Therapeutic monitoring of antibiotics and antifungals based on pharmacokinetic and pharmacodynamic (PK/PD) parameters is a strategy increasingly used for the optimization of therapy to improve efficacy, reduce the occurrence of toxicities, and prevent the selection of antimicrobial resistance, particularly in vulnerable patients including neonates and the critical or immunocompromised paediatric host.
In neonates and children, infections account for a high percentage of hospital admissions, and anti-infectives are the most used drugs. However, paediatric PK/PD studies and the evidence regarding the efficacy and safety of some newly marketed antibiotics and antifungals—usually used off-label in paediatrics—to determine the optimal drug dosage regimens are limited. It is widely known that this population presents important differences in the PK parameters (especially in drug clearance and volume of distribution) in comparison with adults that may alter antimicrobial exposure and, therefore, compromise treatment success. In addition, paediatric patients are more susceptible to potential adverse drug effects and they need closer monitoring.
The aim of this document, developed jointly by the Spanish Society of Hospital Pharmacy and the Spanish Society of Paediatric Infectious Diseases, is to describe the available evidence on the indications for therapeutic drug monitoring (TDM) of antibiotics and antifungals in newborn and paediatric patients, and to provide practical recommendations for TDM in routine clinical practice to optimise their dosing, efficacy and safety. Of antibiotics and antifungals in the paediatric population.
La monitorización terapéutica de antibióticos y antifúngicos en el paciente pediátrico, basada en parámetros farmacocinéticos y farmacodinámicos (PK/PD), es una estrategia cada vez más utilizada para la optimización del tratamiento de infecciones con el fin de mejorar la eficacia, reducir la aparición de toxicidades y prevenir la selección de resistencias antimicrobianas, especialmente en poblaciones vulnerables como neonatos, pacientes críticos o inmunodeprimidos.
En la población neonatal y pediátrica, las infecciones representan un porcentaje elevado de los ingresos hospitalarios, siendo los antimicrobianos los fármacos más utilizados en este grupo poblacional. Sin embargo, establecer los regímenes posológicos óptimos en esta población es complejo debido a la menor evidencia respecto a la eficacia y seguridad de algunos antimicrobianos, especialmente en los de reciente comercialización que son utilizados habitualmente fuera de ficha técnica y al limitado número de estudios PK/PD disponibles en pediatría. Es bien conocido que el paciente pediátrico se caracteriza por presentar una serie de variaciones de los parámetros farmacocinéticos (como el aclaramiento o el volumen de distribución) debido a los propios cambios madurativos que pueden modificar la exposición a los antimicrobianos y comprometer el éxito del tratamiento. A su vez, es un grupo poblacional más susceptible a presentar potenciales efectos adversos a los fármacos, hecho que conlleva la necesidad de una monitorización más estricta.
El objetivo de este documento de consenso, elaborado entre la Sociedad Española de Farmacia Hospitalaria (SEFH) y Sociedad Española de Infectología Pediátrica (SEIP), es describir la evidencia disponible sobre las indicaciones de la monitorización terapéutica de antibióticos y antifúngicos en pacientes neonatales y pediátricos, y proporcionar recomendaciones prácticas para su monitorización y ajuste de dosis, con el fin de optimizar el tratamiento, maximizando su eficacia y seguridad.
Knowledge of the pharmacokinetic and pharmacodynamic (PK/PD) parameters of antibiotic or antifungal agents is essential in selecting appropriate antimicrobial treatments. In addition, their activity and susceptibility against the microorganisms involved, their penetration and diffusion at the infection site, and the patients' clinical conditions must also be taken into account. PK/PD indices help to optimise clinical and microbiological efficacy, minimise selective pressure for resistance development, and are crucial for selecting an appropriate dosing regimen.1
Determining the plasma concentration of a drug provides an understanding of the exposure achieved with the administered dose and allows dosing adjustments according to the estimated PK/PD indices. This is called therapeutic drug monitoring (TDM), and is based on the premise that there is a quantitative and predictable relationship between blood drug concentrations and therapeutic or toxic response.2
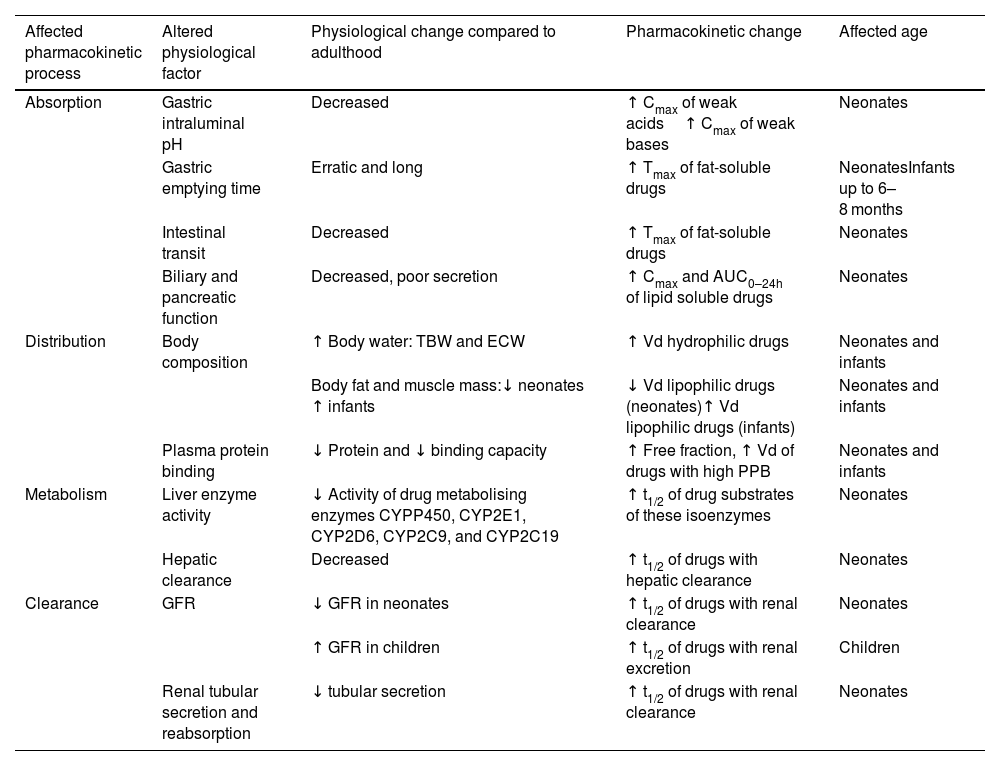
Optimising dosing according to PK/PD parameters is particularly important in the paediatric population.3 Many physiological changes that occur during the first years of life cause large intra- and inter-individual variability in exposure after administration of a given dose (Table 1).3–5 Exposure may also be affected by diseases or clinical situations that can modify the PK profile of drugs, mainly by increasing or decreasing plasma clearance (CL) or volume of distribution (Vd). Other factors include drug interactions, abnormal renal or hepatic function, or certain interventions such as extra-renal CL techniques, extracorporeal membrane oxygenation (ECMO), or therapeutic hypothermia.6
Age-related physiological changes that affect antibiotic and antifungal pharmacokinetics in paediatric patients.
| Affected pharmacokinetic process | Altered physiological factor | Physiological change compared to adulthood | Pharmacokinetic change | Affected age |
|---|---|---|---|---|
| Absorption | Gastric intraluminal pH | Decreased | ↑ Cmax of weak acids↑ Cmax of weak bases | Neonates |
| Gastric emptying time | Erratic and long | ↑ Tmax of fat-soluble drugs | NeonatesInfants up to 6–8 months | |
| Intestinal transit | Decreased | ↑ Tmax of fat-soluble drugs | Neonates | |
| Biliary and pancreatic function | Decreased, poor secretion | ↑ Cmax and AUC0–24h of lipid soluble drugs | Neonates | |
| Distribution | Body composition | ↑ Body water: TBW and ECW | ↑ Vd hydrophilic drugs | Neonates and infants |
| Body fat and muscle mass:↓ neonates ↑ infants | ↓ Vd lipophilic drugs (neonates)↑ Vd lipophilic drugs (infants) | Neonates and infants | ||
| Plasma protein binding | ↓ Protein and ↓ binding capacity | ↑ Free fraction, ↑ Vd of drugs with high PPB | Neonates and infants | |
| Metabolism | Liver enzyme activity | ↓ Activity of drug metabolising enzymes CYPP450, CYP2E1, CYP2D6, CYP2C9, and CYP2C19 | ↑ t1/2 of drug substrates of these isoenzymes | Neonates |
| Hepatic clearance | Decreased | ↑ t1/2 of drugs with hepatic clearance | Neonates | |
| Clearance | GFR | ↓ GFR in neonates | ↑ t1/2 of drugs with renal clearance | Neonates |
| ↑ GFR in children | ↑ t1/2 of drugs with renal excretion | Children | ||
| Renal tubular secretion and reabsorption | ↓ tubular secretion | ↑ t1/2 of drugs with renal clearance | Neonates |
Adapted from Le et al.3
TBW, total body water; AUC0–24h, area under the plasma concentration curve over 24 h; Cmax, maximum concentration; ECW, extracellular water; GFR, glomerular filtration rate; Tmax, time to reach Cmax; t1/2, elimination half-life; PPB, plasma protein binding; Vd, volume of distribution; ↑, increase compared to adulthood; ↓, decrease compared to adulthood.
Therefore, paediatric patients are a group in which antimicrobial TDM may play a key role, especially when there are comorbidities and/or they are receiving therapies that affect drug PK. However, it is important to be aware of the current evidence regarding the need for monitoring individual antibiotics or antifungals. The criteria that justify the TDM of a given drug include the following aspects: a narrow therapeutic margin, significant inter- and intra-individual PK variability, and, ideally, a proven relationship between plasma concentrations and toxicity and/or efficacy.1,2,7
When a drug meets these criteria and TDM is recommended, it is essential to know the therapeutic index, the monitoring guidelines (type and timing of sample collection), as well as the specific recommendations on adjusting dosage based on the sampling data. Interpreting, comparing, and standardising the methods used in routine healthcare practice for TDM is challenging due to significant variations in the level of experience in TDM, availability of different TDM techniques, and the methods employed in different hospitals. Furthermore, there is a lack of evidence on the benefits and clinical implications of TDM for some antibiotics and antifungals in the paediatric population.
The aim of this consensus document is to provide evidence-based recommendations for the most commonly monitored antibiotics and antifungals in paediatrics, in order to establish a consistent working methodology. A working group was created and members were appointed by the participating scientific societies: the Spanish Society of Hospital Pharmacy (SEFH) and the Spanish Society of Paediatric Infectious Diseases (SEIP). A literature search was performed in PubMed for articles published during the last 15 years (2007–2022), without language restrictions, using the following MeSH terms: (“antibacterial agents” or “antifungal agents”) and (“drug monitoring” or “pharmacokinetics” or “pharmacodynamics”) and (“children” or “paediatrics” or “neonates”). The search was extended to antimicrobials and classes of antimicrobials of interest. In addition, a manual search of the references from the articles identified in the initial search was conducted, resulting in the inclusion of additional articles. The working group discussed the findings and reached consensus on formulating the recommendations.
Initial dosing guidelines are not addressed in this document, as they are defined in another consensus document (https://www.seipweb.es/dosisantibioticos/).
RecommendationsTable 2 presents the recommendations on TDM rationales and indications, while Table 3 provides interpretations and dosing adjustments. Table 3 also shows the PK/PD characteristics of each drug group to provide context for these recommendations. It should be noted that recommendations for dosing adjustments should always be tailored to the clinical context, specific patient characteristics, micro-organism, and type of infection, and should be used as an additional tool to support clinical decision-making.
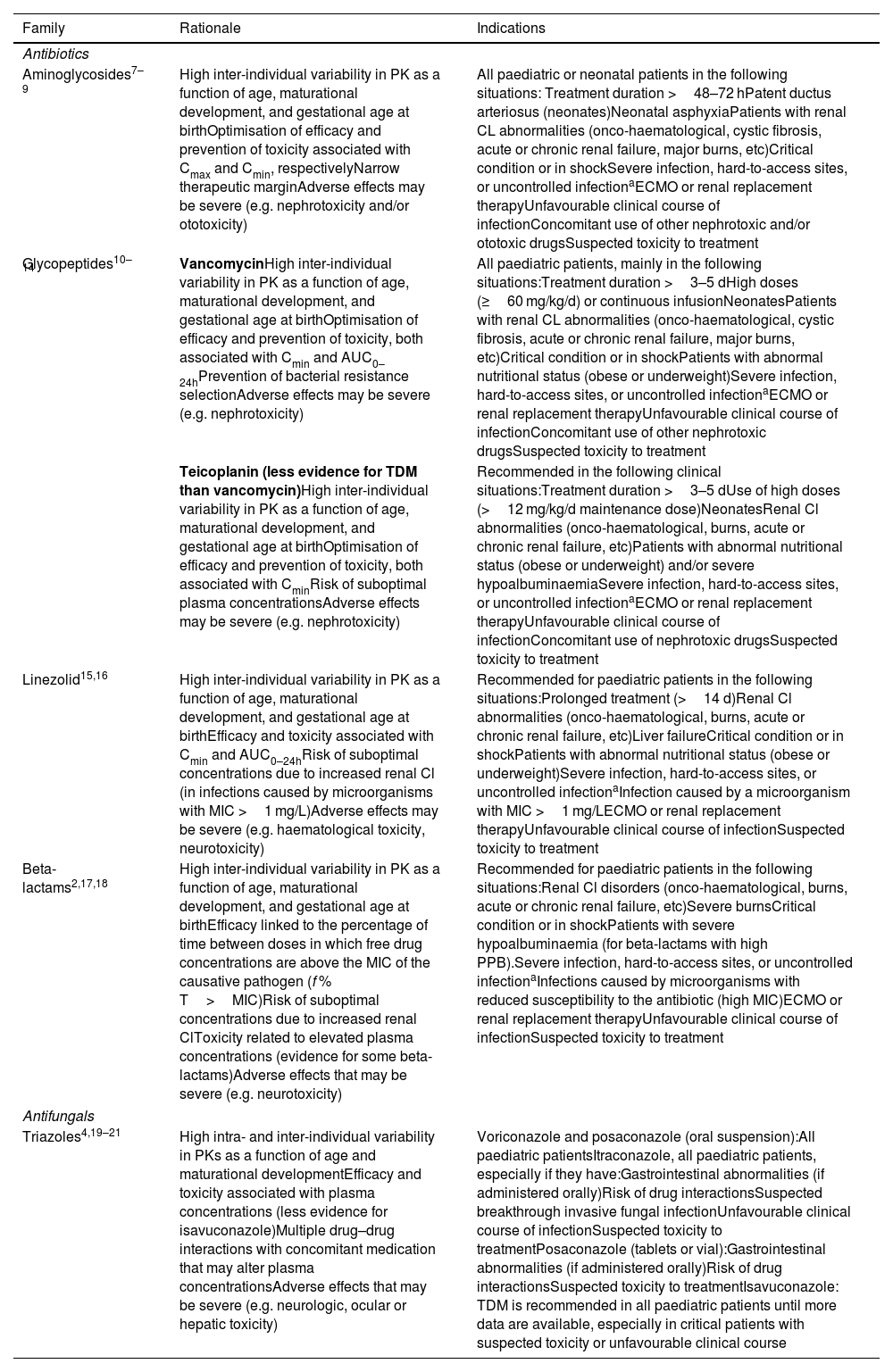
Rationale and indications for antibiotic and antifungal TDM in paediatric patients.
| Family | Rationale | Indications |
|---|---|---|
| Antibiotics | ||
| Aminoglycosides7–9 | High inter-individual variability in PK as a function of age, maturational development, and gestational age at birthOptimisation of efficacy and prevention of toxicity associated with Cmax and Cmin, respectivelyNarrow therapeutic marginAdverse effects may be severe (e.g. nephrotoxicity and/or ototoxicity) | All paediatric or neonatal patients in the following situations: Treatment duration >48–72 hPatent ductus arteriosus (neonates)Neonatal asphyxiaPatients with renal CL abnormalities (onco-haematological, cystic fibrosis, acute or chronic renal failure, major burns, etc)Critical condition or in shockSevere infection, hard-to-access sites, or uncontrolled infectionaECMO or renal replacement therapyUnfavourable clinical course of infectionConcomitant use of other nephrotoxic and/or ototoxic drugsSuspected toxicity to treatment |
| Glycopeptides10–14 | VancomycinHigh inter-individual variability in PK as a function of age, maturational development, and gestational age at birthOptimisation of efficacy and prevention of toxicity, both associated with Cmin and AUC0–24hPrevention of bacterial resistance selectionAdverse effects may be severe (e.g. nephrotoxicity) | All paediatric patients, mainly in the following situations:Treatment duration >3–5 dHigh doses (≥60 mg/kg/d) or continuous infusionNeonatesPatients with renal CL abnormalities (onco-haematological, cystic fibrosis, acute or chronic renal failure, major burns, etc)Critical condition or in shockPatients with abnormal nutritional status (obese or underweight)Severe infection, hard-to-access sites, or uncontrolled infectionaECMO or renal replacement therapyUnfavourable clinical course of infectionConcomitant use of other nephrotoxic drugsSuspected toxicity to treatment |
| Teicoplanin (less evidence for TDM than vancomycin)High inter-individual variability in PK as a function of age, maturational development, and gestational age at birthOptimisation of efficacy and prevention of toxicity, both associated with CminRisk of suboptimal plasma concentrationsAdverse effects may be severe (e.g. nephrotoxicity) | Recommended in the following clinical situations:Treatment duration >3–5 dUse of high doses (>12 mg/kg/d maintenance dose)NeonatesRenal Cl abnormalities (onco-haematological, burns, acute or chronic renal failure, etc)Patients with abnormal nutritional status (obese or underweight) and/or severe hypoalbuminaemiaSevere infection, hard-to-access sites, or uncontrolled infectionaECMO or renal replacement therapyUnfavourable clinical course of infectionConcomitant use of nephrotoxic drugsSuspected toxicity to treatment | |
| Linezolid15,16 | High inter-individual variability in PK as a function of age, maturational development, and gestational age at birthEfficacy and toxicity associated with Cmin and AUC0–24hRisk of suboptimal concentrations due to increased renal Cl (in infections caused by microorganisms with MIC >1 mg/L)Adverse effects may be severe (e.g. haematological toxicity, neurotoxicity) | Recommended for paediatric patients in the following situations:Prolonged treatment (>14 d)Renal Cl abnormalities (onco-haematological, burns, acute or chronic renal failure, etc)Liver failureCritical condition or in shockPatients with abnormal nutritional status (obese or underweight)Severe infection, hard-to-access sites, or uncontrolled infectionaInfection caused by a microorganism with MIC >1 mg/LECMO or renal replacement therapyUnfavourable clinical course of infectionSuspected toxicity to treatment |
| Beta-lactams2,17,18 | High inter-individual variability in PK as a function of age, maturational development, and gestational age at birthEfficacy linked to the percentage of time between doses in which free drug concentrations are above the MIC of the causative pathogen (f % T>MIC)Risk of suboptimal concentrations due to increased renal ClToxicity related to elevated plasma concentrations (evidence for some beta-lactams)Adverse effects that may be severe (e.g. neurotoxicity) | Recommended for paediatric patients in the following situations:Renal Cl disorders (onco-haematological, burns, acute or chronic renal failure, etc)Severe burnsCritical condition or in shockPatients with severe hypoalbuminaemia (for beta-lactams with high PPB).Severe infection, hard-to-access sites, or uncontrolled infectionaInfections caused by microorganisms with reduced susceptibility to the antibiotic (high MIC)ECMO or renal replacement therapyUnfavourable clinical course of infectionSuspected toxicity to treatment |
| Antifungals | ||
| Triazoles4,19–21 | High intra- and inter-individual variability in PKs as a function of age and maturational developmentEfficacy and toxicity associated with plasma concentrations (less evidence for isavuconazole)Multiple drug–drug interactions with concomitant medication that may alter plasma concentrationsAdverse effects that may be severe (e.g. neurologic, ocular or hepatic toxicity) | Voriconazole and posaconazole (oral suspension):All paediatric patientsItraconazole, all paediatric patients, especially if they have:Gastrointestinal abnormalities (if administered orally)Risk of drug interactionsSuspected breakthrough invasive fungal infectionUnfavourable clinical course of infectionSuspected toxicity to treatmentPosaconazole (tablets or vial):Gastrointestinal abnormalities (if administered orally)Risk of drug interactionsSuspected toxicity to treatmentIsavuconazole: TDM is recommended in all paediatric patients until more data are available, especially in critical patients with suspected toxicity or unfavourable clinical course |
AUC0–24h, area under the plasma concentration curve over 24 h; renal Cl, renal clearance; MIC, minimum inhibitory concentration; Cmax, maximum or peak concentration; Cmin, minimum or trough concentration (to be drawn just before administration of the next dose); ECMO, extracorporeal membrane oxygenation; f, free fraction of drug; TDM, therapeutic drug monitoring; PPB, plasma protein binding; Vd, volume of distribution.
Complicated infections or difficult-to-access site: Those with high inoculum and uncontrolled source of infection (pneumonia, non-surgical peritonitis, endocarditis) or those in which antibiotic penetration is limited by the characteristics of the site of infection (osteoarticular, ocular, cardiac, pulmonary, or central nervous system).
Recommendations for antibiotic and antifungal therapeutic drug monitoring in paediatric patients: determination, interpretation, and dosing adjustment.
| Drug | Time of determination | First and subsequent determinations | Target PK/PD index | Potentially toxic plasma exposure | Recommended dosing adjustment |
|---|---|---|---|---|---|
| Antibiotics | |||||
| Aminoglycosides | |||||
| Amikacin (extended interval administration)7,8,22,23 | C8–12h: 8–12 h after start of infusion (to estimate Cmin and Cmax using a population PK model) ±Cmax (30 min after end of 30 min IV infusion) (only in patients with increased Vd such as critical, septic, or burn patients)If minimising the number of samples is required, collect a single sample at 8–12 hIn patients with increased GFR (critical with correct renal function, CF, or oncohaematoma), measuring at 8 h is recommended to avoid undetectable levels | First determination after administering the first doseMeasure weekly or sooner if dosing has been previously adjusted or toxicity is suspected | Cmax/MIC: 8–10 mg/LCmax: 24–35 mg/LA higher Cmax (up to 60–80 mg/L) may be needed in complicated infections or in hard-to access sitesa caused by microorganisms with high MICs, or in septic patients Cmin<4 mg/L to prevent toxicity (ideally undetectable) | Cmin > 4 mg/L (nephrotoxicity)Cmin > 10 mg/L (ototoxicity) | Dosing adjustments should be made using a population PK model included in a Bayesian prediction programIf unavailable, estimates could be made based on nomograms adapted from the adult population (Hartford or Urban-Craig)24Consider reducing the dosing interval if Cmin remains below MIC for more than 12 h between consecutive doses |
| Gentamicin/Tobramycin (extended interval administration)7,25 | C8–12h: 8–12 h after start of infusion (to estimate Cmin and Cmax using a population PK model) ±Cmax (30 min after end of 30 min IV infusion) (only in patients with increased Vd such as critical, septic, or burn patients)If minimising the number of samples is required, collect a single sample at 8–12 hMeasurement at 8 h is recommended to avoid undetectable levels in patients with increased GFR (critical patients with correct renal function, CF, or oncohaematoma) | First determination after administering the first doseMeasure weekly or sooner if dosing has been previously adjusted or toxicity is suspected | Cmax/MIC: 8–10 mg/LA higher Cmax (up to 15–20 mg/L) may be needed in complicated or hard-to-reach infectionsa caused by microorganisms with high MICs, or in septic patients Cmin<1 mg/L to prevent nephrotoxicity (ideally undetectable) | Cmin > 2 mg/L (nephrotoxicity) | Dosing adjustments should be made using a population PK model included in a Bayesian prediction programIf unavailable, estimates could be made based on nomograms adapted from the adult population (Hartford or Urban-Craig)24Consider reducing the dosing interval if Cmin remains below MIC for more than 12 h between consecutive doses |
| Glycopeptides | |||||
| Vancomycin (intermittent infusion)26,27 | Cmin±Cmax (1–2 h after end of 1 h intravenous infusion)2 to estimate AUC0–24h (Cmin+Cmax if unable to estimate AUC0–24h via PK modelling program, or in patients with suspected Vd alteration, such as critical, septic, or burn patients) | First determination 24–48 h after starting treatmentMeasure at 3–5 d or sooner in the case of changes in renal function or suspected toxicity | AUC0–24h/MIC: 400–600b (preferably in the lower range)For neonates with late sepsis due to CoNS, an AUC0–24h/MIC of 240–480 is sufficientIn case AUC0–24h cannot be estimated, it is recommended to use CminCmin: 10–15 mg/LIn hard-to-access infectionsa, Cmin up to 15–20 mg/LFor neonates with late sepsis due to CoNS, a reduced Cmin of 5–10 mg/L is acceptable | AUC0–24h/MIC >600-800bCmin>20 mg/L | AUC0–24h estimations and dosing adjustments should be made using a population PK model within a Bayesian prediction programIf AUC0–24h cannot be estimated, the following options are recommended:If Cmin is sub-therapeutic: increase the dose by 20% (in uncomplicated infections) or 20%–25% (in complicated infections).If Cmin is supratherapeutic: reduce the dose by 15%–20% or prolong the dosing interval Depending on type of infection and/or presence of toxicity, consider skipping the next dose, repeat monitoring at 6–8 h until therapeutic concentration is reached, and restart with adjusted dosesIn patients who fail to achieve target PK/PD despite administration of maximum doses, consider continuous infusion of vancomycinc |
| Vancomycin (continuous infusion)26,27 | Css: measure at any time after steady-state equilibrium is reached and draw from the arm contralateral to the infusion pump | First determination 24–48 h after starting treatmentMeasure at 3–5 d or sooner in the case of changes in renal function or suspected toxicity | AUC0–24h/MIC: 400–600b (preferably in the lower range)Equivalent to the following Css values:Neonates and infants up to 90 d:Css: 15–25 mg/LPatients aged >90 d: Css: 20–25 mg/L | AUC0–24h/MIC >600–800bCss>25 mg/L | AUC0–24h estimations and dosing adjustments should be made using a population PK model within a Bayesian prediction programIf AUC0–24h cannot be estimated, the new dose can be calculated assuming linear PK with the following formula, provided there are no significant changes in clinical status or renal function: new dose (mg/d)=target Css/measured Css · previous dose (mg/d) |
| Teicoplanin12 | Cmin | First determination 3–5 d after starting treatment (with loading dose)Measure weekly or sooner if dosing has been previously adjusted, there are changes in renal function, or suspected toxicityd | Cmin: 10–20 mg/LCmin: 20–40 mg/L in complicated or difficult-to-reach infectionsa | Cmin>40 mg/L (thrombocytopenia)Cmin ≥ 60 mg/L (nephrotoxicity) | Dosing adjustments should be made using a population PK model within a Bayesian prediction program.If unavailable, there are no specific recommendations. Adjust the dosage according to clinical criteria assuming a linear PK or consider discontinuation in the presence of toxicity |
| Oxazolidinones | |||||
| Linezolid,15,16,28 | CminAUC0–24h (uncommon in routine clinical practice) | First determination 48 h after starting treatmentMeasure weekly or sooner if dosing has been previously adjusted or toxicity is suspected | Cmin: 2–7 mg/L (definitions of the upper limit vary from 7 to 10 mg/L)AUC0–24h>80–100 mg·h/L | Cmin>7–10 mg/L (haematological toxicity data in adults)AUC0–24h>300–400 mg·h/L (in adults) (can be estimated from Cmin using a population PK program) | Dosing adjustments should be made using a population PK model within a Bayesian prediction programIf unavailable, adjust the dosage according to clinical criteria assuming a linear PK or consider discontinuation in the presence of toxicity In adults, it is recommended to adjust dosing by modifying the dosing interval while maintaining the same dose |
| Beta-lactams | |||||
| Cefepime29 | Intermittent or extended infusion:Continuous infusion Cmin:Css (measure any time after steady-state equilibrium is reached and draw from the arm contralateral to the infusion pump)Piperacillin Cmin > 128 mg/LfCmin > 44.5 mg/Lf | First determination 24–48 h after starting treatmentMeasure weekly or sooner if dosing has been previously adjusted, there is an unfavourable clinical course, changes in renal function, or suspected toxicity | fT>MIC (beta-lactam free concentration (f Cmin/f Css)>MIC of the infecting organism during 100% of the inter-dose intervaleIn critical or septic patients, or those with complicated or hard-to-reach infections, it is recommended to achieve a f Cmin/f Css>4–5 times MIC of the infecting organism during 100% of the inter-dose intervale (fT >4–5 · MIC)An fCmin or fCss>8–10 times MIC of the infecting organism for 100% of the inter-dose interval has been suggested as an upper limit for adult patientse (fT >8–10 · MIC) | Cmin>20–22 mg/LfCss > 35 mg/Lf | Dosing adjustments should be made using a population PK model within a Bayesian prediction program.If unavailable, a linear PK can be assumed and dosing can be adjusted proportionally to achieve the target Cmin or Css according to clinical criteria or consider discontinuing treatment in the case of toxicityIn adults, dose adjustments of 25%–50% or modifications in the dosing interval have both been suggested |
| Ceftazidime30,31 | Cmin > 64–100 mg/Lf | ||||
| Piperacillin-tazobactam17,32,33 | |||||
| Meropenem34 | |||||
| Antifungals | |||||
| Triazoles | |||||
| Itraconazole1,19,21 | Cmin | First determination 5–7 d after starting treatmentCheck weekly or sooner in the case of previous dosage adjustment, unfavourable clinical course, or introduction or withdrawal of concomitant drugs that may interact with the antifungal, and/or suspected toxicity | Prophylaxis:Cmin > 0.5 mg/LTreatment:Cmin: 1–2 mg/L (sum values of itraconazole and hydroxy-itraconazole) | Cmin>17 mg/L (sum values of itraconazole and hydroxy-itraconazole) | Dosing adjustments should be made using a population PK model within a Bayesian prediction program (available for oncohematology and cystic fibrosis patients) If unavailable, adjust the dosage according to clinical criteria or consider discontinuation in the presence of toxicity |
| Posaconazole1,19,21 | Cmin | Prophylaxis:Cmin: 0.7–2.5 mg/LTreatment:Cmin: 1–2.5 mg/L (if refractory IFI >1.3 mg/L)Consider a higher Cmin in fungal infections with high MICs | Cmin > 3.75 mg/L | ||
| Voriconazole19–21 | Cmin | First measurement 3–4 d after starting treatmentCheck weekly or sooner in the case of previous dosage adjustment, unfavourable clinical course, or introduction or withdrawal of concomitant drugs that may interact with the antifungal, and/or suspected toxicity | Cmin: 1–5 mg/L (both treatment and prophylaxis)Cmin value should reach the upper range in the case of complicated or difficult-to-access infectionsa | Cmin>6 mg/L | Dosing adjustments should be made using a population PK model within a Bayesian prediction program.If unavailable, the following recommendations can be followed:If Cmin<1 mg/L: 50% increase in daily dose. In the case of dose increase on 2 or more consecutive occasions without achieving the desired Cmin: 50% increase of the daily dose, and adjust the frequency of administration from every 12 h to every 8 hIf Cmin>5 mg/L: reduce the dose according to clinical criteria or consider discontinuation in the presence of toxicity |
| Isavuconazole19,21,35,36 | Cmin | First determination 4–6 d after starting treatmentCheck weekly or sooner in the case of previous dosage adjustment, unfavourable clinical course, or introduction or withdrawal of concomitant drugs that may interact with the antifungal, and/or suspected toxicityIn treatments >21 d, consider spacing controls | Prophylaxis and treatment:Cmin>2.5–5.0 mg/LConsider higher Cmin (between 5 and 10 mg/L) in hard-to-reach infectionsa or those caused by fungi with high MICs | Not defined | There are no specific recommendations for dosing adjustments in paediatrics, so it is recommended to adjust dosing according to clinical criteria or to consider discontinuation in the presence of toxicity |
AUC0–24h, area under curve for plasma concentration over 24 h; MIC, minimum inhibitory concentration; Cmax, maximum or peak concentration; Cmin, minimum or trough concentration (measure just before administration of the next dose); Css, average steady-state concentration; C8–12h, plasma concentration 8–12 h after start of intravenous infusion; f, free fraction; CF, cystic fibrosis; IFI, invasive fungal infection; IV, intravenous route; PK, pharmacokinetic; PK/PD, pharmacokinetic-pharmacodynamic; GFR, glomerular filtration rate; CoNS, coagulase-negative Staphylococcus.
Complicated or hard-to-reach infections: Cases with high inoculum and uncontrolled source of infection (pneumonia, non-surgical peritonitis, endocarditis) or cases in which antibiotic penetration is limited by the characteristics of the infection site (osteoarticular, ocular, cardiac, pulmonary, or central nervous system).
Vancomycin: If the MIC of the causative pathogen is unknown, a value of 1 mg/L is assumed by default. If a population model is used for the estimation of AUC0–24h, a sample can be drawn at any time as no Cmax measurement is required.
Vancomycin: When considering the continuous infusion of vancomycin, near-exclusive venous access should be ensured, as its physicochemical incompatibilities with other drugs may prevent co-infusion through the same lumen.
Teicoplanin: Critical patients with hypoalbuminaemia, onco-haematoma, or unstable renal function should be monitored every 2–3 d.
Beta-lactams: If MICs are unknown (due to empirical treatment or while pending results), the epidemiological cut-off value (ECOFF) or the sensitivity cut-off value (EUCAST) can be used, or TDM can be deferred until the results of microbiological cultures are available. Another option is to use the most frequent MIC value for that strain, taking local epidemiology into account.
The following section provides some general considerations on the recommended methodology for TDM and estimation of PK/PD parameters in routine clinical practice.
Blood sample collection for TDMIdeally, the blood sample for determination of plasma concentrations should not be obtained via the same vascular catheter used to administer the drug. The preferred collection method is venipuncture. If this is not possible, alternative methods include discarding a small volume of the collected blood before sampling, or ensuring that as many hours as possible have elapsed between the end of the infusion and blood collection.
A number of methods are currently under study that will facilitate sampling and/or minimise draw volume using different matrices and less invasive techniques (e.g. dried blood on paper, saliva).
Pharmacodynamic parameters (MIC)Ideally, PK/PD indices should be estimated using the established MIC value of the microorganism causing the infection. However, this value may not be available or the pathogen may be unknown and empirical treatment may be needed. In these cases, consider using ECOFF or EUCAST, or defer TDM until microbiological culture results are available. Another option is to use the most frequent MIC value for that strain, taking local epidemiology into account.
Dosing adjustments based on the results of TDMRegarding the estimation of individual PK parameters for each paediatric patient and dosing recommendations based on TDM results, it is recommended, whenever possible, to use a population PK model within a Bayesian prediction program that has been specifically validated for paediatric and/or neonatal populations. Subsequently, plasma concentrations should be rechecked whenever dose adjustments are made, especially for drugs with nonlinear kinetics.
AntibioticsAminoglycosidesAminoglycosides are hydrophilic drugs with high polarity. Thus, they have poor capacity to cross biological membranes (blood–brain barrier, bronchial secretions, vitreous humour). Their antibacterial activity is compromised in acidic media, presence of cations, hyperosmolar environments, and the presence of pus or mucus. Elimination is via the renal route.8
Neonates show great intra- and inter-individual variability associated with changes in aminoglycoside Vd and renal Cl. These parameters are influenced by body weight (direct relationship with Vd), gestational age at delivery (maturational state of the elimination organs), and post-natal age (renal Cl increases more rapidly after birth). Weight-adjusted Vd will be higher in preterm infants because they have a higher proportion of body water in their tissues.8,37
Aminoglycosides exhibit concentration-dependent bactericidal activity with moderate/prolonged post-antibiotic effect. Increased efficacy of aminoglycosides is associated with (Cmax)/MIC=8–10, while toxicity is associated with Cmin.7 To minimise blood draws in the paediatric and neonatal population, a single draw at 8–12 h is usually made to allow estimation of the Cmin and Cmax using population kinetic models. Recently, AUC0–24h/MIC has been suggested as an ratio index for dosing adjustment, especially in complicated infections.38
GlycopeptidesGlycopeptides are water-soluble antibiotics with low oral bioavailability (BA) and therefore require intravenous administration. Vancomycin is well distributed in most body fluids and tissues. It has low/moderate plasma protein binding (PPB) (25%–50%) and moderate to variable penetration into bone (5%–67%) and the central nervous system (CNS) (20%, increased in case of meningitis). In contrast, teicoplanin has a higher PPB (90%) and highly variable tissue concentrations.10 Elimination of glycopeptides is mainly renal. The presence of comorbidities and concomitant medications may influence the tissue distribution, elimination, and toxicity of vancomycin.39 As with aminoglycosides, neonates exhibit significant variability in plasma vancomycin concentrations. Several major factors may cause nephrotoxicity and reduced renal function, including body weight and height, post-menstrual and post-natal age, and intrinsic neonatal factors such as immaturity, or certain drug treatments.
The bactericidal activity of glycopeptides is time- and concentration-dependent.
The PK/PD parameter associated with increased efficiency is the AUC0–24h/MIC ratio. The established value for methicillin-resistant Staphylococcus aureus is 400–600 mg·h/L, but it is less well defined for other species. Although vancomycin has traditionally been considered time-dependent and Cmin has been the main PK/PD parameter, the new American guidelines recommend the use of AUC0–24h/MIC for monitoring to avoid possible overexposure and associated nephrotoxicity.11,40 CoNS infections in neonates are noteworthy, as there is a low risk of therapeutic failure of vancomycin, and a lower AUC0–24h/MIC value (between 240 and 480) may be appropriate.41
Regarding teicoplanin, although the target of AUC0–24h/MIC in adults is well defined (750–900), it is currently recommended to monitor Cmin in both adults and paediatric patients.12
LinezolidLinezolid is a lipophilic antibiotic with 100% oral BA independent of food intake and good tissue distribution (90% to CNS, 20% to bone). PPB is low (30%) and 60% of the metabolism is hepatic through non-enzymatic oxidation rather than via cytochrome p-450, leading to the production of practically inactive metabolites. Renal excretion is 80% (30% unchanged and 50% as inactive metabolites). Population studies have identified body weight and aspartate aminotransferase (AST) values as the covariates with the greatest impact on linezolid Cl. Higher AST values have been associated with higher AUC0–24h, and higher body weight with higher CL.15
Linezolid has bacteriostatic activity against staphylococci and enterococci, and bactericidal activity against streptococci and pneumococci. These activities are time- and concentration-dependent. The PK/PD parameters predictive of efficacy of linezolid are an AUC0–24h/MIC between 80 and 120 or the percentage of time between 2 consecutive doses during which the drug concentration is above the MIC (fT>MIC), which should be between 80% and 100%. However, in routine clinical practice, it is usually considered as time-dependent and Cmin is used.16
In cases of infections caused by microorganisms with a MIC >1 mg/L, this target cannot be reached with the standard dose (10 mg/kg/8 h) and a larger one should be administered.16
Beta-lactamsBeta-lactam antibiotics are primarily hydrophilic. PPB varies significantly between drugs (20%–30% for piperacillin-tazobactam, 16%–19% for cefepime, 10%–15% for ceftazidime, and 2% for meropenem), while renal excretion is the primary route of elimination. Their hydrophilic nature means that Vd is affected by systemic inflammatory response syndrome, fluid therapy, or the use of vasopressors. This effect more pronounced in critical patients and particularly in septic, neutropenic, and/or burn patients.
Beta-lactam bactericidal activity shows time-dependent PDs. The most important PK/PD parameter for efficacy is fT>MIC. Currently, there is disagreement on the optimal value of the steady-state free concentration of the drug, ranging from 1 to 4–6 times above the MIC at 100% fT>MIC. Several studies have shown a higher probability of achieving target PK/PD with extended or continuous infusion of piperacillin-tazobactam, ceftazidime, and meropenem in cancer patients with febrile neutropenia, infections caused by microorganisms classified as “susceptible, increased exposure” by EUCAST, or in critical patients.17,30,32,34,42,43
Currently, there is limited evidence on the impact of beta-lactam TDM on clinical outcomes in the paediatric population. For this reason, monitoring is mainly recommended for certain antibiotics, targeted therapy for difficult-to-treat infections, certain clinical situations (e.g. septic shock), patients with devices that alter PK parameters (e.g. ECMO, renal replacement therapy), and/or suspected beta-lactam toxicity (especially in the case of carbapenemics).34
AntifungalsThere are varying levels of recommendation and evidence regarding the need for TDM for different families of antifungals. This consensus document focuses on the triazoles because they have the strongest evidence of the clinical benefit of TDM and because they are widely used in immunocompromised paediatric patients. Notably, isavuconazole has been included even though it is currently not approved for paediatric use. Approval is expected soon and its use in clinical practice is increasing.
Finally, monitoring of all patients on flucytosine, although its use is currently rare, is recommended to ensure efficacy and because of its high toxicity.19,44
TriazolesTriazoles (and azoles in general) are lipophilic drugs, with varying degrees of lipophilicity depending on their structure. For example, itraconazole has a very lipophilic tail compared to other azoles. Their oral BA is generally good (>50%). Food intake can alter BA: it is increased by the use of itraconazole capsules and posaconazole oral suspension (especially with high-fat foods), and decreased by voriconazole and itraconazole (oral suspension).44 Unlike fluconazole and isavuconazole, food intake does not affect posaconazole absorption when administered as a gastro-resistant tablet. Triazoles are widely distributed in the body with good penetration into most tissues. Elimination of fluconazole is mainly renal (80%), whereas for others such as itraconazole, voriconazole, posaconazole, and isavuconazole, elimination occurs via hepatic metabolism. Several factors may influence elimination, including age, concomitant medication, and genetic polymorphisms of cytochrome P450, particularly the CYP2C19 isoenzyme for voriconazole.45 Due to its long plasma elimination half-life (t1/2), loading doses are needed to reduce the time required to reach steady state. Some of these drugs have nonlinear PK due to potential saturation of hepatic metabolism. As a result, small increases in dose can significantly increase plasma concentrations and t1/2. Most relevant interactions of azoles are due to their ability to inhibit cytochrome P450 enzymes. They all inhibit CYP3A4 isoenzyme. Isavuconazole also inhibits CYP3A5 and voriconazole inhibits CYP2C9. A number of them also have an inducing effect.46
Azoles have concentration- and time-dependent fungistatic activity against Candida spp. and time-dependent fungicidal activity against Aspergillus spp. Cmin is the main TDM PK/PD parameter. The optimal value of Cmin depends on the type of azole and its prophylactic or therapeutic indication.47
Tables 2 and 3 show the indications, procedures, interpretation, and use of TDM for posaconazole, itraconazole, voriconazole, and isavuconazole. Although TDM is not typically recommended for fluconazole, monitoring may be needed in specific patients with renal impairment, and/or on renal replacement therapy, with severe burns, and with infections in hard-to-access sites.19,44
ConclusionsOptimised PK/PD parameters are fundamental tools in the selection, use, and dosing of antibiotics and antifungals in paediatrics. TDM of these drugs can be very useful in clinical decision-making to maximise the clinical efficacy of antimicrobial therapy, minimise the likelihood of adverse effects, and prevent the emergence of resistance. The evidence supporting TDM in paediatrics varies between different families of antibiotics and antifungals. It is well established for aminoglycosides, vancomycin and voriconazole, and it is recommended in all paediatric patients. However, the evidence is much weaker for others, such as beta-lactams, linezolid, and isavuconazole, where TDM is recommended only in selected patients, pending further clinical evidence in the paediatric population to justify TDM on a routine basis.
The availability and type of analytical techniques used in TDM, as well as their implementation or interpretation, may vary widely between hospitals, making it difficult to standardise this clinical practice. To ensure consistent practice, a working methodology and evidence-based recommendations for dosing adjustments should be established. In addition, the implementation of clinical PK units within antimicrobial therapy optimization programs is essential. This consensus document aims to provide standardised protocols for the implementation of antibiotic and antifungal TDM in paediatrics, taking into account the variability of the paediatric population and the PK characteristics of each drug. Finally, although TDM is a very useful tool for optimising antimicrobial therapy in this population, any recommendation for dose adjustment must be made by consensus in multidisciplinary teams, while taking into account the microorganism involved, the individual characteristics of each patient, and their particular clinical situation.
Ethical responsibilitiesThe authors accept the responsibilities defined by the International Committee of Medical Journal Editors (available at http://www.icmje.org/ and in Hospital Pharmacy).
FundingNone of the authors have received funding for this work.
CRediT authorship contribution statementSonia Luque: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Methodology, Investigation, Formal analysis, Conceptualization. Natalia Mendoza-Palomar: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Formal analysis, Conceptualization. David Aguilera-Alonso: Writing – review & editing, Visualization, Validation, Methodology, Investigation, Formal analysis. Beatriz Garrido: Writing – review & editing, Visualization, Validation, Methodology, Investigation, Formal analysis. Marta Miarons: Writing – review & editing, Visualization, Supervision, Investigation, Formal analysis. Ana Isabel Piqueras: Writing – review & editing, Validation, Methodology, Investigation, Formal analysis. Enrique Tévar: Writing – review & editing, Visualization, Validation, Methodology, Investigation, Formal analysis. Eneritz Velasco-Arnaiz: Writing – review & editing, Visualization, Validation, Investigation, Formal analysis. Aurora Fernàndez-Polo: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
The above-mentioned authors have written the article on behalf of the following groups:
SEFH Working Group on Pharmaceutical Care in Infectious Diseases:
Coordinator: Leonor Periañez Parraga (Hospital Universitari Son Espases, Mallorca)
Deputy Coordinator/Secretary: Sònia Luque Pardos (Hospital del Mar, Barcelona)
Advisory spokespersons:
M. Victoria Gil Navarro (Hospital Universitario Virgen del Rocío, Sevilla)
José María Gutiérrez Urbón (Hospital Juan Canalejo, La Coruña)
Coordinating Committee:
Carmen Rodríguez González (Hospital General Universitario Gregorio Marañón, Madrid)
Beatriz Mejuto Perez del Molino (Complejo Hospitalario Universitario de Santiago de Compostela, Santiago de Compostela)
Eva Campelo Sanchez (Hospital Álvaro Cunqueiro, Eoxi Vigo)
Francisco Moreno Ramos (Hospital Universitario La Paz)
Maria Eugenia Martínez Núñez (Hospital Universitario de Getafe, Madrid)
María Eugenia Rodríguez Mateos (Hospital Puerta del Mar, Cádiz)
María Núñez Núñez (Hospital Universitario Clínico San Cecilio, Granada)
Svetlana Sadyrbaeva Dolgova (Hospital Universitario Virgen de las Nieves, Granada)
SEFH Spokesperson: Aurora Fernàndez Polo (Hospital Universitari Vall d'Hebron, Barcelona)
Resident: Alba Pau Parra (Hospital Universitari Vall d'Hebron, Barcelona)
SEFH Pharmacokinetics and Pharmacogenetics Working Group (PKGen):
Coordinator: Enrique Tévar Alfonso (Hospital Universitario Nuestra Señora de la Candelaria, S/C de Tenerife)
Vice-coordinator: Begoña Porta Oltra (Hospital Universitario Doctor Peset, Valencia)
Team members:
Azucena Aldaz Pastor (Clínica Universitaria, Navarra)
M. Dolores Aumente Rubio (Hospital Reina Sofía, Córdoba)
M. Dolores Bellés Medall (Hospital General Universitario, Castellón)
Remedios Marqués Miñana (Hospital La Fe, Valencia)
Patricio Mas Serrano (Hospital General Universitario, Alicante)
Jose Germán Sánchez Hernández (Complejo Asistencial Universitario, Salamanca)
Javier Milara Paya (Hospital General, Valencia)
Dolors Soy Muner (Hospital Clinic, Barcelona)
SEFH Paediatric Pharmacy Working Group:
Coordinator:M. Teresa Pozas del Río (Hospital Infantil Universitario Niño Jesús, Madrid)
Secretary: Belén Rodríguez Marrodán (Hospital Universitario Puerta de Hierro, Madrid)
Team members:
Concha Álvarez del Vayo (Hospital Universitario Virgen del Rocío, Sevilla)
María Teresa Bosch Peligero (Hospital Germans Trias i Pujol, Barcelona)
Maria José Cabañas Poy (Hospital Universitario Vall d'Hebron, Barcelona)
Raquel Escrig (Hospital Universitario y Politécnico La Fe, Valencia)
Cecilia M Fernández-Llamazares (Hospital General Universitario Gregorio Marañón, Madrid)
Carmen Gallego Fernández (Hospital Materno Infantil HUR, Málaga)
Ana García Robles (Hospital Universitario y Politécnico La Fe, Valencia)
Yolanda Hernández Gago (Complejo Hospitalario Universitario Insular, Gran Canaria)
Cristina Martínez Roca (Complejo Hospitalario Universitario de A Coruña, A Coruña)
Miquel Villaronga Flaqué (Hospital Sant Joan de Déu, Barcelona)
Marta Miarons Font (Consorci Hospitalari de Vic, Barcelona)
Beatriz Garrido Corro (Hospital Virgen de la Arrixaca, Murcia)
SEIP Bacterial Infections Working Group:
Coordinator: Leticia Martínez Campos (Hospital Universitario Torrecárdenas, Almería)
Secretary: Jesús Saavedra Lozano (Hospital Gregorio Marañón, Madrid)
Team members:
David Aguilera Alonso (Hospital Gregorio Marañón, Madrid)
Cristina Calvo Rey (Hospital la Paz, Madrid)
Jaime Carrasco Colom (Hospital Universitario Son Espases, Mallorca)
Elena Colino Gil (Hospital de las Palmas, Gran Canaria)
David López Martín (Hospital Costa del Sol, Marbella)
Ana Isabel Menasalvas Ruiz (Hospital Virgen de la Arrixaca, Murcia)
Esmeralda Núñez Cuadros (Hospital Carlos Haya, Málaga)
Carlos Rodrigo Gonzalo de Liria (Hospital Vall d'Hebrón, Barcelona)
SEIP Invasive Fungal Infection Working Group:
Coordinator: Peter Olbrich (Hospital Virgen del Rocío, Sevilla)
Secretary: Begoña Carazo Gallego (Hospital Carlos Haya, Málaga)
Team members:
Laura Ferreras Antolín (St. George's Hospital, London)
Carlos Daniel Grasa Lozano (Hospital La Paz, Madrid)
Natalia Mendoza Palomar (Hospital Vall d'Hebron, Barcelona)
Marisa Navarro Gómez (Hospital Gregorio Marañón, Madrid)
Olaf Neth (Hospital Virgen del Rocío, Sevilla)
José Tomás Ramos Amador (Hospital Clínico San Carlos, Madrid)
Elena Rincón López (Hospital Gregorio Marañon, Madrid)
Sílvia Simó Nebot (Hospital Sant Joan de Déu, Barcelona)
Pere Soler Palacín (Hospital Vall d'Hebrón, Barcelona)
SEIP Antimicrobial Optimisation Programmes Working Group (PROA):
Coordinator: Fernando Baquero Artigao (JD SEIP, Hospital La Paz, Madrid)
Secretary: Leticia Martínez Campos (JD SEIP- GT IA SEIP, H. Torrecárdenas, Almería)
Team members:
Carlos Rodrigo Gonzalo de Liria (GT-IB SEIP, Hospital Germans Trias i Pujol, Barcelona)
José Tomás Ramos Amador (GT-IF-SEIP, Hospital Clínico San Carlos, Madrid)
Cristian Launes Montaña (GT-IR-SEIP, Hospital Sant Joan de Deu, Barcelona)
Maria Carmen Suarez Arrabal (GT IyF-SEIP, C.S. Sardinero, Santander)
Luis Escosa García (GT IRAS, Hospital La Paz, Madrid)
Susana Melendo Pérez (Hospital Vall d'Hebron, Barcelona)
David Aguilera Alonso (Hospital Gregorio Marañón, Madrid)
Walter Goycoechea Valdivia (Hospital Virgen del Rocío, Sevilla)
Eneritz Velasco Arnaiz (Hospital Sant Joan de Déu, Barcelona)
Cristina Epalza Ibarrondo (Hospital 12 de Octubre, Madrid)
Marta García Ascaso (Hospital Niño Jesús, Madrid)
SEIP Healthcare-Related Infections Working Group:
Coordinator: Luis Escosa (Hospital la Paz, Madrid)
Team members:
Walter Alfredo Goycochea Valdivia (Hospital Virgen del Rocío, Sevilla)
Ana Isabel Menasalvas Ruiz (Hospital Virgen de la Arrixaca, Murcia)
Olaf Neth (Hospital Virgen del Rocío, Sevilla)
Anabel Piqueras (Hospital la Fe, Valencia)
María del Mar Santos Sebastián (Hospital Gregorio Marañón, Madrid)
Eneritz Velasco (Hospital Sant Joan de Deu, Barcelona)
David Aguilera Alonso (Hospital Gregorio Marañón, Madrid)
Laura Martín (Hospital Regional de Málaga)
Daniel Blázquez Gamero (Hospital 12 de Octubre, Madrid)