syringes with intravitreal Anti-VEGF drugs: particle burden and protein aggregates in brolucizumab, aflibercept and bevacizumab. Schargus et al.57 | 2021 | |
| High particle variability across siliconized and oil-free syringes and needles from the same lots. do Monte Agra et al.58 | 2021 | |
| Particulate matter from syringes used for intravitreal injections. Dounce et al.59 | 2021 | |
| Pharmaceutical compounding and storage of faricimab in a syringe for intravitreal injection do not impair stability and bi-specific binding properties. Jørstad et al.60 | 2023 |
Articles related to the technical specifications of syringes.
| Article | Year | Conclusions |
|---|---|---|
| SCORE Study Report 7: Incidence of intravitreal silicone oil droplets associated with staked-on vs luer cone syringe design. Scott et al.61 | 2009 | Recommendations: the use of silicone-free syringes, accuracy of dosing, compatibility of primary packaging materials, stability of the formulation, avoidance of temperature variations.Beneficial characteristics of pre-filled syringes in sterile environments: fewer bubbles, increased dosing accuracy, reduced silicone release, and reduced risk of endophthalmitis.It is recommended to optimise transport, storage, and syringe selection to reduce silicone deposits in the eye.Importance of dead space and cone in the choice of syringe to reduce the frequency of silicone droplets in the eye. |
| Prefilled syringes for intravitreal drug delivery. Sassalos et al.62 | 2019 | |
| Characterisation of polymeric syringes used for intravitreal injection. Peláez et al.63 | 2020 | |
| Critical analysis of techniques and materials used in devices, syringes, and needles used for intravitreal injections. Melo et al.64 | 2021 | |
| Container closure and delivery considerations for intravitreal drug administration. Parenky et al.65 | 2021 | |
| Observation of silicone oil within the vitreous and sclera following intravitreal administration of biotherapeutics using insulin syringes in cynomolgus monkeys. Huet et al.66 | 2021 | |
| Evaluation of a novel prefilled syringe concept for ophthalmic applications: a formative human factors study. Franzese et al.67 | 2022 | |
| Accuracy, precision, and residual volume of commonly used syringes for intravitreal injections and the impact on intraocular pressure. Agra et al.68 | 2023 |
Most of the articles conclude by highlighting the need for silicone-free syringes and needles specific for intraocular use to be available on the market.
After reviewing the current manufacturers and technical specifications of syringes marketed nationally and internationally, we identified the most suitable syringes for this use (i.e., those with an approved indication for intraocular use and those that are free of silicone as a lubricant), as well as some that are currently being used without an indication (Table 5).
List of current syringes used in clinical practice with or without intraocular indication.
| Name (Manufacturer/Distributor) | Intraocular indication | Total volume, graduation | Silicone | CE marking | Dead space | Luer lock | Parts |
|---|---|---|---|---|---|---|---|
| VitreJect14(Ocuject LLC) | SI | 1-ml syringeScale: 0.05 ml | NO | SI | ≤0.02 ml | SI | 2 |
| Zero Residual, 0.2 ml, silicone oil-free15(SJJ Solutions) | SI | 0.2-ml syringeScale: 0.05 ml | NO | SI | ≤0.01 ml | SI | 2 |
| Zero Residual, 0.3 ml, low silicone oil16(SJJ Solutions) | SI | 0.3-ml syringeScale: 0.05 ml | SI | SI | ≤0.01 ml | NO | 3 |
| StaClear SC250 and SC250-LS17(Tribofilm Research) | SI | 0.25-ml syringeScale: 0.01 ml | SI | In process | ≤0.07 ml | SI | 3 |
| Daikyo Crystal Zenith Ophthalmic18(West Pharmaceutical) | SI | 0.5-ml syringeScale: 0.05 ml | NO | NO | ≤0.07 ml | SI | 2 |
| PLAJEX 0.5 ml19(Terumo) | SI | 0.5-ml syringeScale: – | NO | NO | ≤0.07 ml | SI | 3 |
| HSW Norm-Ject20(VWR - Air Tite) | NO | 1-ml syringeScale: 0.01 ml | NO | SI | ≤0.07 ml | SI | 2 |
| Injekt-F21(B. Braun - Fisher) | NO | 1-ml syringeScale: 0.01 ml | NO | SI | ≤0.01 ml | NO | 2 |
| BD Plastipak22(Becton Dickinson) | NO | 1-ml syringeScale: 0.01 ml | SI | SI | 0.07 ml | SI | 3 |
| Jeringa Caress tuberculina 1 ml23(Nacatur 2) | NO | 1-ml syringeScale: 0.01 ml | SI | SI | SI | NO | 3 |
This systematic review identified and summarised the clinical, technical, and physicochemical effects of using of syringes with or without silicone oil as a lubricant for in the setting of intraocular drug delivery.
Such use is a concern for healthcare professionals due to the implications and consequences for patients, with adverse reactions being among the most important.
In 2019 and 2020, 7 and 8 articles, respectively, were published addressing the problem of silicone droplets found in the vitreous of patients after intraocular drug injections. As a result, the Spanish Agency for Medicines and Health Products (AEMPS) issued a warning on this issue in January 2021.6 However, the number of publications on this topic decreased in 2022 and 2023, with 5 and 4 publications, respectively.
In all the articles reviewed, silicone oil emerges as the central focus of the study due to the varied consequences it induces in clinical practice. The most extensively studied consequences are adverse reactions due to patient discomfort and an associated increase in care burden, which may manifest as an increase in the number of patients requiring consultations and check-ups, or an increase in the number of interventions to remove silicone particles in certain patients.24–43
Other studies have addressed the issue of minimising or eliminating silicone oil release in the absence of suitable syringes. They have found that the handling and skill of healthcare staff is critical, as sudden movements or agitation during transport, storage, and/or intervention increase the number of silicone droplets within the patients' vitreous cavities.44–50
The results of this review also highlight the fact that factors such as temperature variations can cause protein aggregation or denaturation. Another aspect to consider is storage time, as indicated by stability studies conducted for each drug. Sudden movements during the transportation of pre-filled syringes and the design of the syringe itself can affect the transfer of silicone.
Due to the legal risks associated with the off-label use of these medical devices, the large volume of preparations involved, and the delicate nature of the technique, which carries a high risk of ocular complications, safe purpose-built syringes and needles need to be marketed with the approved indication for this specific use. This will ensure adherence to appropriate quality standards and help prevent off-label use.
In the light of these clinical concerns and all the aforementioned issues, it is imperative to establish technical recommendations for syringes and needles intended for intravitreal use. This will facilitate the attainment of ideal intraocular puncture. The following recommendations have been extracted from the reviewed scientific literature,69,70 and are also based on medical device legislation and clinical experience. They can be summarised as follows:
- •
CE marking, required for use in Europe.
- •
Approval for intraocular use.
- •
Syringe preferably ≤1 ml, lubricant-free, 3-part (with a piston to ensure leak-tightness and smooth gliding).
- •
Syringe barrel preferably made of glass.
- •
Syringe cone preferably with luer lock for safer connection to the needle.
- •
Minimal or zero dead space to avoid loss of dosage.
- •
Syringe body printed with indelible, clear, visible marking, numbered in tenths of millilitres.
- •
Needles preferably silicone-oil free, with luer cone and atraumatic bevel.
- •
Injection needle preferably 30G × ½ inch gauge (0.3 mm × 12.7 mm).3
In addition to selecting the correct syringe for intraocular use, other relevant aspects must be taken into account in order to avoid complications and ensure the efficacy of ophthalmologic treatments. These aspects include the following:
- •
Ensure an aseptic technique during vial fractionation and a sterile environment during the administration procedure.
- •
Intravitreal injections should only be administered by qualified physicians trained in this technique.71
- •
Careful handling to avoid vigorous shaking of pre-filled syringes, which significantly increases the number of silicone oil particles.72
- •
Ensure proper storage away from light and avoid temperature fluctuations.
- •
Maintain detailed records of all procedures, documenting the type of substance injected, the quantity administered, the date and time of the procedure, as well as any relevant observations.
In Spain, syringes are in use that are not compliant with legislation; in the best-case scenarios, these are silicone- or trace-free syringes with no indication for intraocular use. Given the clinical and legal implications of this situation, the absence of silicone oil as a lubricant has been prioritised over the other aspects outlined in the aforementioned recommendations. This is evident in the case of HSW Norm-Ject20 and Injekt-F21 syringes, which have been widely used until now. Such off-label use has been driven by the lack of approved syringes on the market for this indication.
Agreements are currently underway to introduce 2 syringes in Spain that meet all the requirements for intraocular use: the Zero Residual 0.2-ml SiO-free syringe15 manufactured in the Netherlands and the American-made VitreJect Ophthalmic Syringe.14 If successful, they will be made available starting in early 2024 through 2 national distributors: Dextromedica will distribute the Zero Residual 0.2-ml SiO-free syringes, while Izasa Medical will distribute the VitreJect Ophthalmic syringes.
These 2 syringes would be positioned in the Spanish market as excellent alternatives to the nonapproved syringes used until now, given that they are indicated for intraocular use, bear the CE marking required for use in Europe, are free of silicone oil, have low or no dead space, and are fitted with luer lock cones. The components of these syringes are polyethylene and polypropylene, and in the case of VitreJect Ophthalmic syringes, silicone oil has been replaced by oleic acid as a lubricant.73
The use of silicone oil-free syringes minimises the risk of silicone sedimentation in the vitreous cavity of patients, but it should be noted that intraocular needles still use silicone oil as a lubricant (<0.25 mg/cm2). 74 Some authors have suggested that needles may also release a certain number of silicone droplets, albeit in fewer quantities compared to those released by syringes.47 It remains unclear whether this aspect could be a determining factor, as the amount of silicone in the needles is minimal as well as being external to the barrel; hence, it would not come into contact with the drug itself. Once these new syringes are implemented in clinical practice, future research could investigate whether such minimal quantities could lead to issues associated with silicone oil release.
Another potential avenue of research would be to investigate whether the lubricants used in the new syringes present any previously unstudied issues and whether they impact patients or pose procedural challenges for ophthalmologists.
This study is limited by the lack of a review of other scientific databases and of articles not indexed in them.
In conclusion, the use of siliconised syringes without approval for intraocular use has undesirable consequences in clinical practice and could potentially lead to legal sanctions. For years, healthcare professionals have been concerned about the off-label use of these types of syringes. Therefore, the launch of the Zero Residual 0.2-ml SiO-free syringe and the VitreJect Ophthalmic syringe on the Spanish market represents a breakthrough and a potential solution to the issues examined in this review.
FundingNone declared.
Liability and assignment of rightsAll authors accept responsibility as defined by the International Committee of Medical Journal Editors (available at http://www.icmje.org/).
In the event of publication, the authors grant exclusive rights of reproduction, distribution, translation, and public communication (by any media or sound, audiovisual or electronic support) of their work to Farmacia Hospitalaria and, by extension, to the SEFH. To this end, a letter of assignment of rights will be signed at the time of submission of the paper through the online manuscript management system.
CRediT authorship contribution statementÁngela Pascual Carrasco: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft. Isabel Espadas García: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Writing – original draft. Ana Ramírez López: Conceptualization, Data curation, Validation, Visualization, Writing – original draft. Juan Selva Otaolaurruchi: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation.