To determine the prevalence of reconciliation errors on admission to hospital in the pediatric onco-hematological population in order to check whether they are similarly susceptible to these reconciliation errors as adults and to describe the characteristics of the patients who suffer them.
MethodsA 12-month prospective, multicentre study of medication reconciliation on admission in the pediatric onco-hematological population to assess the incidence of reconciliation errors and to describe the characteristics of the patients.
ResultsMedication reconciliation was performed in 157 patients. At least a medication discrepancy was detected in 96 patients. Of the discrepancies detected, 52.1% were related to patient's new clinical situation or by the physician, while 48.9% were determined to be reconciliation errors. The most frequent type of reconciliation error was the “omission of a medication”, followed by “a different dose, frequency or route of administration”. A total of 77 pharmaceutical interventions were carried out, 94.2% of which were accepted. In the group of patients with a number equal to or greater than 4 drugs in home treatment, there was a 2.1-fold increase in the probability of suffering a reconciliation error.
ConclusionsIn order to avoid or reduce errors in one of the critical safety points such as transitions of care, there are measures such as medication reconciliation. In the case of complex chronic pediatric patients, such as onco-hematological patients, the number of drugs as part of home treatment is the variable that has been associated with the presence of medication reconciliation errors on admission to hospital, and the omission of some medication was the main cause of these errors.
Determinar la prevalencia de errores de conciliación al ingreso hospitalario en la población pediátrica onco-hematológica para comprobar si ésta presenta una susceptibilidad similar a la de los adultos para sufrir estos errores de conciliación y describir las características de los pacientes que los sufren.
MétodoEstudio prospectivo y multicéntrico, de 12 meses de duración, de conciliación de medicación al ingreso en población pediátrica onco-hematológica para evaluar la incidencia de errores de conciliación y describir las características de los pacientes en los que se producen.
ResultadosSe concilió la medicación de 157 pacientes. En 96 pacientes se detectó al menos 1 discrepancia de la medicación. De las discrepancias detectadas el 52,1% fueron justificadas por la nueva situación clínica del paciente o por el médico responsable mientras que el 48,9% se consideraron errores de conciliación. El tipo de error de conciliación más frecuente fue la «omisión de algún medicamento», seguido por «una dosis, frecuencia o vía de administración diferente». Se efectuaron un total de 77 intervenciones farmacéuticas, de las que se aceptaron el 94,2%. En el grupo de pacientes con un número igual o mayor a 4 fármacos en tratamiento domiciliario se observó un incremento de 2,1 veces la probabilidad de sufrir un error de conciliación.
ConclusionesPara evitar o reducir los errores en uno de los puntos críticos de seguridad como son las transiciones asistenciales, existen medidas, como la conciliación de la medicación. En el caso de los pacientes pediátricos crónicos complejos, como los pacientes onco-hematológicos, el número de fármacos como parte del tratamiento domiciliario es la variable que se ha asociado a la presencia de errores de conciliación al ingreso hospitalario, siendo la omisión de algún medicamento la causa principal de estos errores.
Este estudio aporta datos sobre la incidencia de errores de conciliación al ingreso hospitalario de pacientes onco-hematológicos pediátricos. Analiza además las posibles variables implicadas en la aparición de este tipo de errores (demográficas, derivadas de la complejidad del tratamiento…) y realiza un análisis univariante para determinar el peso que cada una de esas variables pudiera tener sobre el riesgo de aparición de un error de conciliación.
Los resultados de este estudio permitirán el desarrollo de estrategias de selección y priorización de pacientes susceptibles de sufrir errores de conciliación en las transiciones asistenciales. De esta manera los farmacéuticos implicados en el cuidado de la población pediátrica onco-hematológica podrán incluir la conciliación de la medicación como una herramienta más en el proceso de atención farmacéutica de estos pacientes.
IntroductionThe drug utilization process is typically complex and error-prone, and involves several stages. Medication errors (MEs) are defined as any preventable event occurring through action, or the lack of action, at any stage of the of the medication use process, which may or may not result in harm to the patient.1 Up to 60% of MEs occur during care transitions.2
According to Medication safety in transitions of care, a technical report published in 2017 by the World Health Organization (WHO),3 between 3 and 97% of adult patients and between 22 and 72% of pediatric patients experience at least one medication discrepancy on admission. The report therefore proposes to improve safety in three main areas, including transitions of care, particularly for vulnerable patients. Pediatric patients play an important role within this category4 as they are exposed to three times more potential adverse events (AEs)5 and are much less tolerant of MEs than adults.
One of the main strategies proposed to reduce the incidence of MEs during care transitions is medication reconciliation (MR), a process whose implementation and development has been promoted by various Spanish and international institutions, such as the WHO,6 the Institute for Safe Medication Practices (ISMP),7 the National Institute for Health and Care Excellence (NICE),8 the Joint Commission on Accreditation of Healthcare Organizations (JCAHO),9 the Spanish Ministry of Health,10 and the Health Service of the Community of Madrid.11
MR has been defined as the formal and standardized process of obtaining a complete list of the medications a patient was taking prior to the last transition of care, comparing it with the patient's active prescription, and resolving any discrepancies to ensure that patients receive the medication required for any chronic conditions they may have, adapted to their current clinical situation.12 The MR process consists of several stages13 aimed at obtaining a complete list of the medications a patient is taking. Obtaining this list is a complex endeavor, and is the key part of the MR process. The rest of the process depends on the quality of the medication list obtained. A discrepancy is any difference between the medication a patient was taking at home before the last transition of care and the medication prescribed in hospital. A discrepancy in itself is not necessarily an error. In fact, most discrepancies are usually due to the need to adjust the patient's chronic medication to their new clinical status or to the need for the patient to undergo tests or procedures with which their usual medication could interfere. These are known as justified discrepancies. When discrepancies do not respond to a voluntary adjustment of the medication to the patient's new clinical situation, they are considered to be reconciliation errors (REs).
Between 2004 and 2019, more than 300 articles were published on on-admission MR in adult patients, while only 17 were published in the pediatric population, and none of them in Spain. Analysis of a representative sample of these articles shows that 23%–87% of the adult patients studied had at least one ME performed on admission. Similarly, the percentage of pediatric patients who had at least one RE ranged from 7% to72%.14–17
The risk of error at the different stages of the drug use process is significantly higher in the case of complex chronic patients. The management of hemato-oncological conditions requires the use of a large number of drugs, many of which have a narrow therapeutic index, a large number of drug–drug interactions, and an increased risk of adverse effects, all of which tend to lead to frequent hospitalizations during the course of treatment because of complications arising from the conditions themselves or the treatment regimens required. In this regard, Iturgoyen et al.18 have shown that patients with hemato-oncologic conditions are at a higher risk of MEs, and should be prioritised in the medication reconciliation process.
For the above reasons, and considering that the published data on pediatric patients, albeit much smaller in number, show a similar prevalence of REs in children and adults, the present study sought to determine the prevalence of on-admission REs in a group of pediatric hemato-oncologic patients to find out whether these patients are as susceptible to on-admission REs, i.e., whether an MR program for newly admitted pediatric hemato-oncologic patients could be as useful in preventing REs as in the adult population.
MethodsThis was a prospective multicenter study on MR carried out in 11 Spanish hospitals with a pediatric hospitalization unit. It lasted 12 months and included pediatric patients with hemato-oncologic diseases aged between 0 and 18 years. Subjects could be admitted at any time during the course of the study.
Patients who were hospitalized for 24 hours or less, and those for whom a clinical interview (with them or their carer) was not possible, or for whom information about their usual treatment could not be reliably obtained, were excluded from the analysis.
To obtain the required information on the patients' pharmacotherapeutic history (PTH), a review of their hospital and primary care records, as well as of medical and nursing reports from other hospitals, was carried out. A clinical interview was also conducted with the patients and/or their carers.
Once sufficient information on each patient's PTH was obtained, an analysis was made of the potential discrepancies between their usual medication regimen and the medication prescribed to them on admission. A comparison was made between each patient's PTH and the medication prescribed to them on admission, taking into consideration their current clinical situation, the reasons given by the prescribing physician and the indications associated with the prescribed pharmacological treatment.
Discrepancies were classified as justified or unjustified according to the Consensus Document on terminology and classification in medication reconciliation published by the Spanish Society of Hospital Pharmacists (2009)13, the latter being considered as REs potentially responsible for MEs. When a RE was identified, the prescriber was contacted. All such communications were recorded for subsequent analysis.
The variables examined in the study, in addition to patients' demographics and place of origin, included the reason for admission and the unit to which they were admitted; the underlying condition and any drug allergies or intolerances; medications they were taking (patients taking four or more home medications were considered polymedicated); the number of narrow therapeutic index drugs (NTIDs) used; and any discrepancies found, the type of discrepancies and the drug involved.
The data werecollected using the free software REDcap (Research Electronic Database Capture), a web-basedtool for the management, design and coordination of multicenter clinical trials.
Statistical analysis of the data was performed using the SAS® 9.4. statistical package. Quantitative variables were described using descriptive statistical measures such as mean and median. The Shapiro-Wilks, the chi-squared test and Fisher's Exact Test were used as appropriate.
The study was classified as a non-post-authorization study by the Spanish Agency for Medicines and Medical Devices and was approved by the Ethics Committee of the lead institution involved in the study.
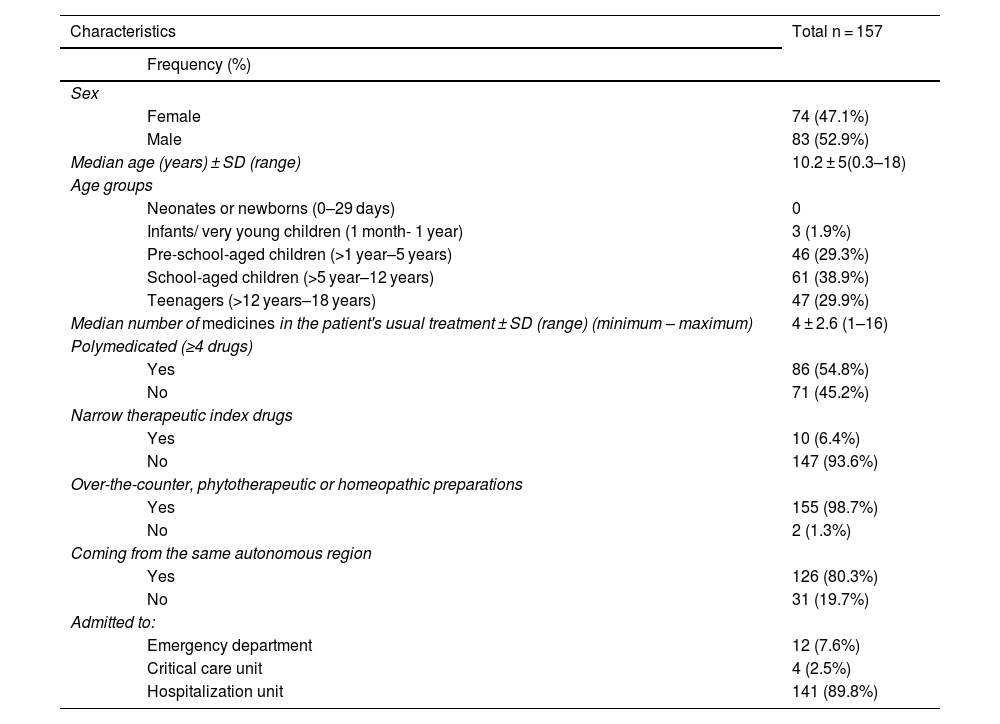
ResultsDescription of the study sampleAdmission MR was performed on157 patients, whose sociodemographic characteristics are summarized in Table 1.
Sociodemographic characteristics of patients included in the study.
| Characteristics | Total n = 157 | |
|---|---|---|
| Frequency (%) | ||
| Sex | ||
| Female | 74 (47.1%) | |
| Male | 83 (52.9%) | |
| Median age (years) ± SD (range) | 10.2 ± 5(0.3–18) | |
| Age groups | ||
| Neonates or newborns (0–29 days) | 0 | |
| Infants/ very young children (1 month- 1 year) | 3 (1.9%) | |
| Pre-school-aged children (>1 year–5 years) | 46 (29.3%) | |
| School-aged children (>5 year–12 years) | 61 (38.9%) | |
| Teenagers (>12 years–18 years) | 47 (29.9%) | |
| Median number of medicines in the patient's usual treatment ± SD (range) (minimum – maximum) | 4 ± 2.6 (1–16) | |
| Polymedicated (≥4 drugs) | ||
| Yes | 86 (54.8%) | |
| No | 71 (45.2%) | |
| Narrow therapeutic index drugs | ||
| Yes | 10 (6.4%) | |
| No | 147 (93.6%) | |
| Over-the-counter, phytotherapeutic or homeopathic preparations | ||
| Yes | 155 (98.7%) | |
| No | 2 (1.3%) | |
| Coming from the same autonomous region | ||
| Yes | 126 (80.3%) | |
| No | 31 (19.7%) | |
| Admitted to: | ||
| Emergency department | 12 (7.6%) | |
| Critical care unit | 4 (2.5%) | |
| Hospitalization unit | 141 (89.8%) | |
SD: standard deviation.
A total of 186 discrepancies were identified, of which 97 (52.1%) were considered justified due to the patient's new clinical situation or on the basis of the treating physician's judgment; 86 (48.9%) were considered to be due to REs. At least one discrepancy was found in 96 patients, representing 61% of the total sample. The maximum number of discrepancies found in a single patient was 6 (range: 1–6).
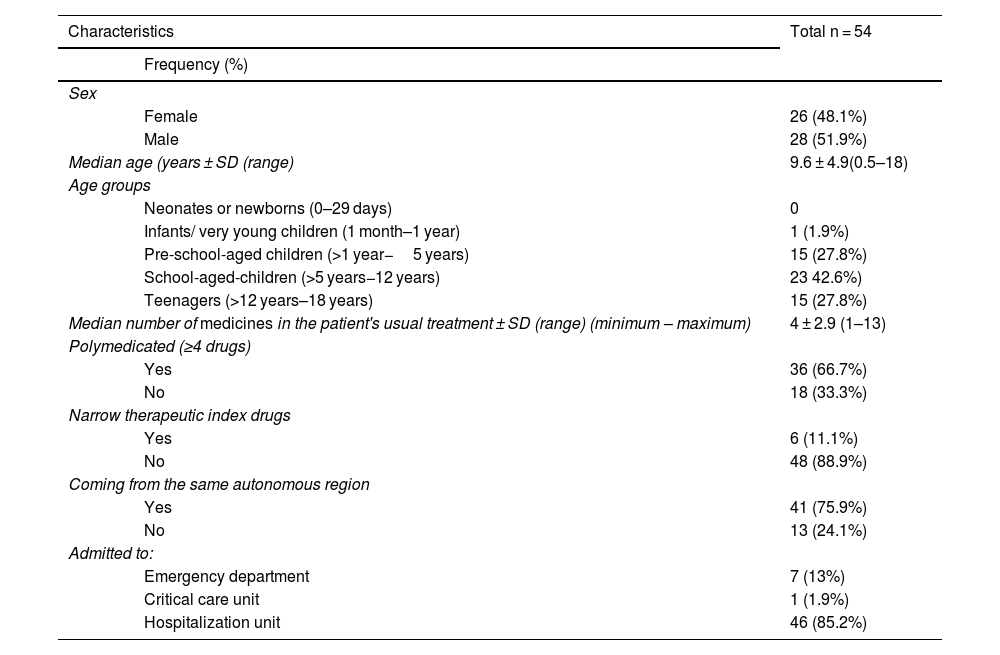
The prevalence of REs was 34.4% (54 of 157 patients with at least one RE). Table 2 shows the characteristics of the patients experiencing with at least one RE. None of the patients in whom REs were observed used homeopathic preparations at home.
Characteristics of patients with reconciliation errors.
| Characteristics | Total n = 54 | |
|---|---|---|
| Frequency (%) | ||
| Sex | ||
| Female | 26 (48.1%) | |
| Male | 28 (51.9%) | |
| Median age (years ± SD (range) | 9.6 ± 4.9(0.5–18) | |
| Age groups | ||
| Neonates or newborns (0–29 days) | 0 | |
| Infants/ very young children (1 month–1 year) | 1 (1.9%) | |
| Pre-school-aged children (>1 year−5 years) | 15 (27.8%) | |
| School-aged-children (>5 years−12 years) | 23 42.6%) | |
| Teenagers (>12 years–18 years) | 15 (27.8%) | |
| Median number of medicines in the patient's usual treatment ± SD (range) (minimum – maximum) | 4 ± 2.9 (1–13) | |
| Polymedicated (≥4 drugs) | ||
| Yes | 36 (66.7%) | |
| No | 18 (33.3%) | |
| Narrow therapeutic index drugs | ||
| Yes | 6 (11.1%) | |
| No | 48 (88.9%) | |
| Coming from the same autonomous region | ||
| Yes | 41 (75.9%) | |
| No | 13 (24.1%) | |
| Admitted to: | ||
| Emergency department | 7 (13%) | |
| Critical care unit | 1 (1.9%) | |
| Hospitalization unit | 46 (85.2%) | |
SD: standard deviation.
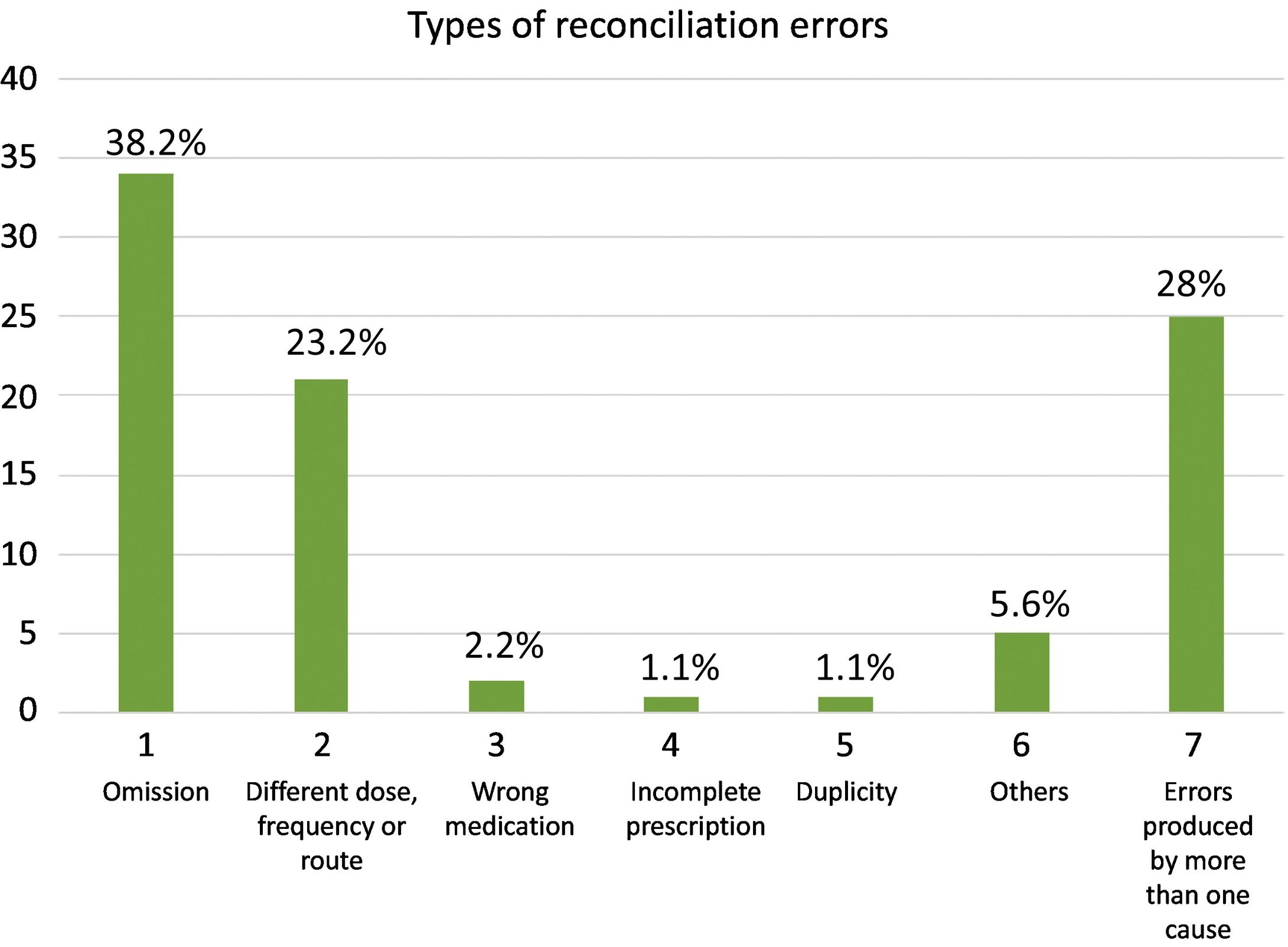
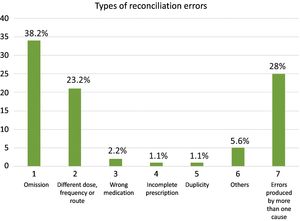
The most common type of RE was “omission of a drug” (38.2%), followed by “error in the frequency or route of administration of a drug” (23.6%). Other REs included «incomplete prescription» (1.1%), «wrong drug» (2.2%) and «therapeutic duplication» (1.1%). Some REs could not be classified in these categories because they were due to more than one cause. Fig. 1 shows the types of REs identified.
The anatomical areas or pharmacological groups most frequently involved in REs were: WHO ATC codes A (alimentary tract and metabolism) (31%), N (nervous system) (25%), R (respiratory system) (20%) and J (anti-infectives for systemic use) (13%). By subgroup, the most frequently implicated drugs were those with codes R03 (drugs for obstructive airway diseases) (12%), N03 (antiepileptics) (11%) and J01 (antibacterials for systemic use) (11%), followed by A2 (drugs for acid-related disorders) (9%), A11 (vitamins) (8%), (psycholeptics) (7%) and N06 (psychoanaleptics) (5%).
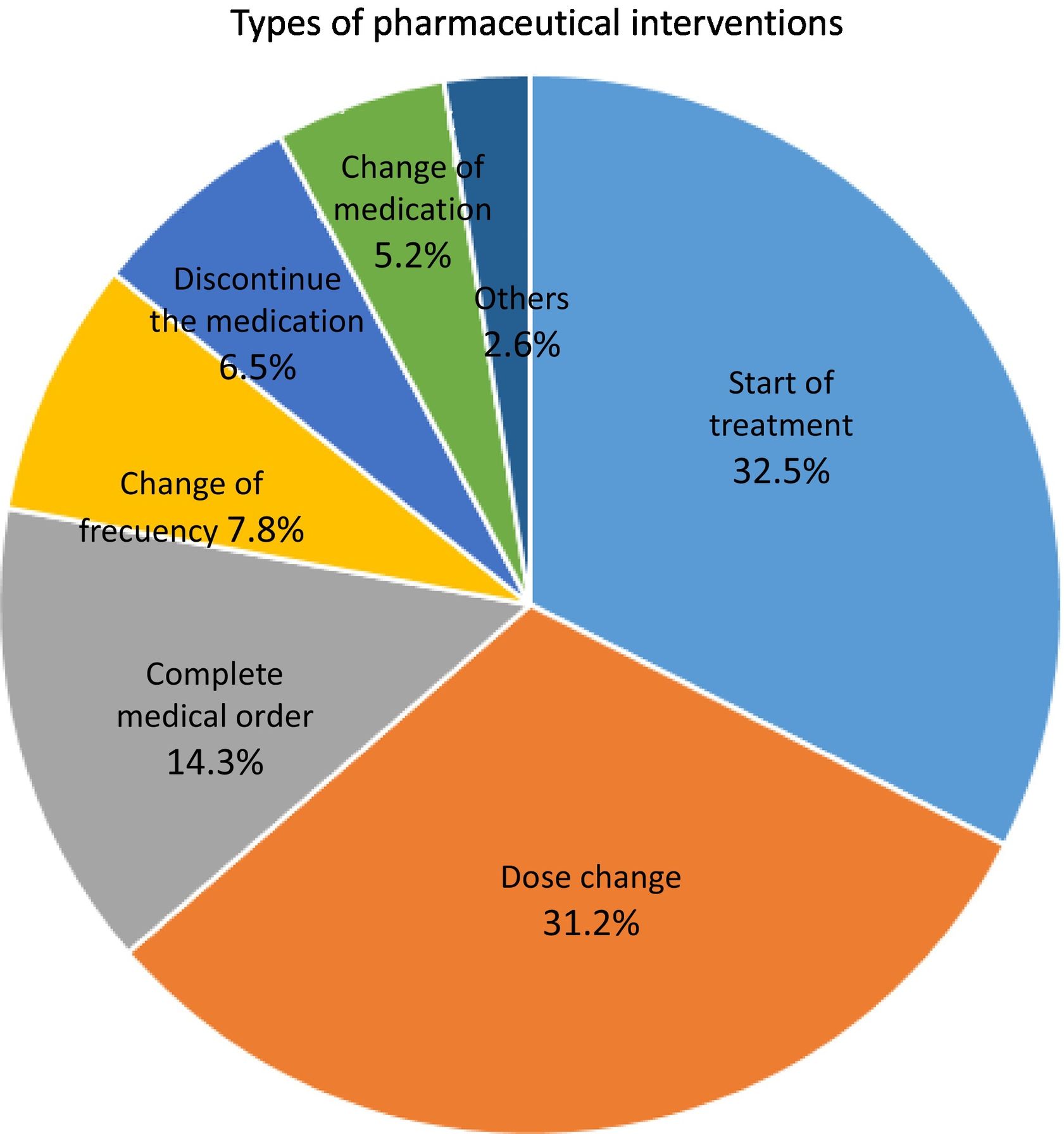
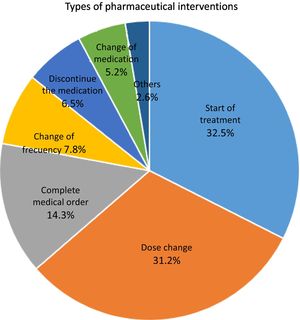
Analysis of the pharmaceutical interventions performedA total of 77 pharmaceutical interventions (PIs) were performed. In 94.2% of these, the attending physician admitted to having incurred an RE. The different types of PI performed are shown in Fig. 2. The most common PIs were: «start of treatment» (32.5% of the total) and «dose change» (31.2% of the total). These PIs coincided with the two most common REs recorded (omission of a drug and use of a different dose). Of the 77 PIs performed in total, 16 were related to safety (19.8%), 42 were related to effectiveness (51.9%) and 23 were related to both safety and effectiveness (28.4%).
Univariate analysisA comparative analysis was performed between patients with and without REs in terms of their demographic characteristics, their usual treatment, the number of home medicines they were taking and the NTIDs they were taking. Only the variable “number of home medications” showed statistically significant differences between patients with and without REs. A total of 48.5% of patients without REs were taking four or more home drugs as compared with 66.7% in the group with REs (p = 0.0424). Patients taking four or more medicines at home were found to be 2.1 times more likely to experience REs.
DiscussionMEs are among the most frequent causes of injuries and preventable harm in the health systems of the world over. Patient safety is of upmost importance in clinical practice.MEs can occur at any stage of the drug utilization process with a greater impact in specific environmentslike the inpatient setting. This could be attributed to the locationof severe and acute clinical cases and the application of complex drug regimens. Adverse drug events are more likely to affect young children and elderly patients.
In 2017, the WHO initiated the “Medication without harm” scheme under its third Global Patient Safety Challenge. The program comprised several measures to ensure safe pharmacological practices and reduce the incidence of severe preventable medication-related harm.
Several studies have shown that on-admission MR in adult hemato-oncologic patients is effective in identifying and addressing MEs and problems related to medication.19–21 In particular, Damlien et al.19 found at least one discrepancy in 80% of patients, and Kraus et al.20 reported an incidence of 63.6%. In the current study, at least one discrepancy was found in 61% of patients.
In a study involving144 hemato-oncologic pediatric patients, Schuch et al.22 found a 14% incidence of REs, with 43% of polymedicated patients experienced at least one RE. The higher percentages found in the present study (49% of REs and 68% of polymedicated patients with at least one RE) could be due to the fact that the percentage of polymedicated patients in Schuch et al. was lower compared to these series (36% vs. 55%).
In their study on hospitalized adult hemato-oncologic patients, Moghli et al.23 found a slightly higher incidence of REs than our study in pediatric patients (66.3% vs. 49%). This may be due to the fact that the mean number of drugs used by patients in Moghli et al. was 6.5 as compared with 5.1 in this study. The most common type of RE in Moghli et al.23 was omission, with the majority of errors ocurring in drugs classified under the ATC code A (alimentary tract and metabolism). These findings are consistent with the outcomes of our study.
Son et al.21 identified that the most frequent PIs were related to the treatment duration and to the requirement to add an extra drug (due to the most frequently detected REs in our study, i.e., omission of a drug). Likewise, the most common RE identified by Schuch et al. 22 was the omission of a drug.
Damlien19 and Kraus20 found a correlation between the amount of medication patients habitually use and the occurrence of discrepancies. In our study, we identified a statistically significant correlation between the number of drugs taken by patients at home and the incidence of REs.
The study's most striking revelation was the overwhelming prevalence of homeopathic/phytotherapeutic preparations available over the counter in our sample (99%). This highlights the crucial role that pharmacists play in reviewing the pre-admission treatments of such patients and identifying potential interactions between these products and the prescribed treatments provided during their hospital stay.
The results of this study may be valuable to hospital pharmacists involved in the area of pediatrics, specifically hemato-oncologic pediatrics, as they may help them in the improved selection of polymedicated patients on admission and facilitate the reconciliation of their medication.
One of the strengths of this study is its multicenter design, covering Spain comprehensively and reducingpotential risks from patient managementduring transitions of care across different regions of the country. However, the COVID-19 pandemic presented the main limitation by occasionally hindering both the reconciliation process and patient interviews, resulting in the potential loss of patients.
This study revealed the existence of a significant amount of involuntary discrepancies, which could lead to REs upon admission. Most of these REs related to home medication being omitted from hospital prescriptions. Our findings emphasize the necessity ofimplementing new approaches to medication management during admission to decrease the occurrence of REs.
The performance of similar studies, but including complex pediatric patients suffering from diseases other than cancer, could offer valuable insights in determining whether the results of this analysis can be generalized to the entire pediatric patient population.
Contribution to the literatureThis study sheds light on the incidence of on-admission reconciliation errors amongpediatric hemato-oncologic patients. It also investigates the potential variables, either demographic or resulting from the complexity of the treatment, that could be associated to the appearance of such errors. A univariate analysis is presented to determine how each variable may influence the occurrence of REs. The results of the study may guide the development of strategies for selecting and prioritizing patients at risk of REs during care transitions. This would allow pharmacists working with hemato-oncologic patients to incorporate REs into the pharmaceutical care protocol.
Ethical responsibilitiesPursuant to Spanish Organic Data Protection Law 3/2018 and Regulation 2016/679 of the European Parliament and of the Council of 27 April 2016, the patients' personal data was protected through a correlative numerical code preceded by the hospital's initials.
AuthorshipMargarita Cuervas-Mons Vendrell participated in the planning and design of the study, as well as in the collection of data, the interpretation of results and the writing of the manuscript.
Dolores Pilar Iturgoyen Fuentes participated in the collection of data, the interpretation of results and in the writing of the manuscript.
Miquel Villaronga Flaque, María José Cabañas Poy, Cecilia M. Fernández-Llamazares, Concha Álvarez Del Vayo, Carmen Gallego Fernández, Cristina Martínez Roca, Yolanda Hernández Gago, Ana García Robles y Beatriz Garrido Corro participated in the collection of data.
All co-authors carried out a critical review of the manuscript, approved the final version and agreed to it being submitted for publication.
FundingNone.
S Manrique Rodriguez, C Martinez Fernandez-Llamazares, I Taladriz Sender (Hospital General Universitario Gregorio Marañón, Madrid). C Salazar Valdebenito, J Arrojo Suarez, A Font Barceló, M Villaronga Flaque (Hospital Sant Joan de Déu, Barcelona).
B Rodriguez Marrodán, JA Alcaraz Lopez, C Lozano Llano (Hospital Universitario Puerta de Hierro, Madrid).
B Garrido Corro, MJ Blazquez Alvarez (Hospital Virgen de la Arrixaca, Murcia). D Gonzalez Andres, AM Agüi Callejas, I García López (Hospital Infantil Universitario Niño Jesús, Madrid). C Martinez Roca, C Fernandez Oliveira (Complejo Hospitalario La Coruña).
AA Garcia Robles, A Ferrada Gasco (Hospital Universitario y Politécnico La Fe, Valencia). C Gallego Fernandez, C Fernandez Cuerva, JM Fernandez Martin, A Pintado Alvarez, L Yunquera Romer, JJ Alcaraz Sanchez (Hospital Regional Universitario Carlos Haya, Málaga).
A Pau Parra, I Jimenez Lozano, B Garcia Palop, MJ Cabañas Poy, CJ Parramón Teixido (Hospital Universitario Vall'Hebron, Barcelona). M Moleon Ruiz, FJ Araujo Rodriguez, M Mejías Trueba, A Tristancho Pérez, C Alvarez Del Vayo Benito, B Fernandez Rubio, H Rodriguez Ramallo, N Báez Gutiérrez (Hospital Universitario Virgen del Rocío, Sevilla). C Otero Villalustre, Y Hernández Gago, D Diego Dorta Vera, D Fernandez Vera, I Ruiz Santos (Complejo Hospitalario Universitario Insular, Gran Canaria).