Ustekinumab is used in moderate-severe plaque psoriasis with inadequate response to anti-tumour necrosis factor a drugs. Recent studies support the need to assess real long-term data. The aim of this study was to evaluate the real long-term effectiveness and safety of ustekinumab in moderate-severe plaque psoriasis refractory to 2 anti-tumour necrosis factor a drugs.
MethodRetrospective descriptive study from January 2010 to March 2019. The study included patients with moderate-severe plaque psoriasis previously treated with at least 2 anti-tumour necrosis factor a biologic drugs. The effectiveness endpoints were Psoriasis Area and Severity Index 90 and 75 response rates at weeks 24, 48, 72, and 96. Safety was assessed using adverse effects and treatment withdrawal.
ResultsA total of 36 patients were included (men, 61%). Ustekinumab was used after treatment with 2 anti-tumour necrosis factor a drugs in 88.9% of patients. The biologic drugs most frequently administered prior to ustekinumab were infliximab (94.4%) and etanercept (91.7%). It was observed that at least 66.7% of patients reached Psoriasis Area and Severity Index 90 at weeks 24, 48, 72, and 96. Adverse effects were recorded in 6 patients. There were no treatment withdrawals.
ConclusionsUstekinumab showed real long-term effectiveness and safety in moderate-severe plaque psoriasis with inadequate response to 2 previous anti-tumour necrosis factor a drugs.
Ustekinumab se usa en psoriasis en placas moderada-grave con respuesta inadecuada a fármacos antifactor de necrosis tumoral α. Recientes estudios sostienen la escasez de resultados en vida real a largo plazo. El objetivo es evaluar la efectividad y seguridad de larga duración de ustekinumab en psoriasis en placas moderada-severa refractaria a dos fármacos antifactor de necrosis tumoral α.
MétodoEstudio descriptivo retrospectivo entre enero de 2010 y marzo de 2019. Se incluyeron pacientes con psoriasis en placas moderada-grave tratados previamente con al menos dos agentes biológicos antifactor de necrosis tumoral α. Las variables de efectividad fueron respuestas Psoriasis Area and Severity Index 90 y 75 a las 24, 48, 72 y 96 semanas. La seguridad fue valorada mediante reacciones adversas y suspensiones de tratamiento.
ResultadosSe incluyeron 36 pacientes. El 61% fueron varones. Ustekinumab fue usado tras dos fármacos antifactor de necrosis tumoral α en el 88,9% de los pacientes. Los agentes biológicos previos más frecuentes fueron infliximab (94,4%) y etanercept (91,7%). Se observó que, al menos, el 66,7% de los pacientes alcanzaron Psoriasis Area and Severity Index 90 en las semanas 24, 48, 72 y 96. Se registraron reacciones adversas asociadas a ustekinumab en 6 pacientes. No hubo suspensiones.
ConclusionesUstekinumab ha demostrado ser efectivo y seguro a largo plazo según resultados de vida real en psoriasis en placas moderada-severa tras respuesta inadecuada a dos fármacos antifactor de necrosis tumoral a.
Psoriasis is a chronic inflammatory autoimmune skin disease that affects 1.5% to 3% of the European population1. The most common type is plaque psoriasis. It is classified as mild, moderate, or severe depending on the extent and location of the lesions. It is moderate or severe in up to 30% of patients with this disease2 and has an enormous impact on the physical, intellectual, social, and emotional wellbeing of patients. Severe psoriasis has also been associated with comorbidities and even with other autoimmune diseases, such as Crohn's disease and psoriatic arthritis3.
Biologic drugs are often used in psoriasis refractory to other systemic treatments, such as cyclosporine, methotrexate, or psoralen plus ultraviolet A radiation. There is a broad therapeutic armamentarium of these drugs, such as etanercept, infliximab, and adalimumab. Ustekinumab is a biologic drug that acts on interleukins 12 and 23, which are involved in inflammatory processes4. This monoclonal antibody was authorised by the European Medicines Agency in 2009 for moderate-severe plaque psoriasis, and has been widely used as a rescue treatment after refractoriness to biologic drugs that act against tumour necrosis factor alpha (anti-TNF-α). Since then, other biologic drugs have been marketed to treat plaque psoriasis5–7.
Despite the variety of biologic drugs in use, there is a lack of data regarding the long-term results of these drugs. Some reviews and network metaanalyses8,9 have reported that there have been few clinical trials (CT) on the efficacy of long-term biologic drugs, despite the long period since these drugs entered the therapeutic armamentarium. These studies also supported the need to measure long-term real-world health outcomes, given that there may be differences between data obtained from CTs and those obtained in routine clinical practice.
Finally, it is relevant to take into account the significant direct and indirect costs associated with biologic drugs. Therefore, it is not surprising to find that decisions are being made based on cost-effectiveness and the sustainability of public health systems5,6. In the light of the foregoing, we suggest that the current approach to the treatment of moderate-severe plaque psoriasis is not easy. Long-term studies could provide information that would clarify some of the current uncertainties regarding biologic drugs.
The objective of this study was to assess the long-term effectiveness and safety of ustekinumab in patients with moderate-severe plaque psoriasis refractory to 2 anti-TNF-α biologic drugs.
MethodsStudy designA retrospective descriptive study was conducted from January 2010 to March 2019.
Study populationThe study included all patients with a diagnosis of moderate-severe plaque psoriasis who were under treatment with ustekinumab and had previously been treated with two or more anti-TNF-α biologic drugs. As described in the technical data sheet10, doses of ustekinumab were based on patient weight: ustekinumab 45 mg at weeks 0 and 4, followed by 45 mg every 12 weeks for patients weighing less than or equal to 100 kg; and ustekinumab 90 mg with the same regimen for patients over 100 kg.
Data collectionPatient data were collected using the digital medical record and outpatient dispensing module of the Dominion Farmatools® application. We recorded baseline population characteristics (age, gender, diagnoses), data on the treatments used (previous therapies, use of methotrexate, ustekinumab regimen, duration of treatment) and the baseline value of the Psoriasis Area and Severity Index (PASI).
Selected variables and analysisThe PASI 90 and PASI 75 response rates are defined as reductions equal to or more than 90% or 75% with respect to their PASI scores relative to the baseline value. The safety of ustekinumab was assessed by recording adverse effects (AE) and AE-associated treatment suspensions in the digital medical record. It was assumed that patients whose treatment was suspended due to lack of efficacy in a given week did not achieve PASI 90 or PASI 75 in the following weeks. Patients with insufficient follow-up in any of the effectiveness metrics were defined as non-evaluable. Patients who did not present efficacy data due to AE or any other pathophysiological state were also defined as non-evaluable.
ResultsA total of 36 patients were included. The majority were men (61.1%). In most cases, ustekinumab was the third biologic drug patients received after they had previously been treated with treatment of two anti-TNF-α drugs (88.9%). However, the study also included patients receiving ustekinumab as the fourth biologic drug (11.1%). Prior to ustekinumab, the two most frequently used biologic drugs were infliximab (34 patients, 94.4%) and etanercept (33 patients, 91.7%). Almost 95% of patients received the ustekinumab 45-mg regimen. The baseline PASI value for over 80% of the patients was equal to or more than 12. Table 1 shows the patients’ baseline characteristics and prior treatments.
Baseline characteristics and treatment data
| Baseline characteristics | |
| Number of patients | 36 |
| Age (mean years, range) | 47 (24-78) |
| Gender (number of patients, %): | |
| Male | 22 (61.1%) |
| Female | 14 (38.9%) |
| Treatments | |
| N° of previous treatments (n° patients, %): | |
| 2 | 32 (88.9%) |
| 3 | 4 (11.1%) |
| Previous treatments (number of patients, %): | |
| Etanercept + adalimumab | 28 (77.7%) |
| Infliximab + etanercept + adalimumab | 4 (11.1%) |
| Infliximab + adalimumab | 2 (5.6%) |
| Infliximab + etanercept | 2 (5.6%) |
| Efalizumab + infliximab | 1 (2.8%) |
| Duration of ustekinumab treatment | |
| (mean months, range): | 30.7 (6-85) |
| Ustekinumab regimen (number of patients, %): | |
| Ustekinumab 45 mg (weight <100 kg) | 34 (94.4%) |
| Ustekinumab 90 mg (weight ≥ 100 kg) | 2 (5.6%) |
| Basal Psoriasis Area and Severity Index Baseline (number of patients, %): | |
| ≥ 12 | 29 (80.5%) |
| 6 | 2 (5.6%) |
| 4 | 2 (5.6%) |
| 2 | 3 (8.3%) |
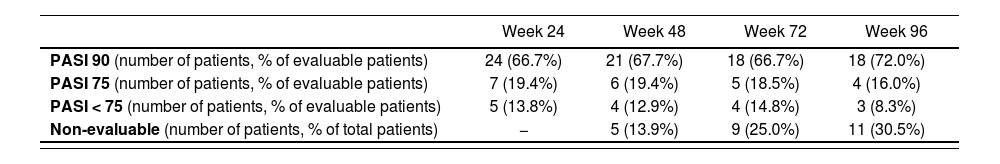
Table 2 shows the effectiveness data for ustekinumab. At weeks 24, 48, 72 and 96, at least 66.7% of the patients reached PASI 90 and between 19.4% and 16.0% of patients reached PASI 75. The percentage of non-evaluable patients increased over time, and reached 30.5% of the total by week 96. One patient was non-evaluable due to temporary withdrawal of ustekinumab at weeks 72 and 96 because of pregnancy. The remaining non-evaluable patients had shorter follow-up than that required by the corresponding effectiveness metrics.
Ustekinumab effectiveness data
| Week 24 | Week 48 | Week 72 | Week 96 | |
|---|---|---|---|---|
| PASI 90 (number of patients, % of evaluable patients) | 24 (66.7%) | 21 (67.7%) | 18 (66.7%) | 18 (72.0%) |
| PASI 75 (number of patients, % of evaluable patients) | 7 (19.4%) | 6 (19.4%) | 5 (18.5%) | 4 (16.0%) |
| PASI < 75 (number of patients, % of evaluable patients) | 5 (13.8%) | 4 (12.9%) | 4 (14.8%) | 3 (8.3%) |
| Non-evaluable (number of patients, % of total patients) | − | 5 (13.9%) | 9 (25.0%) | 11 (30.5%) |
PASI: Basal Psoriasis Area and Severity Index.
Regarding safety, six patients experienced ustekinumab- associated AEs, all of which were related to abnormal laboratory values: increased blood glucose (4 cases), increased plasma creatinine (2 cases), increased transaminase enzymes (1 case), increased plasma urea (1 case), increased uric acid (1 case), and kidney deterioration (1 case). No treatment was suspended due to the use of ustekinumab.
DiscussionThis study showed the considerable long-term effectiveness of ustekinumab in moderate-severe plaque psoriasis as the third or even fourth biologic drug refractory to 2 anti-TNF-α drugs. Some reviews and network meta-analyses8,9 have suggested that studies such as the present one are needed to corroborate the results obtained in CTs7,12. Relative to the results of these studies, the present study shows a higher percentage of patients who achieved and maintained PASI 90 in the short and long term. Few CTs have provided long-term data. The present study showed that the effectiveness of ustekinumab was maintained up to week 96. The long-term assessment of patients who have received so many lines of treatment is difficult. Nevertheless, despite some losses to follow-up, we believe that the long-term results of this study are relevant.
Furthermore, ustekinumab was generally well tolerated, as shown by the fact that few AEs were recorded and no treatments were suspended because of them. This result is in line with reports suggesting that ustekinumab has a good safety profile13. However, this study has some of the limitations associated with its retrospective descriptive design, such as the loss of any information not recorded in the digital medical record. An example of this limitation is the absence of certain AEs such as infections. The ustekinumab technical data sheet and CTs of ustekinumab describe fever as a frequent AE with up to 20% of patients being affected7,10,12. Therefore, further studies are needed to corroborate the safety data obtained in this study.
The main limitations of this study are the sample size and study design. The lack of randomization and a control group in retrospective descriptive studies makes it difficult to infer causality. Therefore, the results should be interpreted with caution and viewed as complementary to those observed in CTs, rather than as a substitute for them.
The scientific evidence supports the discontinuation of ustekinumab treatment in patients with an adequate response14. Although some studies have demonstrated the rescue treatment is needed in the first 12 months in a high percentage of patients with moderate-severe psoriasis following the withdrawal of biological treatment15, others studies have shown that the efficacy of new biologic drugs following ustekinumab treatment16. Treatment with ustekinumab after 2 anti-TNF-α drugs appears to be a strategy that achieves a good response in patients. It also has an acceptable safety profile, could allow the discontinuation of treatment in certain patients, keeps in reserve new biologic drugs for later lines of treatment, and increases cost effectiveness. In the future, there could be a decrease in the costs associated with ustekinumab treatment due to the possibility of discontinuing treatment, price competition with new drugs, and, in particular, the expiration of its patent.
Despite extensive experience with the use of biologic drugs to treat psoriasis, there are still substantial gaps in knowledge. Further long-term studies of other biologic drugs in refractory moderate-severe plaque psoriasis are needed to compare the retention of different therapeutic alternatives. On such study was that conducted by González-Fernández et al.17. Other studies have highlighted the lack of head-to-head trials, safety data, and patient-related variables18. Based on real-world results, the present study shows that the long-term use of ustekinumab in patients with moderate-severe plaque psoriasis refractory to 2 anti-TNF-α biologic drugs is both effective and safe.
FundingNo funding.
Presentation at CongressesPreliminary data from part of the study was shown in a report under the title “Use of ustekinumab in refractory patients with psoriasis”. European Association of Hospital Pharmacists (EAHP) 24th Annual Congress. Barcelona, Spain: 27-29 March, 2019.
Conflicts of interestManuel David Gil-Sierra participated in an advisory board for Janssen Pharmaceutica. The remaining authors declare no conflicts of interest.
Contribution to the scientific literatureThis study provides long-term real-world data on ustekinumab in moderate-severe psoriasis.
Implications of the results for medical practice, research, health policies, or hospital pharmacy in general: Contribution to knowledge of data on ustekinumab under routine clinical practice conditions.