To evaluate in patients with a history of keratitis by herpes simplex virus, ocular recurrences, visual acuity, non-ocular recurrences, stromal keratitis and adverse effects after prolonged treatment with antiviral agents. Registered in Prospero CRD42024556228.
MethodsSystematic review of trials identified in CENTRAL, Embase, Medline, www.ClinicalTrials.gov and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp). Trials of patients with a history of at least one episode of herpes simplex keratitis were included. Participants had to be free of active herpetic disease at the time of enrollment in the trial. Trials had to include oral and/or topical antiviral agents versus placebo or other antivirals, administered for at least 4 weeks. A data extraction was performed by pairs with risk of bias assessment for each trial using Cochrane Risk of Bias; GRADE was used to provide the certainty of evidence for each outcome.
ResultsFour trials were found that included 1,017 patients. Antivirals in prolonged use protected from recurrences of ocular herpetic disease better than placebo (RR 0.56; 95% CI 0.45–0.70) NNT 6 (4–11); acyclovir was better than placebo in this same action (RR 0.59; 95% CI 0.46–0.74) NNT 8 (5–14), but not different from valacyclovir (RR 1.0; 95% CI 0.37–2.70) NNT not calculated. Prolonged use of antivirals also decreased recurrences of non-ocular herpetic disease (RR 0.56; 95% CI 0.44–0.71) NNT 6 (5–11) and recurrences with stromal keratitis (RR 0.55; 95% CI 0.35–0.85) NNT 17 (10–50). No data were found on visual acuity. Regarding adverse effects, there were no differences between interventions (RR 0.96; 95% CI 0.60–1.54) NNT not calculated. The certainty of evidence was high for ocular and non-ocular recurrences, and low for adverse effects, due to imprecision and inconsistency of the findings.
ConclusionsProlonged use of antivirals protects from ocular, non-ocular recurrences and stromal keratitis safely. The effects on visual acuity are not known.
evaluar en pacientes con antecedentes de queratitis por el virus herpes simple las recurrencias oculares, agudeza visual, recurrencias no oculares, la queratitis estromal y los efectos adversos tras el tratamiento prolongado con agentes antivirales. Registrada en Prospero CRD42024556228.
Métodorevisión sistemática de ensayos identificados en CENTRAL, Embase, Medline, www.ClinicalTrials.gov y World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp). Se incluyeron ensayos de pacientes con antecedentes de al menos un episodio de queratitis por herpes simple. Los participantes debían estar libres de enfermedad herpética activa en el momento de inscribirse en el ensayo. Los ensayos debían incluir agentes antivirales orales y tópicos versus placebo u otros antivirales, administrados durante al menos 4 semanas. Se realizó una extracción de datos por pares con evaluación de riesgo de sesgo para cada ensayo usando Riesgo de Sesgo de Cochrane; se usó GRADE para proporcionar la certeza de evidencia de cada resultado.
Resultadosse encontraron 4 ensayos que incluyeron a 1.017 pacientes. Los antivirales en uso prolongado protegieron de las recurrencias de la enfermedad herpética ocular mejor que el placebo (RR 0,56; IC 95% 0,45-0,70) NNT 6 (4–11); aciclovir fue mejor que el placebo en esta misma acción (RR 0,59; IC 95% 0,46-0,74) NNT 8 (5–14), pero no diferente de valaciclovir (RR 1,0; IC 95% 0,37-2,70) NNT no calculado. El uso prolongado de antivirales disminuyó también las recurrencias de enfermedad herpética no ocular (RR 0,56; IC 95% 0,44-0,71) NNT 6 (5–11) y de recurrencias con queratitis estromal (RR 0,55; IC 95% 0,35-0,85) NNT 17 (10–50). No se encontraron datos sobre agudeza visual. En cuanto a los efectos adversos, no hubo diferencias entre intervenciones (RR 0,96; IC 95% 0,60-1,54) NNT no calculado. La certeza de evidencia fue alta para las recurrencias oculares y no oculares, y baja para efectos adversos, por imprecisión e inconsistencia de los hallazgos.
Conclusionesel uso prolongado de antivirales protege de recurrencias oculares, no oculares y queratitis estromal de forma segura. No se conocen los efectos sobre la agudeza visual.
Herpes simplex virus (HSV) is a leading cause of infection-related corneal opacification and loss of vision in the USA and other high-income countries1. Following initial primary exposure to ophthalmic HSVA, latent infection is established, resulting in frequent asymptomatic ocular HSV recurrences.
Herpes simplex keratitis (HSK) is a corneal infection secondary to the reactivation of a latent HSV2. HSK is primarily caused by VHS-1, with VHS-2 being rarely the causative agent3. HSK recurrences are very frequent and may cause corneal opacification.4 The estimated incidence/prevalence of HSK is 500,000 new cases reported in the USA annually and 1.54 million new cases reported worldwide, including 40,000 new cases of severe visual impairment or blindness5.
Complications of recurrent HSK primarily include corneal blindness secondary to scarring or perforation, and a dry eye6.
The management of recurrent HSK ranges from no active (antiviral) treatment, as episodes are self-limiting even when left untreated, through antiviral therapies that may relieve symptoms but not eliminate the infection (“lifelong latency”)2. Oral antiviral agents include acyclovir, famciclovir and valacyclovir. Topical ophthalmic antivirals include trifluridine (most commonly prescribed in the USA), ganciclovir, and acyclovir (first-line treatment in Europe but not available in the USA).
A systematic review of the efficacy and safety of the antivirals currently available would help prevent one of the leading causes of blindness and improve the quality of life of patients. Guidelines recommend the prophylactic use of antiviral agents (oral, ophthalmic or combinations of the two) to prevent herpes recurrences7. However, these guidelines are obsolete and are based on the results of clinical trials, not on a systematic review.
The objective of this review is to determine whether long-term use of antiviral therapies in patients with a history of ocular HSV infection is effective in reducing ocular and extra-ocular HSV recurrences, preventing loss of vision, reducing stromal keratitis recurrences, and preventing the occurrence of adverse events (AEs), as compared to placebo or other medications (including other antivirals).
MethodsThe protocol for this systematic review and meta-analysis was prospectively registered to PROSPERO (CRD42024556228)8. Results are reported in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines9.
Types of studiesOur meta-analysis included randomized clinical trials involving patients with one or several episodes of ocular HSV infection in one or two eyes, without age- or sex-based differences. The first episode was not considered an ocular disease for recurrent HSV, but only the subsequent episode(s) of ocular HSV recurrence(s). Participants were required to be free of active herpetic disease before and at registration in the clinical trial. The clinical trials (CTs) were required to involve the use of oral or topical antiviral agents versus an oral or ophthalmic topical agent other than the ones administered in the intervention, placebo (oral or topical), and no active treatment. In the case of CTs with more than two intervention arms, the arms not relevant to our comparative study were excluded. Cross-sectional studies were excluded, as a critical event may occur long after the end of the intervention. Moreover, determining whether recurrence was prevented by the first or the second period of intervention (carry-over period) was not possible. The included CTs involved treatments with a minimum duration of 4 weeks and a minimum follow-up period of three months.
Data collectionCochrane's Database of Controlled Clinical Trials (CENTRAL) at Cochrane's Library (latest issue); Embase.com (from 1947 to June 1, 2024); Medline via PubMed (from 1948 to June 1, 2024); National Database of Ongoing Clinical Trials of the USA National Institutes of Health ClinicalTrials.gov (www.ClinicalTrials.gov) and the WHO International Clinical Trials Registry Platform (www.who.int/ictrp). All records were searched until June 1, 2024.
To gather as much relevant evidence as possible, additional relevant articles were identified by manual inspection of reference lists from retrieved papers and systematic reviews.
Search wordsMESH descriptors and free text were used with the following Boolean combinations: [Keratitis, Herpetic], [Keratitis, Dendritic] [Herpes Simplex] [Eye Diseases] [Acyclovir] [Gancyclovir] [Valaciclovir] [Famciclovir]. [Randomized Controlled Trial]. The search strategies and filters used, along with the characteristics of studies (Annex 2), are included in the Supplementary Material (Annex 1).
Study selection and data extractionPotentially eligible studies were selected by two independent reviewers using Rayyan® software. Following title and abstract screening, eligible studies were selected for full-text reading by two independent reviewers. Data were peer reviewed and entered into a spreadsheet. Assessment of risk of bias was also performed. Discrepancies were resolved by an independent third reviewer.
Primary outcomesPrimary outcomes included the proportion of participants with active recurrent HSV infection; decline (reduction) of the best-corrected visual acuity (BCVA), defined as mean differences in BCVA of 0.2LogMAR or 2 Snellen lines with respect to baseline status; and the proportion of participants with a BCVA decline >0.2LogMAR or two Snellen lines with respect to baseline status.
Secondary outcomesProportion of participants with extra-ocular HSV recurrences including, but not limited to, genital and orofacial HSV infection; proportion of participants with ocular disease secondary to HSV recurrences who develop stromal keratitis; and frequency of AEs and serious AEs.
Risk of bias in clinical trialsThe risk of bias in the selected CTs was assessed using Cochrane's ROB1 tool, which includes random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessors; incomplete outcome data; selective reporting, and other sources of bias10,11.
Effect measuresThe effect of the intervention on dichotomous variables was assessed using relative risk (RR) and the associated 95% confidence intervals (95%CI). The number needed to treat for an additional beneficial effect (NNTB) was calculated from the RR. The NNT was calculated using the NNT = 1/ACR * (1 − RR) formula, where ACT, also known as the baseline risk or risk for an AE, is the outcome of interest with the comparator intervention. To assess differences in continuous variables, differences between means with their 95% CI were estimated11. Estimators were calculated using Revman 5.4® software.
Subgroup analysisSubgroup analysis was conducted to identify potential sources of differences (where more than 10 CTs were available) in the effect of the intervention on primary outcomes based on the following factors:
- •
Duration of treatment, considering 6 months as short-term; 6–12 months as intermediate term; and 12 months as long-term treatment.
- •
Duration of follow-up for outcome analysis: considering 6 months as short-term; 6–12 months as intermediate term; and 12 months as long-term.
- •
Type of intervention, according to whether the intervention involved an oral antiviral agent alone; a topical ophthalmic antiviral agent alone, or a combination of the two.
- •
Dosage of the antiviral agent.
The GRADE tool was used to assess the overall certainty of evidence12. Two authors of this review independently assessed the certainty of the evidence available, including the risk of bias in the CTs, imprecision, indirectness, inconsistency and potential publication bias.
Summary table of outcomesTables were constructed to summarize the findings for each outcome. The tables included RR and NNT estimates for the pre-defined outcomes with their 95% CIs. The certainty of evidence based on the Overall Summary of Findings is also detailed in the tables13.
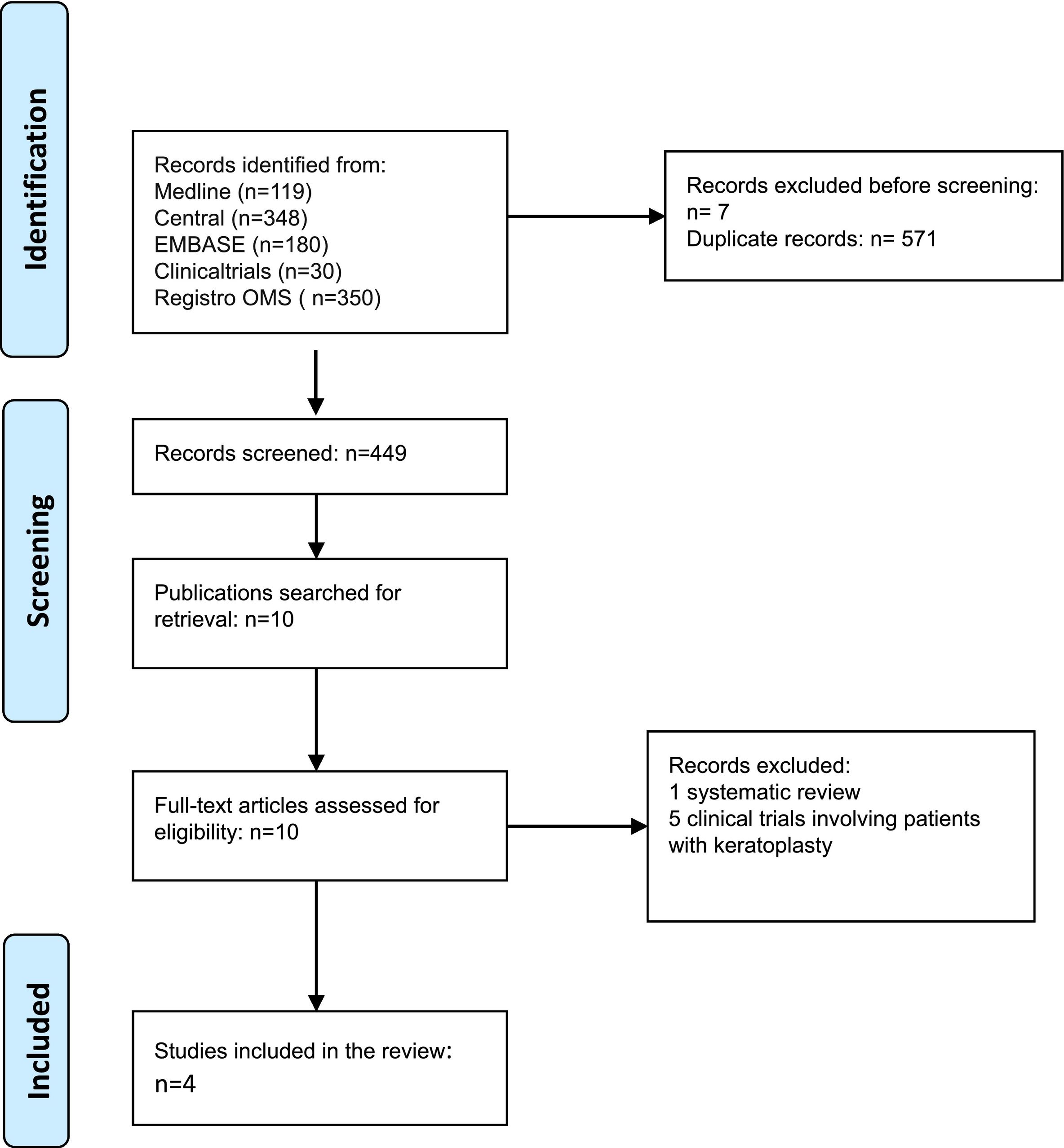
ResultsThe meta-analysis included four CTs14–17 involving a total of 1017 patients, with sample sizes ranging from 5215 to 70314 patients. The characteristics of the studies are detailed in the Annex to the Supplementary Material. All CTs reported effectiveness and AE data. The patients were free of active herpes infection at the start of the CT and had a history of one or several episodes of herpetic keratitis. No age limit was applied in this review. A systematic review18 and 5 trials19–23 were excluded, as they included patients with a previous keratoplasty. Appendix 1 summarizes the characteristics of the studies included. The search strategies used on each database are detailed in Appendix 2.
The studies were conducted in the USA, Italy and China and were published between 199414 and 201516. The follow-up period ranged from 12 to 32 months16.
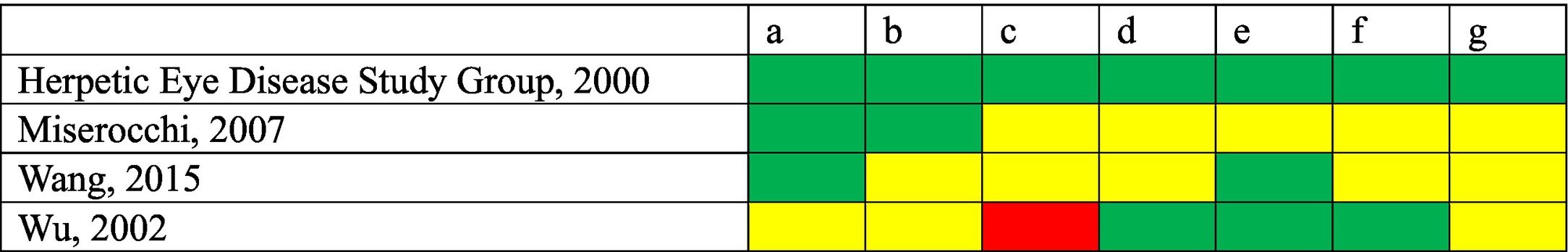
Table 1 contains the assessment of risk of bias in the CTs included. No competing interests were declared in any of the three studies15–17. Competing interests were declared in a study14, which was the study with the lowest risk of bias due to its design and sample size.
Risk of bias of the clinical trials included.
(a) Random sequence generation (selection bias).
(b) Allocation concealment (selection bias).
(c) Blinding of participants and pesonnel (intervention bias).
(d) Blinding of outcome assessor (detection bias).
(e) Incomplete outcome data.
(f) Selective outcome reporting.
(g) Other sources of bias.
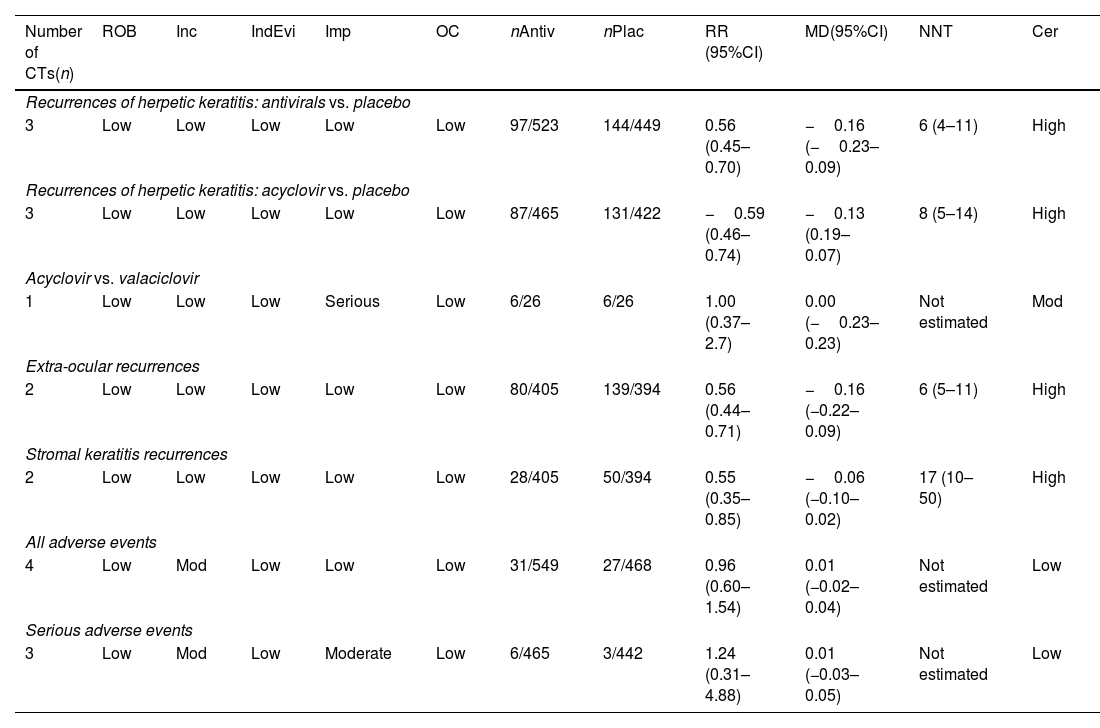
Fig. 2 contains efficacy and safety results. The GRADE certainty of evidence is detailed in Table 2.
GRADE certainty of evidence.
| Number of CTs(n) | ROB | Inc | IndEvi | Imp | OC | nAntiv | nPlac | RR (95%CI) | MD(95%CI) | NNT | Cer |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recurrences of herpetic keratitis: antivirals vs. placebo | |||||||||||
| 3 | Low | Low | Low | Low | Low | 97/523 | 144/449 | 0.56 (0.45–0.70) | −0.16 (−0.23–0.09) | 6 (4–11) | High |
| Recurrences of herpetic keratitis: acyclovir vs. placebo | |||||||||||
| 3 | Low | Low | Low | Low | Low | 87/465 | 131/422 | −0.59 (0.46–0.74) | −0.13 (0.19–0.07) | 8 (5–14) | High |
| Acyclovir vs. valaciclovir | |||||||||||
| 1 | Low | Low | Low | Serious | Low | 6/26 | 6/26 | 1.00 (0.37–2.7) | 0.00 (−0.23–0.23) | Not estimated | Mod |
| Extra-ocular recurrences | |||||||||||
| 2 | Low | Low | Low | Low | Low | 80/405 | 139/394 | 0.56 (0.44–0.71) | −0.16 (−0.22–0.09) | 6 (5–11) | High |
| Stromal keratitis recurrences | |||||||||||
| 2 | Low | Low | Low | Low | Low | 28/405 | 50/394 | 0.55 (0.35–0.85) | −0.06 (−0.10–0.02) | 17 (10–50) | High |
| All adverse events | |||||||||||
| 4 | Low | Mod | Low | Low | Low | 31/549 | 27/468 | 0.96 (0.60–1.54) | 0.01 (−0.02–0.04) | Not estimated | Low |
| Serious adverse events | |||||||||||
| 3 | Low | Mod | Low | Moderate | Low | 6/465 | 3/442 | 1.24 (0.31–4.88) | 0.01 (−0.03–0.05) | Not estimated | Low |
Cer: Certainty; Indirect Evidence; Imp: Imprecision; Inc.: Inconsistency; Mod: Moderate; OC: Other considerations; ROB: Risk of bias in clinical trials.
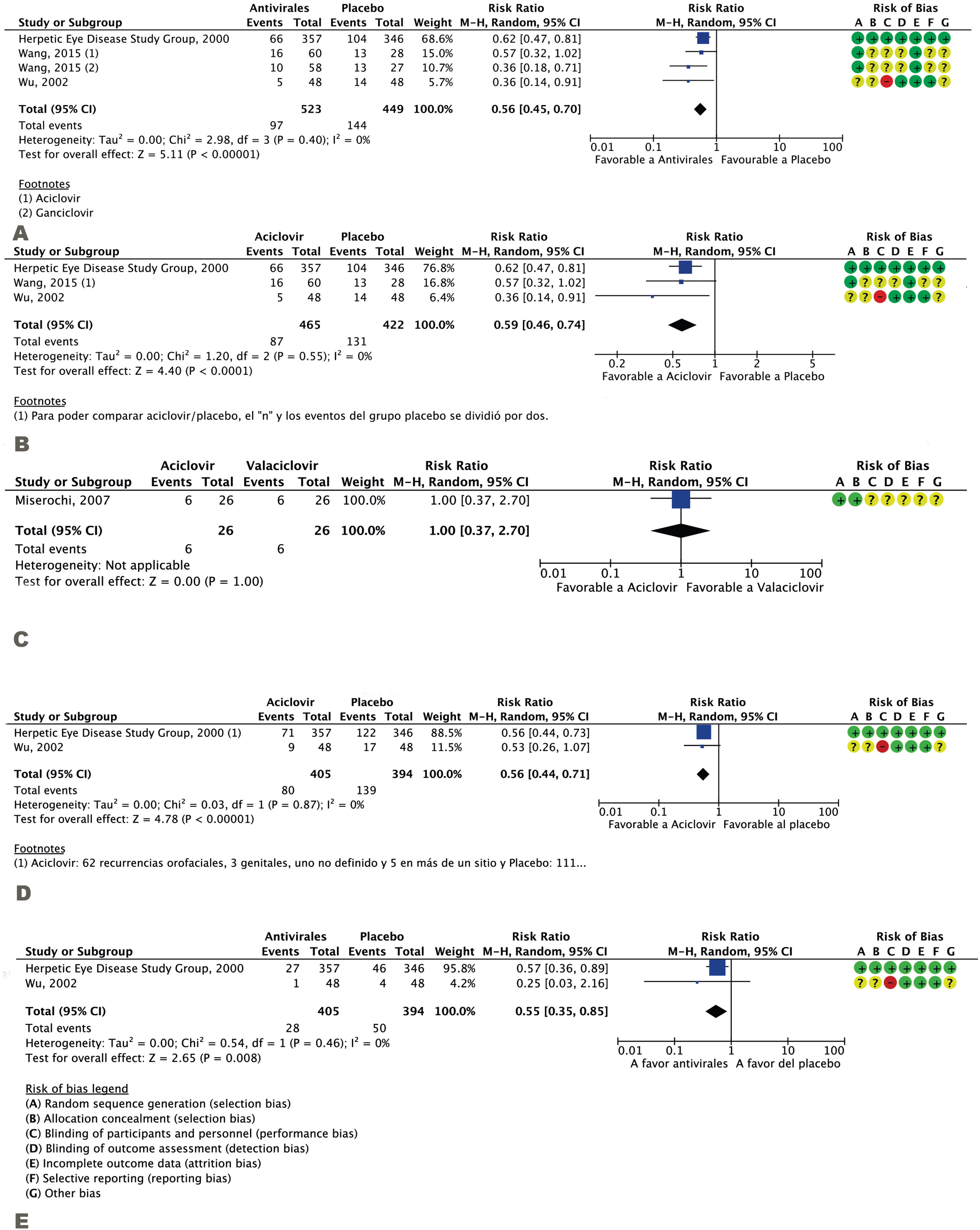
Three studies, including a total of 972 participants, assessed ocular HSV recurrence13,15,16. The incidence of ocular HSV recurrences decreased in the antiviral group (RR 0.56; 95%CI 0.45–0.70; I2 = 0%; NNT 6; 95%CI 4–11) (Fig. 1A). The certainty of evidence for this outcome was high. Of all antivirals, acyclovir was the most extensively studied, as its effects were assessed in three studies aggregately including 887 patients13,15,16. This agent also induced a reduction in ocular HSV recurrences (RR 0.59; 95%CI 0.46–0.74; I2 = 0%; NNT 8; 95%CI 5–14), with a high certainty of evidence (Fig. 1B).
We only found a comparative study assessing two antivirals (acyclovir and valaciclovir)15, which included 52 patients. The certainty of evidence in this study was moderate (certainty was downgraded by one level due to imprecision). No significant differences were observed between the two antivirals in the reduction of ocular HSV recurrences (RR 1; 95%CI 0.37–2.70; I2 not applicable) (Fig. 1C).
The other pre-defined primary outcome was a decline in visual acuity, but this outcome was not reported in any study.
Secondary endpointsAcyclovir exerted protective effects against extra-ocular HSV recurrences, as compared to placebo, with a high certainty of evidence: two studies involving 799 participants14,17; RR 0.56; 95%CI 0.44–0.71; I2 = 0%; NNT 6 (95%CI 5–11) (Fig. 1D), with a high certainty of evidence. The low NNT observed suggests that this agent is very effective. Otherwise, with very few patients treated (5–10), recurrences will decrease, as compared to not treating the patients with antivirals.
As stromal keratitis is the most likely AE to cause a permanent loss of vision, studies were screened for this AE, and two studies including 799 participants were identified14,17: RR 0.55; 95%CI 0.35–0.85; I2 = 0%; NNT 17; 95%CI 10–50 (Fig. 1E), with a high certainty of evidence.
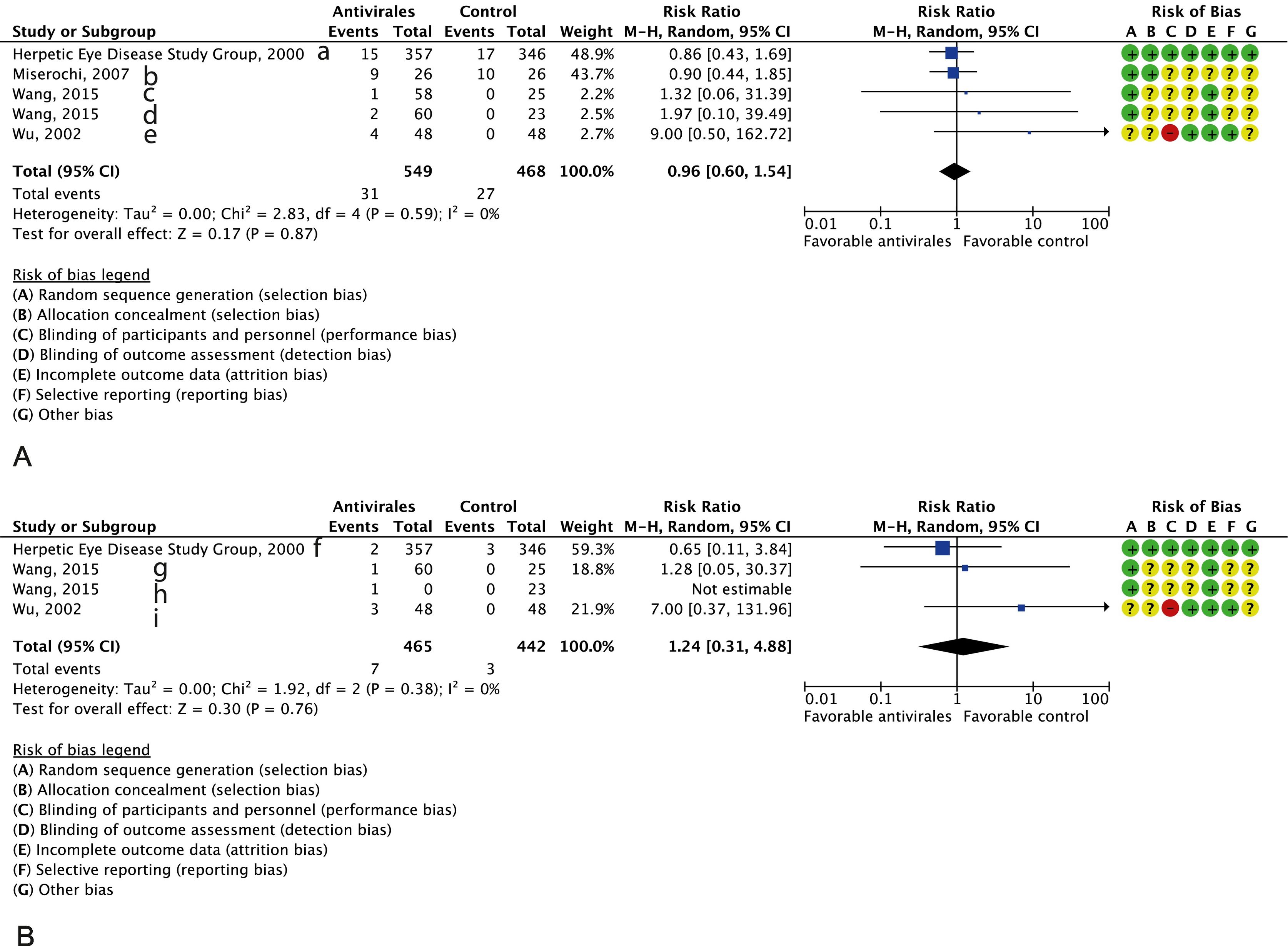
Patients exhibited good tolerance to treatment. No differences were observed between groups in the occurrence of all AEs14–17 (four studies including 1017 participants, RR 0.96; 95%CI 0.60–1.54; I2 = 0%, with a moderate certainty of evidence (imprecision and inconsistency caused two-level downgrading). No differences were observed either regarding the occurrence of AEs (three studies14,16,17 with 907 participants, RR 1.24; 95%CI 0.31–4.88; I2 = 0%, with low certainty of evidence due to imprecision and inconsistency) (Fig. 2A and B, respectively). (See Fig. 3.)
An analysis of sensitivity for the primary outcome was performed (antivirals vs. placebo). Following the removal of the largest study11, a RR of 0.40 was obtained, with a 95%CI 0.27–0.58; I2 = 0%, which does not change the direction of effect and demonstrates the consistency of the analysis.
The limited number of studies included hindered subgroup analysis, and no tools could be used to detect publication bias.
DiscussionComprehensivenessThe studies included did not report data for all the primary outcomes of this review.
Certainty of evidenceThe certainty of evidence was high for the outcome ‘clinical effectiveness’ and low for ‘AEs’. Considering the risk of bias inherent to the design of CTs, the blinding limitations in three of the four studies did not result in downgrading, as this limitation did not affect the outcome in most variables. In the comparative study of acyclovir versus valaciclovir, imprecision caused certainty to be downgraded by one level. In the two safety outcomes, certainty was lowered two levels due to imprecision and inconsistency.
Sensitivity analysisRemoval of the study with the largest sample and best design did not influence outcomes, as antivirals retained their protective effects against recurrences.
Concordance and discordance with other studies and reviewsA Cochrane systematic review published in 2016 was retrieved during the search process. This study assessed recurrences reported for acyclovir versus placebo in patients with keratoplasty18. The results of this review are consistent with the 2016 Cochrane review, which recommended the prophylactic use of antivirals to prevent recurrences in patients with keratoplasty.
Clinical discussionThe evidence gathered in this review is not solid enough to conclusively establish the dosage and duration of antiviral treatments to prevent recurrences. However, as most studies included a follow-up period longer than one year, apparently, acyclovir (400 mg/12 h) can be safely administered, since it is the most extensively studied agent and was used for more than a year in all CTs.
On the other hand, further studies are needed to determine whether discontinuance of antiviral therapy is associated or not with an increased incidence of recurrences.
Future researchFurther clinical trials with different antivirals at different doses and follow-up periods are needed where visual acuity is also assessed.
The evidence currently available demonstrates that antivirals have protective effects against ocular (and extra-ocular) herpetic recurrences. This protective effect was confirmed for acyclovir and other antivirals (valaciclovir/ganciclovir).
The AEs related to the use of antivirals were similar to those reported in the placebo group.
Current knowledgeHerpes simplex generally remains latent, thereby causing frequent recurrences. Herpetic keratitis is the leading cause of decline in visual acuity and blindness in developed countries.
Beyond management of the acute phase, prophylactic antiviral treatment is not administered on a routine basis.
Contribution to the scientific literatureThis original study is a thorough review of the evidence available on the prophylactic use of antivirals to prevent ocular herpetic recurrences.
This study reveals that long-term use of antivirals after the acute phase reduces the incidence of recurrences by a half.
Additional AEs were not identified in the patients treated. This finding is consistent with other studies in corneal transplant recipients.
Assignment of rightsAll authors assign the rights to Hospital Pharmacy.
CRediT authorship contribution statementLaura Ruiz Sifre: Writing – review & editing, Writing – original draft, Validation, Methodology, Formal analysis, Conceptualization. Sylvia Bort Martí: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. Vicente Ruiz García: Writing – review & editing, Writing – original draft, Validation, Supervision, Formal analysis, Data curation, Conceptualization. Ángeles Ruth Bort Martí: Writing – review & editing, Writing – original draft, Validation, Methodology, Formal analysis, Conceptualization.
FundingThe authors did not receive any funding for the preparation of this manuscript.