To describe the experience of home antibiotic infusion therapy using elastomeric infusion pumps, administered to patients admitted to the Home Hospitalisation Unit of a tertiary hospital for 3 years and to analyse clinical evolution and mortality.
MethodRetrospective observational study. The medical history of the patients included in the study was reviewed. Information was obtained on personal history, antimicrobial therapy received, and clinical evolution. Statistical analysis was performed using SPSS® 19 software.
Results81 patients were included, 61.7% men, with a mean age of 73.5±17.5 years. The most frequent comorbidities were diabetes mellitus (30.9%) and chronic kidney disease (28.4%). Patients received a mean of 11.9±8.5 days of antibiotic treatment in an elastomeric infusion pump. The main focus of infection was respiratory (27.2%), followed by bacteremia (16%) and skin and soft tissue infections (12.3%). Of the infections, 65.4% were monomicrobial, with Pseudomonas aeruginosa being the main microorganism involved (39.6%). The most commonly used antimicrobial was piperacillin/tazobactam (33.3%). The clinical course was good in 85.2% of the patients, but the mortality rate in the 30 days following the end of treatment was 24.7%. In the univariate analysis, a history of neoplasia in the last 5 years (p=.01) and having received fewer days of antibiotic therapy prior to the start of outpatient antimicrobial therapy in infusion pump (p=.04) were associated with worse clinical outcome.
Age over 80 years was associated with better outcome (p=.03). The diagnosis of heart failure was associated with higher mortality (p=.026) and patients from surgical services, with lower mortality (p=.047). In the multivariate analysis, the presence of neoplasia was associated with unfavourable evolution (p=.012) and heart failure with higher mortality (p=.027).
ConclusionsOutpatient antimicrobial therapy in elastomeric infusion pumps is an alternative in patients requiring prolonged intravenous treatment, and age is not a conditioning factor for inclusion in these programs. However, the presence of certain comorbidities can negatively affect the clinical course and mortality of patients.
Describir la experiencia de uso de la terapia de infusión domiciliaria de antibióticos mediante bombas de infusión elastoméricas, administrada a pacientes ingresados en la Unidad de Hospitalización a Domicilio de un hospital de tercer nivel durante tres años y analizar evolución clínica y mortalidad.
MétodoEstudio observacional retrospectivo mediante revisión de las historias clínicas de los pacientes incluidos. Se obtuvo información sobre antecedentes personales, terapia antimicrobiana recibida y evolución clínica. Análisis estadístico realizado mediante el software SPSS® 19.
ResultadosSe incluyeron 81 pacientes, 61,7% hombres, con una media de edad de 73,5 ± 17,5 años. Las comorbilidades más frecuentes fueron diabetes mellitus (30,9%) y enfermedad renal crónica (28,4%). Los pacientes recibieron de media 11,9 ± 8,5 días de antibiótico en bombas de infusión elastoméricas. El principal foco infeccioso fue el respiratorio (27,2%), seguido de bacteriemia (16%) e infecciones de piel y partes blandas (12,3%). El 65,4% de las infecciones fueron monomicrobianas, siendo la Pseudomonas aeruginosa el principal microorganismo implicado (39,6%). El antimicrobiano más utilizado fue piperacilina/tazobactam (33,3%). El 85,2% de los pacientes presentó buena evolución clínica pero la tasa de mortalidad en los 30 días posteriores a la finalización del tratamiento fue de 24,7%. En el análisis univariante, se asociaron a peor evolución clínica los antecedentes de neoplasia en los últimos 5 años (p = 0,01) y haber recibido menos días de antibioterapia previo al inicio del tratamiento antibiótico domiciliario en infusor (p = 0,04). La edad mayor de 80 años se asoció a mejor evolución (p = 0,03). Se asoció a mayor mortalidad el diagnóstico de insuficiencia cardíaca (p = 0,026) y a menor mortalidad los pacientes procedentes de servicios quirúrgicos (p = 0,047). En el análisis multivariante, la presencia de neoplasia se asoció a evolución desfavorable (p = 0,012) y la insuficiencia cardíaca, a mayor mortalidad (p = 0,027).
ConclusionesLa terapia antibiótica domiciliaria en bombas de infusión elastoméricas es una alternativa en pacientes que necesitan tratamiento intravenoso prolongado, sin ser condicionante para la inclusión en estos programas la edad. No obstante, la presencia de ciertas comorbilidades puede afectar negativamente a la evolución clínica y mortalidad de los pacientes.
In recent decades, there has been a significant interest in developing programmes to enable the home-based management of conditions that traditionally require hospitalisation. One example is the development of outpatient parenteral antimicrobial therapy (OPAT).
This approach is defined as the administration of parenteral antimicrobial therapy in at least 2 doses on different days in patients' homes.1 Since its emergence in the 1970s,2 OPAT has become increasingly relevant in the management of patients requiring prolonged antibiotic therapy.3
A key element in the success of this programme is the right choice of administration method. The most commonly used antimicrobials are those administered as a single daily dose. The use of elastomeric infusion pumps for drug administration is of great interest for drugs that require more than one daily dose.4 These systems are sterile transparent devices that have an internal balloon or reservoir that allows the drug to be released at a constant rate. They are lightweight and require no external power source, so patients have full mobility.5 The flow rate of elastomeric infusion pumps can be affected by various factors, including temperature variation, fluid viscosity, position of the device relative to the vascular access point, and concentration of the antibiotic in the solution.6–8
The design of these devices represents a major breakthrough in OPAT. The main uses of these ambulatory continuous infusion pumps are the home-based administration of antimicrobial therapy and chemotherapy, symptomatic treatment in palliative care patients, and patient-controlled analgesia.9 In the case of antibiotic administration, continuous infusion has proven to be an effective strategy for optimising the pharmacokinetics and pharmacodynamics of time-dependent drugs, such as β-lactam antibiotics. The rationale for the efficacy of these antibiotics is directly related to the time in which the drug concentration exceeds the minimum inhibitory concentration (MIC) specific to the pathogen.10,11 Therefore, prolonging the infusion time provides more consistent serum levels while maintaining plasma drug concentrations above the MIC for a longer period of time, thereby improving the efficacy and likelihood of treatment success.12
In Spain, this form of therapy has been developed in different ways, depending on the needs and resources of each area. In Galicia, it is usually administered by home hospitalisation services (HHS); in fact, the Spanish Society of Home Hospitalisation has developed clinical guidelines for the administration of OPAT.4
The use of OPAT has several advantages, such as avoiding prolonged hospital stays, reducing the risk of nosocomial infections,13 and being a cost-effective treatment option.14 However, it also has its limitations, such as the requirement for patients to be in a stable clinical situation, have adequate family support, the availability of telephones/mobiles, and, in the case of our hospital, reside within the catchment area of the HHS.15
The present study is a result of the significant development of OPAT, and describes the experience of using home antibiotic infusion therapy with elastomeric infusion pumps. This therapy was administered to patients under the care of the HHS unit of a tertiary-level hospital over 3 years. The study also sought to identify potential variables that could help predict the clinical evolution and mortality of patients.
MethodRetrospective observational study based on a review of the clinical histories of patients receiving OPAT via elastomeric infusion pumps under the care of the Complejo Hospitalario Universitario de Lugo (Spain) from January 2019 to December 2022.
The study included all paediatric and adult patients transferred to the HHS for continuous antibiotic therapy via elastomeric infusion pumps following admission to conventional inpatient units. No exclusion criteria were defined.
We collected sociodemographic data (age and sex) and clinical data (originating service, comorbidities, site, type, and clinical course of infection, isolated microorganism, type and duration of antibiotic therapy, vascular access used, and treatment-related complications).
The following comorbidities were recorded: history of acute myocardial infarction, heart failure (HF), cerebrovascular disease, peripheral arterial disease, cognitive impairment, chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM), chronic kidney disease (CKD; defined as the presence of a glomerular filtration rate <60 mL/min/m2), liver disease, human immunodeficiency virus (HIV) infection, and current or past 5-year history of cancer. The age-adjusted Charlson Comorbidity Index (CCI) was used to measure the degree of comorbidity and analyse its impact on clinical evolution and mortality.
After the conclusion of OPAT for each patient, we reviewed the clinical histories and documented the clinical situation upon admission, the course of the patient's condition, and the resolution of the infection (or lack thereof) at discharge from the HHS. To assess mortality, these data were collected again after 30 days. At the scheduled follow-up visits, the HHS team assessed the evolution of infections using physical and laboratory tests, including blood pressure, temperature, leukocytosis, neutrophilia, C-reactive protein, and procalcitonin. Good clinical evolution was defined as the absence or disappearance of fever or signs or symptoms of infection, absence of unplanned hospital readmission, and no death in the month after stopping OPAT. Mortality was defined as death of the patient within 30 days of stopping treatment, whether related or unrelated to the treated infection.
Different elastomeric infusion pumps (Dosi-Fuser, Leventon [150 mL/24 h]; Dosi-Fuser, Leventon [250 mL/12 h]; Accufuser, Woo Young Medical [300 mL/24 h]; and Accufuser, Woo Young Medical [300 mL, variable rate]) were used depending on the antibiotic to be administered and the volume required for its dilution. They were prepared in laminar flow cabinets in the hospital pharmacy service. OPAT with elastomeric infusion pumps was started 1 h after a loading dose or 1 h after the last intermittent dose administered in the hospital. For the 24-h infusers, the HHS team was responsible for changing the infusers on a daily basis. However, for the 12-h infusors, a family member sometimes changed the infusers (self-administration).
The data obtained were used to create a database in Microsoft Excel. All subsequent statistical analyses were performed using SPSS 19 software. Quantitative variables are expressed as mean±standard deviation (SD) and qualitative variables are expressed as absolute value and percentage. In the univariate analysis, the 2 means were compared using the Student t-test and/or Mann–Whitney test. A p-value of ≤.05 was used as a cut-off for statistical significance. Odds ratios (OR) with their corresponding 95% confidence intervals (CIs) were calculated for qualitative variables. A multivariate analysis was then performed using logistic regression to assess the degree of association between clinical evolution and mortality: (1) the variables identified as significant in the univariate analysis and (2) the clinically relevant variables that could influence the final outcome. Hazard ratios (HRs) with their corresponding 95% CIs were calculated.
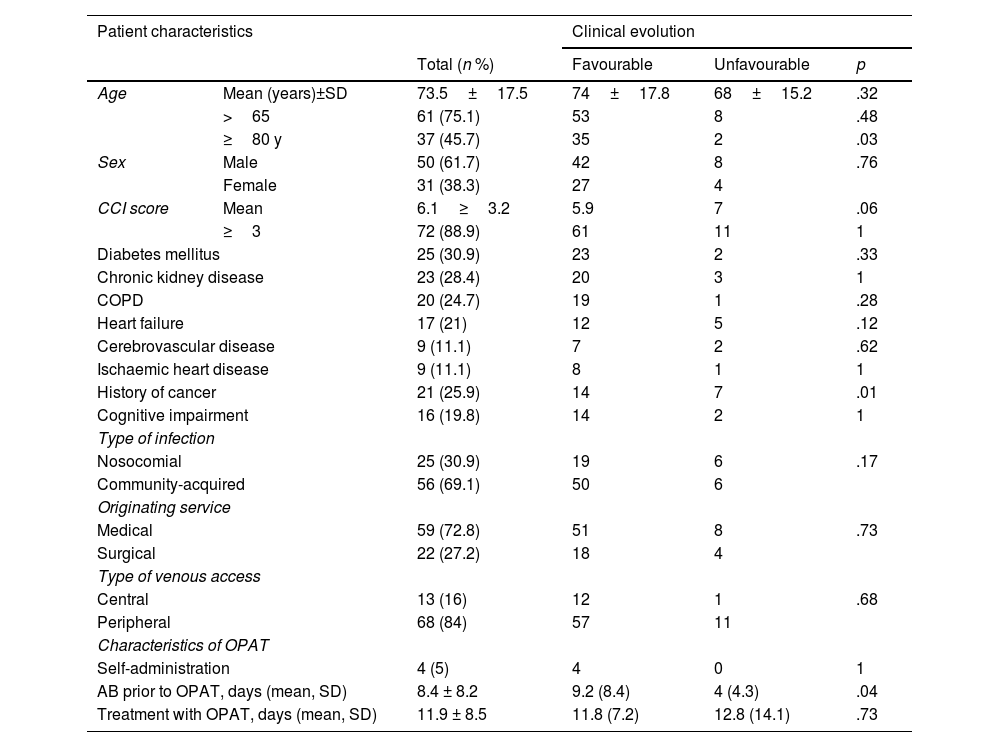
ResultsThe study included 81 patients receiving antibiotic treatment with elastomeric infusion pumps. Mean age was 73.5±17.5 years (age range: 6–102 years). Of the patients, 61.7% were male. Table 1 shows the main characteristics of the patients.
Patient characteristics and association with clinical evolution.
| Patient characteristics | Clinical evolution | ||||
|---|---|---|---|---|---|
| Total (n %) | Favourable | Unfavourable | p | ||
| Age | Mean (years)±SD | 73.5±17.5 | 74±17.8 | 68±15.2 | .32 |
| >65 | 61 (75.1) | 53 | 8 | .48 | |
| ≥80 y | 37 (45.7) | 35 | 2 | .03 | |
| Sex | Male | 50 (61.7) | 42 | 8 | .76 |
| Female | 31 (38.3) | 27 | 4 | ||
| CCI score | Mean | 6.1≥3.2 | 5.9 | 7 | .06 |
| ≥3 | 72 (88.9) | 61 | 11 | 1 | |
| Diabetes mellitus | 25 (30.9) | 23 | 2 | .33 | |
| Chronic kidney disease | 23 (28.4) | 20 | 3 | 1 | |
| COPD | 20 (24.7) | 19 | 1 | .28 | |
| Heart failure | 17 (21) | 12 | 5 | .12 | |
| Cerebrovascular disease | 9 (11.1) | 7 | 2 | .62 | |
| Ischaemic heart disease | 9 (11.1) | 8 | 1 | 1 | |
| History of cancer | 21 (25.9) | 14 | 7 | .01 | |
| Cognitive impairment | 16 (19.8) | 14 | 2 | 1 | |
| Type of infection | |||||
| Nosocomial | 25 (30.9) | 19 | 6 | .17 | |
| Community-acquired | 56 (69.1) | 50 | 6 | ||
| Originating service | |||||
| Medical | 59 (72.8) | 51 | 8 | .73 | |
| Surgical | 22 (27.2) | 18 | 4 | ||
| Type of venous access | |||||
| Central | 13 (16) | 12 | 1 | .68 | |
| Peripheral | 68 (84) | 57 | 11 | ||
| Characteristics of OPAT | |||||
| Self-administration | 4 (5) | 4 | 0 | 1 | |
| AB prior to OPAT, days (mean, SD) | 8.4 ± 8.2 | 9.2 (8.4) | 4 (4.3) | .04 | |
| Treatment with OPAT, days (mean, SD) | 11.9 ± 8.5 | 11.8 (7.2) | 12.8 (14.1) | .73 | |
AB, antibiotic; CCI, Charlson Comorbidity Index; COPD, chronic obstructive pulmonary disease; OPAT, outpatient parenteral antibiotic treatment; SD, standard deviation.
Regarding the originating service, 59 patients (72.8%) were from medical services and 22 (27.2%) were from surgical services.
The most frequent comorbidities were DM (30.9%), CKD (28.4%), a history of cancer in the last 5 years (25.9%), and COPD (24.7%). The patients had a mean CCI score of 6.1 and 88.9% had a CCI score of 3 or more.
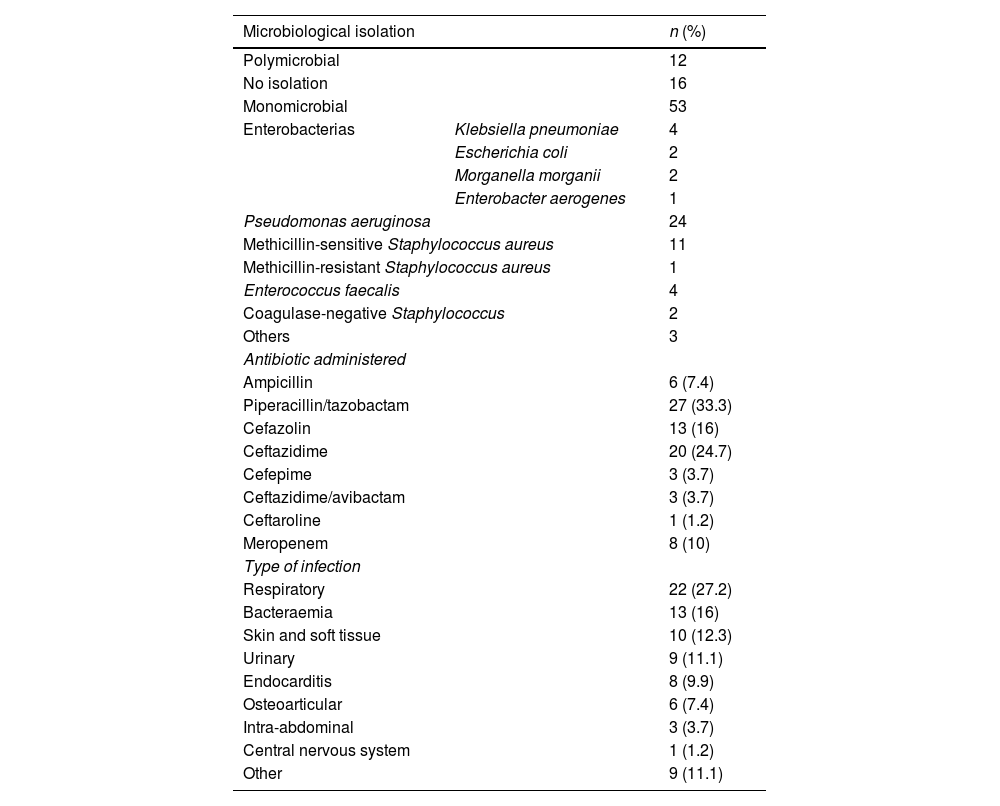
Overall, 69.1% of infections were community-acquired, the most common being respiratory infections (27.2%), bacteraemia (16%), skin and soft tissue infections (12.3%), and urinary tract infections (UTI) (11.1%). Treatment was empirical, without microbiological isolation, in 19.8% of cases, whereas treatment was targeted in 80.2% of cases. Most patients (65.4%) had a monomicrobial infection, whereas only 12 patients (14.8%) had a polymicrobial infection. Pseudomonas aeruginosa was the most commonly isolated organism (39.6%), followed by enterobacteria (23.6%). Within this group, Escherichia coli was isolated in 9 patients, 5 of whom had strains producing extended-spectrum β-lactamases.
The most commonly administered antimicrobial was piperacillin/tazobactam (33.3%), then ceftazidime (24.7%), and cefazolin (16%) (Table 2). Patients had received a mean of 8.4±8.2 days of antibiotic therapy prior to starting OPAT and subsequently received a mean of 11.9±8.5 days of home therapy using elastomeric infusion pumps. The mean duration of antibiotic treatment, both empirical and targeted, was approximately 20 days. If only intra-abdominal infections, osteoarticular infections, and endocarditis are considered, the mean duration of antibiotic therapy was 36.2±11 days.
Characteristics of treated infections.
| Microbiological isolation | n (%) | |
|---|---|---|
| Polymicrobial | 12 | |
| No isolation | 16 | |
| Monomicrobial | 53 | |
| Enterobacterias | Klebsiella pneumoniae | 4 |
| Escherichia coli | 2 | |
| Morganella morganii | 2 | |
| Enterobacter aerogenes | 1 | |
| Pseudomonas aeruginosa | 24 | |
| Methicillin-sensitive Staphylococcus aureus | 11 | |
| Methicillin-resistant Staphylococcus aureus | 1 | |
| Enterococcus faecalis | 4 | |
| Coagulase-negative Staphylococcus | 2 | |
| Others | 3 | |
| Antibiotic administered | ||
| Ampicillin | 6 (7.4) | |
| Piperacillin/tazobactam | 27 (33.3) | |
| Cefazolin | 13 (16) | |
| Ceftazidime | 20 (24.7) | |
| Cefepime | 3 (3.7) | |
| Ceftazidime/avibactam | 3 (3.7) | |
| Ceftaroline | 1 (1.2) | |
| Meropenem | 8 (10) | |
| Type of infection | ||
| Respiratory | 22 (27.2) | |
| Bacteraemia | 13 (16) | |
| Skin and soft tissue | 10 (12.3) | |
| Urinary | 9 (11.1) | |
| Endocarditis | 8 (9.9) | |
| Osteoarticular | 6 (7.4) | |
| Intra-abdominal | 3 (3.7) | |
| Central nervous system | 1 (1.2) | |
| Other | 9 (11.1) | |
The main methods of administration were peripheral venous access (84%), followed by peripherally inserted central venous catheter (PICC) (12.3%), and the use of an implanted reservoir (3.7%). Antibiotics were self-administered by 4.9% of the patients. Venous access complications occurred in 16% of patients, mostly leakage (n=5) or extravasation (n=5); 2 cases of mild phlebitis were reported.
In 85.2% of the patients, the course of the infection was favourable. When analysed by age group, 94% of patients older than 80 years had a favourable evolution. However, this percentage fell to 86% when analysing all patients older than 65 years. Overall, 50% of the patients with a poor clinical course had a nosocomial infection, and 41.6% received empirical antibiotic therapy. In the group of patients with a poor clinical course, the mean duration of antibiotic therapy before infuser placement was 4 days.
Only one patient experienced antimicrobial-related complications (nephrotoxicity) requiring dose adjustment. The 30-day mortality rate after completion of antibiotic treatment was almost 25% and the 30-day readmission rate was 22.2%.
In the univariate analysis, a history of cancer in the last 5 years (OR 5.50; 95% CI [1.51–19.96]; p=.01) and having received fewer days of antibiotic therapy before OPAT (OR 3.50; 95% CI [1.46–12.32]; p=.04) were associated with a worse outcome. Age over 80 years was associated with better clinical outcome (OR 0.19; 95% CI [0.40–0.95]; p=.03). Heart failure was the comorbidity associated with higher mortality (OR 4.10; 95% CI [1.46–13.79]; p=.026). Patients from surgical services had a lower mortality rate (OR 0.76; 95% CI [0.62–0.95]; p=.047).
In the multivariate analysis, only a history of cancer was associated with an unfavourable outcome (HR 5.35; 95% CI [1.45–19.74]; p=.012) and the presence of HF with higher mortality (HR 3.80; 95% CI [1.23–12.05]; p=.027).
DiscussionOPAT programmes were first implemented in the 1970s in the United States.1 Since that time, they have grown and evolved, with clear benefits for patients and healthcare systems. Within this therapy modality, the use of elastomeric infusion pumps is of great interest for the administration of antibiotics, especially time-dependent antibiotics.11
In our study, we found that several types of infections were treated by OPAT, with respiratory infections being the most common. This result is consistent with those of previous studies conducted in areas close to our centre,16 although a higher incidence of bacteraemia and a lower incidence of UTIs was observed.
The microbiological results of our study are also consistent with those of other studies, in which the main microorganism isolated was Pseudomonas aeruginosa and the main antibiotic used was piperacillin/tazobactam.17 Given that there are virtually no oral treatment options for this pathogen and that resistance to quinolones is increasing,18 it is logical that this is the most commonly used antimicrobial.
The mean total duration of antibiotic treatment was around 20 days, taking into account both empirical and targeted antibiotic treatment. Taking into account the recommendations outlined in infectious disease guidelines for certain diagnosed diseases in our patients (respiratory infection, UTI, or bacteraemia), it might appear that treatment duration was longer than necessary. However, it should be borne in mind that this figure is an average and is substantially influenced by the presence of complicated infections (e.g., necrotising pneumonia or intra-abdominal infections), along with cases of endocarditis and osteoarticular infections, where the recommended treatment duration is typically 4–6 weeks.19
One notable feature of our sample, as reflected in the results, is that it comprised an older population, with 45% of patients aged 80 years or more. This aspect contrasts with the age composition observed in other studies, where a significantly higher percentage of the population was under the age of 70 years.20 Although our sample consisted of an elderly population with a substantial burden of comorbidities, a high percentage of patients had a good clinical outcome. However, the 30-day readmission rate was higher than that found in other studies of similar populations.16 This variability may stem from a limitation in our study, as we did not analyse the cause of readmission, and therefore could not differentiate whether it was due to the original infectious process or another cause. In fact, previous studies have shown that of patients treated with OPAT who experience readmission, only 30% are readmitted due to a worsening of the original infection.21
It is striking that a worse clinical course was observed in patients aged between 65 and 80 years than in those older than 80 years. This finding is not in line with those of other studies, where worse outcomes were observed in older age groups,21 or in studies in which such an association22 was not observed in patients treated with OPAT. This difference is probably due to several factors. Firstly, the mean CCI score in the subgroup with the worst clinical outcome (7.8) was higher than the overall mean CCI score (6.1). Secondly, a high percentage of patients had nosocomial infections and received empirical treatment. Finally, the mean duration of antibiotic treatment before infuser placement was much shorter in the group of patients with a poor clinical course of infection (4 days vs 8.4 days). Complications arising from the infection may not have been detected early, possibly due to the lower frequency of patient observation by healthcare staff in HHS units.
In terms of the variables of interest for predicting the evolution of infections, our results are generally consistent with the available evidence, in that we found worse evolution in individuals with a current or past 5-year history of cancer.23 It is noteworthy that the patients from medical services had a higher mortality rate. This may be due to the fact that in our hospital, care is shared between the infectious disease service and the surgical service, which optimises antibiotic treatment. Moreover, this type of therapy was prescribed by various medical services, with a significant percentage of patients receiving it from the palliative care service.
The 30-day mortality rate was high at almost 25%, but it should be taken into account that almost 90% of the patients had a CCI score of 3 or more, 25.9% had a previous history of cancer, and 45.7% came from the palliative care service.
This study has several limitations, including its design. A prospective randomised study should be conducted to confirm our results and obtain more consistent data. The sample size may be seen as another limitation and an explanation for the lack of statistically significant associations between certain comorbidities and evolution and/or mortality, as found in other studies.24
Finally, another important aspect not addressed in this study is the monitoring of specific conditions that affect the flow rate of elastomeric infusion pumps. Poorly controlled elastomeric infusion pumps can lead to undermedication with the possibility of therapeutic failure, increased hospital readmissions, and the emergence of antibiotic resistance.8
Future studies of this type of therapy could incorporate the degree of patient and relative satisfaction—including reported results such as those collected through quality of life surveys—as well as cost analyses comparing the costs of this type of treatment with those of conventional hospitalisation.16
In conclusion, the use of elastomeric infusion pumps in OPAT programmes represents an enormous advance, enabling the therapeutic management of infections in the outpatient setting and serving as a viable option to consider in patients requiring prolonged intravenous antibiotic treatment. It has been widely reported in the literature that this modality has many benefits, including increased comfort, improved quality of life for patients and caregivers, reduced costs, and reduced hospitalisation-related complications without compromising treatment efficacy. Moreover, as mentioned above, it can be a useful and effective alternative in the elderly population. However, it is important to note that certain diseases or conditions may adversely affect the clinical outcome and mortality of patients treated with OPAT on elastomeric infusion pumps.
Contribution to the scientific literatureThis study presents efficacy and safety data on OPAT using elastomeric infusion pumps administered to patients under the care of the HHS unit of a tertiary-level hospital.
The study provides new evidence showing that it is a good option for managing patients who require intravenous antibiotic treatment over an extended period of time. It has many benefits for patients and the healthcare system.
Ethical responsibilitiesThe authors of this study declare that they have fulfilled all the ethical standards required for conducting the study.
Approval was granted by the Santiago-Lugo Research Ethics Committee. Registration code 2019/245.
FundingNone declared.
CRediT authorship contribution statementSara Ferro Rodríguez: Writing – review & editing, Writing – original draft, Software, Resources, Project administration, Methodology, Investigation, Data curation, Conceptualization. Yelco Chantres Legaspi: Writing – review & editing, Writing – original draft, Software, Project administration, Methodology, Investigation, Data curation, Conceptualization. Eva María Romay Lema: Visualization, Validation, Supervision. Blanca Ayuso García: Visualization, Validation, Supervision. Paloma Castellano Copa: Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Methodology, Investigation, Formal analysis. Pedro Peinó Camba: Visualization, Validation. Andrea Barcia Losada: Visualization, Validation, Supervision. Cristina Rodríguez Díaz: Visualization, Validation, Supervision.
We would like to thank Ramón Rabuñal Rey for his invaluable assistance with the statistical analysis and interpretation of the results.