To descrive the use of therapeutic plasma exchange in several pathologies and its adjustment to international reference guides.
MethodObservational, descriptive, retrospective study, of all the patients that received plasmapheresis between January 2014-December 2015. We analized the appropriate indication according to the bibliography consulted, and the therapeutic outcome. Indication, replaced volume of plasma, number of sessions and periodicity were established by the Hematology Service depending on the disease and its clinical course.
Results10 patients (8 women), between 28-72 years old, received therapeutic plasma exchange. The pathologies treated were neurological (9 patients), Waldenstrom disease (1 patient). The technique used was continuous centrifugation with albumin 5% as replacement fluid.
ConclusionsThe therapeutic plasma exchange in reviewed patients agreed to reference guides. There was not a direct relation between the recommendation grade and the response obtained. The reduced number of patients is a limitation to obtain conclusive results.
Describir la utilización del recambio plasmático terapéutico (RPT) en distintas patologías y su ajuste a las guías internacionales de referencia.
MétodoEstudio observacional, descriptivo y retrospectivo en pacientes que recibieron plasmaféresis entre enero de 2014 y diciembre de 2015. Se analizó la adecuación de su indicación según la bibliografía consultada, así como la respuesta obtenida. El Servicio de Hematología estableció la indicación, el volumen plasmático a recambiar, el número de sesiones y la periodicidad según la enfermedad de base y su evolución clínica.
ResultadosDiez pacientes (8 mujeres) entre 28 y 72 años de edad, recibieron RPT. Las patologías eran de origen neurológico (9 pacientes), enfermedad de Waldenström (1 paciente). La técnica utilizada fue centrifugación continua con albúmina 5% como líquido de reposición.
ConclusionesEl RPT en los pacientes revisados se ajustó a las guías de referencia. No se observó correlación directa entre el grado de recomendación establecido por dichas guías y la respuesta obtenida. El número reducido de pacientes supone una limitación a la hora de extraer resultados concluyentes.
Therapeutic Plasma Exchange (TPE) is an extracorporeal blood purification technique, which consists in the extraction of a specific volume of plasma that is exchanged for a replacement fluid. The aim of this technique is to remove large-molecular-weight substances, pathogens, or immunocomplexes circulating in the plasma that intervene in the pathological immune response and that are considered responsible for a disease or its clinical manifestations.
Although TPE has been used in more than 80 diseases, such as renal, metabolic, autoimmune, rheumatologic, haematologic, neurologic, digestive, and hepatic disease, there is relatively little experience worldwide with this technique. Because these diseases are uncommon, the use of TPE is supported by case series rather than by controlled studies, thus making it difficult to obtain a solid level of evidence.
Currently, TPE plays an important role mainly in the treatment of autoimmune disease and is a valid option in the case of disease refractory to conventional treatment. However, depending on the indications, great variations in outcomes have been observed in clinical practice. The American Society for Apheresis (ASFA) has published guidelines on the use of TPE. The guidelines establish 4 categories that are constantly reviewed and updated taking into account the data provided by published clinical research1.
The objective of this work was to describe the use of TPE in several pathologies and to determine adherence to accepted international guidelines.
MethodsAn observational descriptive retrospective study which included all patients who received TPE from January 2014 to December 2015 in a public tertiary hospital.
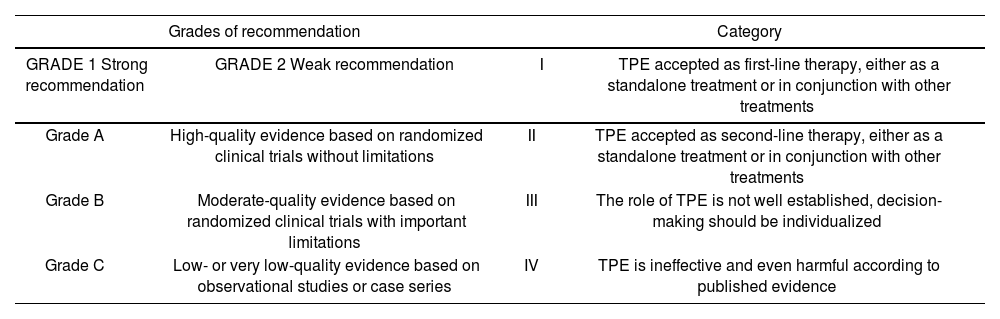
The ASFA guidelines were used to verify the appropriateness of the indications for TPE1,2. The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system was also used. This system assigns grades of recommendation based on the quality of the published evidence (Table 1)3.
Grades of recommendation and category for TPE of the American Society for Apheresis (ASFA)3
| Grades of recommendation | Category | ||
|---|---|---|---|
| GRADE 1 Strong recommendation | GRADE 2 Weak recommendation | I | TPE accepted as first-line therapy, either as a standalone treatment or in conjunction with other treatments |
| Grade A | High-quality evidence based on randomized clinical trials without limitations | II | TPE accepted as second-line therapy, either as a standalone treatment or in conjunction with other treatments |
| Grade B | Moderate-quality evidence based on randomized clinical trials with important limitations | III | The role of TPE is not well established, decision-making should be individualized |
| Grade C | Low- or very low-quality evidence based on observational studies or case series | IV | TPE is ineffective and even harmful according to published evidence |
TPE, Therapeutic plasma exchange. Extracted from: Laínez-Andrés JM et al. Recambio plasmático terapéutico: aplicaciones en Neurología. Rev Neurol. 2015;60(3): 120-31
The IANUS® electronic clinical record platform was used to obtain the following variables: age, sex, pathology, number of TPEs received in 1 year, replacement fluid used, duration of treatment, concomitant medication, adverse reactions, and response. All data were anonymised according to the procedure established by Law 41/2002 of November 14, 20024.
TPE was performed in the hospital blood bank using a Spectra Optia Apheresis System® with continuous flow centrifugation through a central catheter. Acid-citrate-dextrose (ACD) was used as the anticoagulant. Oral calcium carbonate was administered before the procedure to prevent hypocalcaemia. The extracted plasma volume (PV) was replaced with 5% albumin5.
The Haematology Service established indications, PV to be replaced, and the number and frequency of sessions in all patients based on the underlying disease and its clinical course.
The Pharmacy Service analysed the use of TPE for different pathologies by evaluating the appropriateness of indications using the criteria established by the ASFA and the available literature.
ResultsDuring the study period, TPE was ordered for 11 patients (8 women and 3 men) between 28 years and 72 years of age (median 46 years). Nine patients had pathologies of neurological origin (81.8% of patients), and 1 patient had Waldenström's macroglobulinemia. However, TPE was not performed in 1 patient with acute hypertriglyceridemia associated with severe pancreatitis (monitored in Intensive Care and Endocrinology and Nutrition Services) due to its successful treatment with standard measures (fluid therapy, insulin, diet).
In the group of 9 patients with neurological indications for different pathologies, TPE was used as first-line treatment in a patient with Guillain-Barré syndrome and as an alternative after the failure of standard treatment in the other 8 patients. In the haematologic patient with Waldenström's macroglobulinemia, TPE was used as a first-line treatment to reduce excess paraproteins prior to initiating specific treatment.
In all patients, a 5% human albumin solution equivalent to 1 to 1.5 PV was used as replacement fluid and adjusted according to patient tolerance. The mean replacement fluid volume was 1.27 PV.
During the study period, only 3 of the 10 treated patients underwent more than 1 TPE procedure. There was a median of 6 sessions per TPE procedure (range 1-12 sessions). The duration of each session ranged from 2 to 3 hours.
Concomitant medications administered during TPE were cyclosporine (1 patient), gentamicin (1 patient), amikacin (4 patients), and vancomycin (2 patients). Antiepileptic agents (phenobarbital, phenytoin, and valproic acid) were administered to 1 patient after TPE. Pharmacokinetic monitoring was used to determine and adjust the plasma levels of these agents5.
In all patients, the haematologist administered prophylactic oral calcium carbonate for hypocalcaemia before TPE and intravenous calcium after TPE as needed.
TPE was well tolerated in 70% of the patients, none of whom experienced adverse reactions. During the first session, 1 patient experienced nausea and vomiting, but subsequently showed improved tolerance to treatment. Two patients experienced central catheter-related infections.
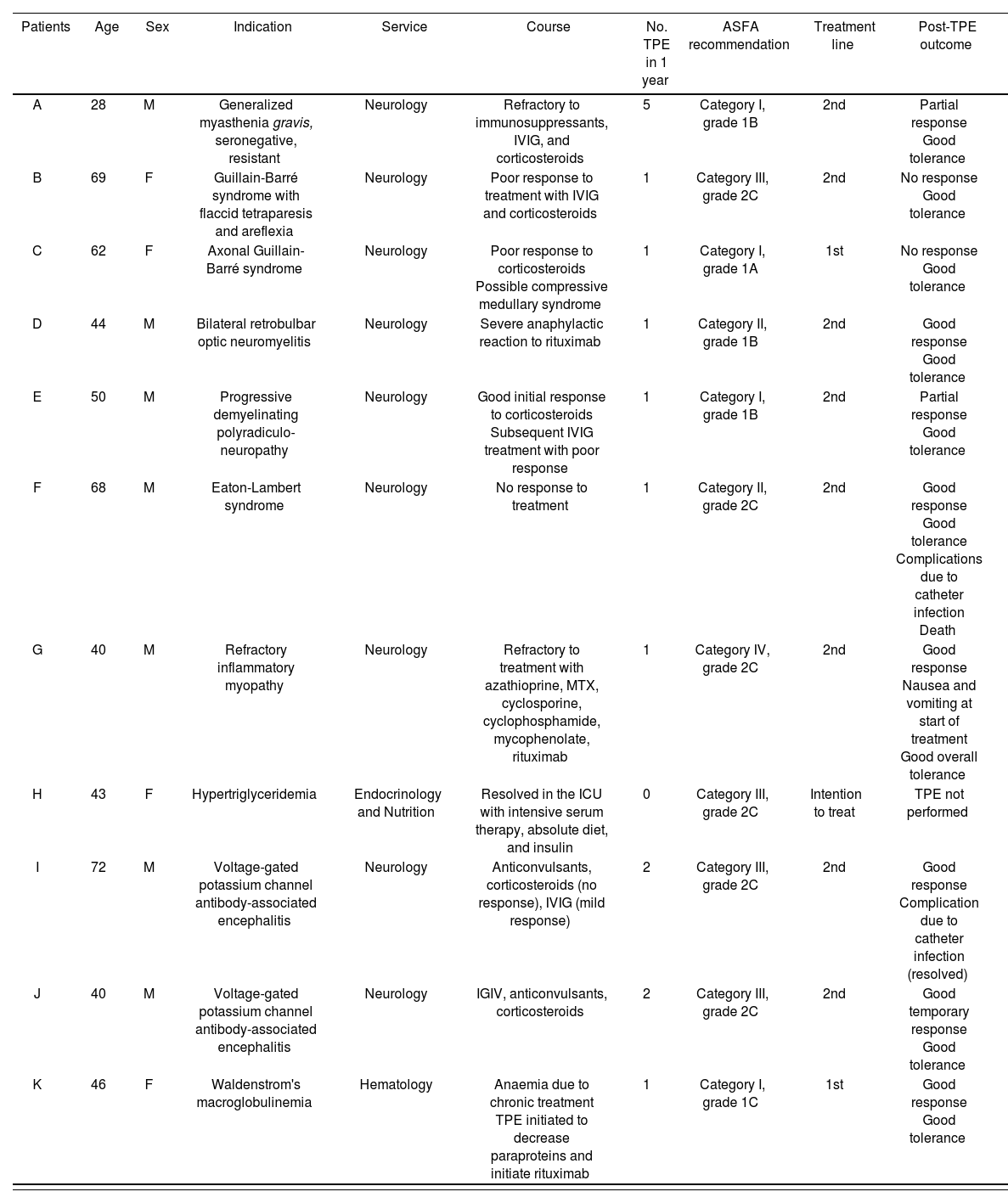
Table 2 shows the characteristics of each patient.
Summary of patient characteristics and outcomes after TPE.
| Patients | Age | Sex | Indication | Service | Course | No. TPE in 1 year | ASFA recommendation | Treatment line | Post-TPE outcome |
|---|---|---|---|---|---|---|---|---|---|
| A | 28 | M | Generalized myasthenia gravis, seronegative, resistant | Neurology | Refractory to immunosuppressants, IVIG, and corticosteroids | 5 | Category I, grade 1B | 2nd | Partial response Good tolerance |
| B | 69 | F | Guillain-Barré syndrome with flaccid tetraparesis and areflexia | Neurology | Poor response to treatment with IVIG and corticosteroids | 1 | Category III, grade 2C | 2nd | No response Good tolerance |
| C | 62 | F | Axonal Guillain-Barré syndrome | Neurology | Poor response to corticosteroids Possible compressive medullary syndrome | 1 | Category I, grade 1A | 1st | No response Good tolerance |
| D | 44 | M | Bilateral retrobulbar optic neuromyelitis | Neurology | Severe anaphylactic reaction to rituximab | 1 | Category II, grade 1B | 2nd | Good response Good tolerance |
| E | 50 | M | Progressive demyelinating polyradiculo-neuropathy | Neurology | Good initial response to corticosteroids Subsequent IVIG treatment with poor response | 1 | Category I, grade 1B | 2nd | Partial response Good tolerance |
| F | 68 | M | Eaton-Lambert syndrome | Neurology | No response to treatment | 1 | Category II, grade 2C | 2nd | Good response Good tolerance Complications due to catheter infection Death |
| G | 40 | M | Refractory inflammatory myopathy | Neurology | Refractory to treatment with azathioprine, MTX, cyclosporine, cyclophosphamide, mycophenolate, rituximab | 1 | Category IV, grade 2C | 2nd | Good response Nausea and vomiting at start of treatment Good overall tolerance |
| H | 43 | F | Hypertriglyceridemia | Endocrinology and Nutrition | Resolved in the ICU with intensive serum therapy, absolute diet, and insulin | 0 | Category III, grade 2C | Intention to treat | TPE not performed |
| I | 72 | M | Voltage-gated potassium channel antibody-associated encephalitis | Neurology | Anticonvulsants, corticosteroids (no response), IVIG (mild response) | 2 | Category III, grade 2C | 2nd | Good response Complication due to catheter infection (resolved) |
| J | 40 | M | Voltage-gated potassium channel antibody-associated encephalitis | Neurology | IGIV, anticonvulsants, corticosteroids | 2 | Category III, grade 2C | 2nd | Good temporary response Good tolerance |
| K | 46 | F | Waldenstrom's macroglobulinemia | Hematology | Anaemia due to chronic treatment TPE initiated to decrease paraproteins and initiate rituximab | 1 | Category I, grade 1C | 1st | Good response Good tolerance |
ASFA, American Society for Apheresis; ICU, Intensive Care Unit; IVIG, Intravenous immunoglobulin; MTX, Methotrexate; TPE, Therapeutic plasma exchange.
In this case series, the most common pathologies were of neurological origin. However, the most favourable result was obtained in the haematologic patient with Waldenström's macroglobulinemia6–8 (patient K; category I, grade 1C)1 in whom hyperviscosity caused by high IgM-protein levels prevented the initiation of treatment. After a single first-line TPE session, treatment with bortezomib and corticosteroids could be initiated. Clinical and analytical stability was achieved, making it unnecessary to initiate treatment with rituximab as originally planned. Despite the complexity of the patient's condition, TPE improved the result of the pharmacological treatment, thus avoiding the use of more complex and expensive therapeutic measures. The literature reports the use of TPE as a pre-treatment measure to reduce hyperviscosity due to high IgM levels, given that hyperviscosity and IgM levels can be reduced by 60% and by 50%, respectively8.
Of the 2 neurological patients diagnosed with Guillain-Barré syndrome3,9, patient C received TPE as a first-line treatment (category I, grade 1A)1. After failing to respond to initial treatment, patient B received TPE as a second-line treatment (category III, grade 2C)1, with poor outcome. Despite the high grade of recommendation for TPE in patient C, the patient did not respond as expected and had to be referred to the Rehabilitation Service. Patient B did not respond to TPE, and the subsequent course of the disease was torpid.
Despite category III and grade 2C1 for TPE, a very favourable initial response was obtained in the 2 patients (I and J) with voltage-gated potassium channel antibody-associated encephalitis10,11. Patient I was discharged with antiepileptic treatment without subsequent readmission, whereas in patient J, despite the good initial response to TPE, the course of the disease was torpid and further TPE procedures were performed after the study period. The patient died. In line with results reported in the literature11, the patients experienced a subjective temporary improvement that was associated with the TPE procedure.
Myasthenia gravis (category I, grade 1B)1 is a neurological indication for which TPE has been shown to be very effective. Patient A had generalized myasthenia gravis, seronegative, and refractory to treatment with immunosuppressants, intravenous immunoglobulins (IVIG), and steroids. TPE led to a partial improvement of symptoms. These results coincide with those of the published literature. A retrospective study by Kumar et al.12 in 35 patients with myasthenia gravis showed short-term improvements in myasthenic seizures. Mandawat et al.13 compared TPE and IVIG for the treatment of myasthenia gravis, and found that both treatments had clinically similar outcomes,
although they recommended IVIG for elderly patients with or without comorbidities and for all other patients with comorbidities. Trantafyllou et al.14 suggested that periodic TPE is safe and effective in controlling the symptoms of patients with moderate to severe myasthenia gravis nonresponsive to immunosuppressive therapy.
TPE was performed in patient G (40 years, with refractory inflammatory myopathy)3,5 who had been previously treated with azathioprine, methotrexate, cyclosporine, cyclophosphamide, mycophenolate, and rituximab without response. Although this disease is an indication (category IV, grade 2C)1 for TPE, this patient benefited from the procedure, with very positive results that led to hospital discharge with prednisone as the only treatment.
Patients D, E, and F had optic neuromyelitis, polyneuropathy, and Eaton-Lambert syndrome3, respectively. They had a good initial response to TPE, although the final outcomes differed between patients (see Table 2).
Adverse reactions to TPE were observed in only 30% of the patients, which is within the range described in the literature (25%-60%)5.
Since TPE may decrease the plasma levels of some drugs5, their pharmacokinetics should be monitored to ensure the efficacy of concomitant treatment.
In conclusion, 80% of the treated patients experienced improvements.
Although the ASFA guidelines were used to verify the appropriateness of the indications for TPE, no direct correlation was observed between the grade of recommendation and the response obtained. This study and its conclusions may be limited by the small sample size.
FundingNo funding declared.
Conflict of interestsNone declared.
Contribution to scientific literature
This study provides information on the use of therapeutic plasma exchange in the treatment of selected pathologies in a tertiary care hospital. The relevance of this information lies in the variability found in the literature on indications, guidelines, results obtained, and interactions with standard treatment.
Therapeutic Plasma Exchange is used in the treatment of several autoimmune diseases. These diseases have a low incidence. Information on this procedure will enhance the provision of more comprehensive pharmaceutical care in Hospital Pharmacy Services and contribute to obtaining the best possible therapeutic outcomes.