Patients with life-limiting illnesses are prone to unnecessary polypharmacy. Deprescribing tools may contribute to minimizing negative outcomes. Thus, the aims of the study were to identify validated instruments for deprescribing inappropriate medications for patients with palliative care needs and to assess the impact on clinical, humanistic, and economic outcomes.
MethodsA systematic review was conducted in LILACS, PUBMED, EMBASE, COCHRANE, and WEB OF SCIENCE databases (until May 2021). A manual search was performed in the references of enrolled articles. The screening, eligibility, extraction, and bias risk assessment were carried out by 2 independent researchers. Experimental and observational studies were eligible for inclusion.
ResultsOut of the 5791 studies retrieved, after excluding duplicates (n = 1050), conducting title/abstract screening (n = 4741), and full reading (n = 41), only 1 study met the inclusion criteria. In this included study, a randomized controlled trial was conducted, which showed a high level of bias risk overall. Adults 75 years or older (n = 130) with limited life expectancy and polypharmacy were allocated to 2 groups [intervention arm (deprescribing); and control arm (usual care)]. Deprescribing was performed with the aid of the STOPPFrail tool. The mean number of inappropriate medications and monthly medication costs were significantly lower in the intervention arm. No statistically significant differences were found in terms of unscheduled hospital presentations, falls, fractures, mortality, and quality of life.
ConclusionsDespite the availability of several instruments to support deprescribing in patients with palliative care needs, only 1 of them has undergone validation and robust assessment for effectiveness in clinical practice. The STOPPFrail tool appears to reduce the number of inappropriate medications for older people with limited life expectancy (and probably palliative care needs) and decrease the monthly costs of pharmacotherapy. Nevertheless, the impact on patient safety and humanistic outcomes remain unclear.
Los pacientes con enfermedades terminales son propensos a la polifarmacia innecesaria. Las herramientas de desprescripción pueden contribuir a minimizar los resultados negativos. Por lo tanto, los objetivos del estudio fueron identificar instrumentos validados para la desprescripción de medicamentos inapropiados en pacientes con necesidades de cuidados paliativos y evaluar el impacto en los resultados clínicos, humanísticos y económicos.
MétodosSe realizó una revisión sistemática en las bases de datos LILACS, PUBMED, EMBASE, COCHRANE y WEB OF SCIENCE (hasta mayo de 2021). Se realizó una búsqueda manual en las referencias de los artículos incluidos. La selección, elegibilidad, extracción y evaluación del riesgo de sesgo se llevaron a cabo por dos investigadores independientes. Se aceptó la inclusión de estudios observacionales y experimentales.
ResultadosDe los 5791 estudios recuperados, después de excluir duplicados (n = 1050), realizar la selección de títulos/resúmenes (n = 4741) y la lectura completa (n = 41), solo un estudio cumplió con los criterios de inclusión. En este estudio incluido, se realizó un ensayo controlado aleatorizado, que mostró un alto nivel de riesgo de sesgo en general. A los adultos de 75 años o más (n = 130) con esperanza de vida limitada y polifarmacia se les asignaron dos grupos [grupo de intervención (desprescripción) y grupo de control (atención habitual)]. Se realizó la desprescripción con la ayuda de la herramienta STOPPFrail. El número promedio de medicamentos inapropiados y los costos mensuales de los medicamentos fueron significativamente más bajos en el grupo de intervención. No se encontraron diferencias estadísticamente significativas en términos de presentaciones hospitalarias no programadas, caídas, fracturas, mortalidad y calidad de vida.
ConclusionesA pesar de la disponibilidad de varias herramientas para apoyar la deprescripción en pacientes con necesidades de cuidados paliativos, solo una de ellas ha sido validada y evaluada de manera sólida en la práctica clínica. La herramienta STOPPFrail parece reducir el número de medicamentos inapropiados en personas mayores con esperanza de vida limitada (y probable necesidades de cuidados paliativos) y disminuir los costos mensuales de la farmacoterapia. Sin embargo, el impacto en la seguridad del paciente y los resultados humanísticos aún no está claro.
- 1)
There are few studies involving patients with palliative care needs that have assessed the impact of deprescribing on clinical, humanistic, and economic outcomes using validated instruments.
- 2)
Deprescribing, using the STOPPFrail tool, contributed to a decrease in the number of inappropriate medications prescribed and the monthly cost of pharmacotherapy among patients with palliative care needs. However, the impact on patient safety and humanistic outcomes, such as quality of life, remains unclear.
- 3)
It is necessary to conduct high-quality studies to assess the impact of deprescribing inappropriate medicines with the aid of validated instruments in this population, regardless of illness, frailty, or age group.
Palliative care is defined as an approach that promotes quality of life (QoL) for patients and their families in the face of diseases that threaten the continuity of life. It focuses on the prevention and relief of suffering through early identification, assessment, and impeccable treatment of physical, psychosocial, and spiritual problems, including pain.1
Several studies have described the inadequate prescribing practices for patients with advanced-stage diseases that pose a threat to their continuity of life.2–4 The prescription of curative medication and/or primary or secondary prophylaxis may be inappropriate for patients with a limited life expectancy.5
Polypharmacy is frequent among patients receiving palliative care.6 In addition to medications prescribed to manage the signs and symptoms of a life-limiting condition, treatments for chronic diseases are also prescribed,7 along with medicines aimed at preventing the deterioration or worsening of the clinical condition associated with underlying comorbidities.2,8
In this context, managing pharmacotherapy poses a challenge for healthcare professionals,2,7,9 as it may contribute to the unsafe and inappropriate use of medications, increase the likelihood of clinically relevant medication interactions,10 iatrogenic cascade,11 serious adverse reactions,12–14 and a decline in QoL.13
Therefore, it is essential to consider the risk/benefit of pharmacological treatment15 by reviewing the pharmacotherapy,16 in order to assess its suitability based on the patient's prognosis, potential safety concerns, therapeutic goals, and objectives.10
According to the World Health Organization, discontinuing, reducing, interrupting, or withdrawing medications should be considered during the review of pharmacotherapy in order to manage polypharmacy and improve outcomes.16 This process is known as deprescribing, which aims to minimize the medication burden in terms of dose, number, and frequency of medications administered, thereby preventing adverse events and clinical deterioration.17
Deprescribing is a patient-centered approach to medicines management.16 It is defined as a systematic process to identify and discontinue medications with the potential to cause harm to the patient. This is done when the risk of using such medications outweigh the benefit, taking into account individualized care based on therapeutic objectives, functionality, life expectancy, values, and individual preferences.18 In palliative care, deprescribing can be applied to any patient, particularly those with limited life expectancy.19
Unspecific or non-validated instruments have been used to measure the impact of deprescribing in patients receiving palliative care,20 including those with life-limiting illnesses,2 limited life expectancy, in the final stage of life,21,22 frailty,23 or cancer24 diagnoses. As a result, the impact of deprescribing remains unclear among patients in any stage of illness with palliative care needs. Therefore, this study aims to identify validated instruments for deprescribing inappropriate medications in patients with palliative care needs; and to assess the impact on clinical, humanistic, and economic outcomes.
MethodsSystematic review protocol registrationThe protocol for this systematic review was registered in the International Prospective Register of Systematic Reviews (CRD42021270337) and conducted in accordance with the recommendations of the Cochrane Collaboration.25 The protocol report follows the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).26
Eligibility criteriaThe inclusion and exclusion criteria for the studies were based on the guiding question of the systematic review, which was formulated using the PICO framework (population, intervention, comparator, and outcome). Therefore, the inclusion criteria were as follows:
- a)
Population: Patients with palliative care needs, of any gender and age, diagnosed with severe, incurable, or in an advanced stage (limited life expectancy <2 years or end of life) of disease, assisted at any level of health care.
- b)
Intervention: Validated instruments/tools for deprescribing, suspending, discontinuing, interrupting, or withdrawing non-essential, inappropriate, unnecessary, or preventive medications.
- c)
Comparator: Usual care or no intervention.
- d)
Outcomes: Clinical (mortality, survival, adverse drug-related events, number of inappropriate medications prescribed, number of medications deprescribed, functional capacity, frequency of hospital admissions, emergency care, maintenance of adherence to prescription); humanistic (satisfaction, QoL, and the experience/perspective of patients, informal caregivers, or family members about the availability or lack of deprescribe); and economic (from the perspective of the health institution).
Observational studies were included, as well as clinical trials that met the inclusion criteria . Editorials, comments, news, conference proceedings abstracts, qualitative studies, and data from theses and dissertations were excluded. Additionally, studies related to the validation of instruments, those that did not use validated instruments for deprescribing, and studies that solely evaluated the clinical, humanistic, and economic outcomes without specifying the instrument used for deprescribing were also excluded.
Information sources and search strategyThe search strategy was reviewed by a librarian (MS) and conducted in online databases: LILACS, PUBMED, EMBASE, COCHRANE, and WEB OF SCIENCE (see Supplementary Material) from inception to May 25, 2021, with no restrictions. In addition, a manual search was performed in the bibliographic references of eligible articles. Articles not written in Portuguese, English, or Spanish were excluded during the screening of titles and abstracts, as well as during full reading. All identified references were entered into Rayyan QCRI, an online tool for systematic reviews.27
Selection of articles and data collection processTwo independent reviewers (FKA, RPIN) screened titles and abstracts to identify potentially eligible studies. Subsequently, the full texts were independently evaluated for eligibility by the same reviewers (FKA, RPIN). It is important to highlight that the assessment validation of the instrument used to perform the deprescribing in patients receiving palliative care was conducted during the full reading stage.
Any disagreements were resolved through discussion and consultation with 2 additional reviewers (MOBZ, FRV) when necessary.
Extraction and tabulation of dataOutcomes investigated: Mortality, survival, withdrawal-related adverse medication events, adverse drug reaction, number of prescribed inappropriate medications, number of medications deprescribed, functional capacity, frequency of hospital admissions, emergency care, maintenance of adherence to deprescribe, satisfaction, QoL, experiences/perspectives of patients, informal caregivers, or family members of the deprescribing process, and costs of deprescribing from the perspective of the health institution.
Two reviewers (FKA, RPIN) independently extracted data from the included studies using a standardized electronic data form. The extracted data were then independently reviewed and checked by a third reviewer (FRV). The extracted variables included the characteristics of: (i) the study (authors, year of publication, country, type of study, level of health care, objectives, sample size); (ii) the patients (inclusion and exclusion criteria, age, gender, serious disease (International Classification of Diseases 10th Revision), incurable disease, disease in an advanced stage that threatens the continuity of life, comorbidities, life expectancy); and (iii) the instruments (method of development and validation, name, type (if they help in the general deprescribing process or only in a specific part, such as the detection of inappropriate medications), domains); (iv) deprescribing process (duration, monitoring and maintenance of adherence, health professionals involved); (v) control (no intervention, type of usual care, monitoring); (vi) clinical outcomes (total number of medications used, number of inappropriate medications, number of medications deprescribed, number of patients who died, number of emergency room visits, number of patients who were hospitalized, number of days the inappropriate medication remained without being prescribed, status of patient functional capacity, frequency of withdrawal adverse events); (vii) humanistic outcomes (degree of patient, family or caregiver satisfaction; QoL score); and, (viii) economic outcomes (direct costs related to medications and health team salary).
Bias risk assessmentTwo reviewers (FKA, RPIN) independently and in duplicate assessed the methodological quality of the included studies using the ROB-2 tool,28 following the guidance of the Cochrane Collaboration.25
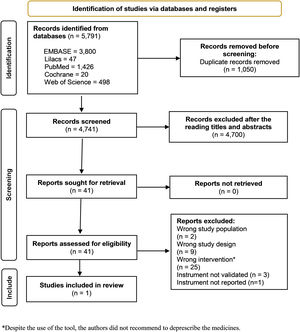
ResultsResults of the searchThe results are summarized in a PRISMA diagram (Fig. 1). Out of the 5791 articles initially retrieved, 4741 unique references remained after removing duplicates. These references were screened based on title and abstract, resulting in 41 full-text articles assessed for eligibility. Finally, 1 article29 was included in the review (Fig. 1). Three studies were excluded30–32 because the authors did not use a validated instrument for deprescribing in palliative care (Table 1).
Non-validated deprescribing instruments for patients receiving palliative care.
| Author (year) | Country | Design of the study | Deprescribing method |
|---|---|---|---|
| Wilder-Smith et al. (2019)30 | USA | Retrospective cross-sectional | Structured judgment review with non-validated instrument [escalation/limitation plan (TELP)] |
| Li et al. (2021)31 | Canada | Retrospective chart review | The authors developed a list of medications possible to be deprescribed which was not validated. |
| Basri et al. (2018)32 | USA | Retrospective review | PhARMD tool, which intended to capture clinical pharmacyinterventions related to pharmaceutical care. |
One study that utilized a validated instrument for deprescribing was included in our review. Curtin et al.29 conducted a parallel-group, unblinded, randomized pragmatic clinical trial in 2 acute hospitals in Ireland. The aim of the study was to examine whether deprescribing guided by The Screening Tool of Older Persons' Potentially Inappropriate Prescriptions (STOPPFrail tool) could reduce the number of medications taken by older people with probable palliative care needs compared to usual pharmaceutical care alone. A proxy for palliative care needs was taken from the use of what is referred to as the surprise question asked of clinicians, “Would I be surprised if this patient died in the next 12 months?”. The secondary objectives of the study were to determine the effect of this intervention on unscheduled hospital admissions, falls, fractures, antipsychotic prescribing, monthly medication costs, QoL, and mortality.
ParticipantsEligible participants were frail older adults (aged ≥75 years) with polypharmacy and limited life expectancy, requiring nursing home care, who were admitted from the community with acute unselected medical or surgical illnesses to 2 acute hospitals in Ireland.
InterventionParticipants were randomized in a 1:1 ratio to receive either usual pharmaceutical care (i.e., care provided by hospital physician and pharmacists) or usual pharmaceutical care supplemented by individualized STOPPFrail-guided deprescribing. The intervention was applied at a single time point during the patient's hospital admission at the time of trial enrollment (3 months).
The groups were homogeneous at baseline in terms of age (P = .24), sex (P = .59), cognitive impairments (P = .67), functional impairments (P=.63), comorbidities (P = .21), and prescribed medications (P = .28), except for the use of analgesics (P = .03). The most prevalent health conditions among the groups were dementia, atrial fibrillation, osteoporosis, and chronic kidney disease.
Primary outcomeData from 98 randomized participants [Intervention arm (n = 51) and Control arm (n = 47)] were available for analysis regarding the number of prescribed medications. After 3 months of follow-up, the intervention arm showed a lower mean number of prescribed medications compared to the control arm (Table 2).
The mean difference in the number of medicines prescribed and the monthly medication costs after 3 months of follow-up (n = 98).
| Mean (±SD) | Baseline | Change after 3 months | Difference±SD (IC 95%) P-value | ||
|---|---|---|---|---|---|
| Intervention (n = 51) | Control (n = 47) | Intervention (n = 51) | Control (n = 47) | ||
| Medicines prescribed | 11.5 (2.7) | 10.9 (3.6) | −2.61 (2.73) | −0.36 (2.60) | 2.25 ± 0.54 (1.8–3.32) P < .001 |
| Monthly costs Dollar $ | 267.04 (117.21) | 250.56 (140.64) | −74.97 (148.32) | −13.22 (110.40) | 61.74 ± 26.60 (8.95–114.53) P = .02 |
No significant statistical difference was observed in mortality [Intervention arm (n = 65) and Control arm (n = 65); RR = 0.67 (95% CI 0.35–1.27), P = .22]. Survival, withdrawal-related adverse medication events, and adverse drug-related events were not reported.
No statistically significant differences were found for patient-related outcomes such as emergency department presentations (not admitted) (P = .72), unplanned hospital admissions (P = .27), unscheduled medical reviews by GPs (P = .82), falls (P = .75), fractures (P = .18), antipsychotic dose reduction (P = .85), or discontinuation (P = .15).
QoL was assessed with the aid of 2 instruments (ICECAP-O and QUALIDEM). At baseline, no statistically significant differences were observed. After the monitoring, the authors noted a deterioration in QoL in both groups, but the mean change did not show a statistical difference [ICECAP-O: Intervention arm (n = 21) and Control arm (n = 29), P = .17; QUALIDEM: Intervention arm (n = 37) and Control arm (n = 38), P = .60].
Regarding the economic impact of deprescribing, there was no statistically significant difference in the extrapolated mean (SD) monthly medication costs between the Intervention and Control arms (Table 2). However, at the 3-month follow-up, the Intervention group showed a significantly lower mean change in monthly medication costs compared to the control group (Table 2).
The study did not report on functional capacity, frequency of hospital admissions, maintenance of adherence to deprescription, or the experiences/perspectives of patients, informal caregivers, or family members regarding the deprescribing process.
Risk of bias in the included studyThe study was vulnerable to bias due to the small sample size, potential confounding factors, and outcome assessment. Additionally, the study was rated as having a critical level of risk of bias on 2 criteria (Fig. 2).
DiscussionThere was 1 study that assessed the impact of deprescribing on clinical, humanistic, and economic outcomes using a validated instrument for patients a limited life expectancy. Nonetheless, considering the limitations of our study, it seems that deprescribing using the STOPPFrail tool appeared to contribute to a decrease in the number of inappropriate medications prescribed and the monthly cost of pharmacotherapy. However, no significant impact was observed on QoL or patient safety.
According to Tjia et al.,33 the best definition of deprescribing in palliative care is “the systematic process of identifying and discontinuing medications in instances in which existing or potential harms outweigh existing or potential benefits within the context of an individual patient's care goals, current level of functioning, life expectancy, values and preferences”. However, decision-making can be challenging due to time constraints for health professionals,15 ethical considerations, prognosis,34 and lack of explicit guidance.15 Consequently, the most commonly used tool to assess the appropriateness of pharmacotherapy prescribed for patients in palliative care is the American Geriatric Association (AGS) Beer's criteria.20
It is important to highlight that medication appropriateness contributes to achieving the goal of improving QoL and patient safety.35 There are 2 approaches to achieving this: with the aid of implicit and/or explicit criteria.33 When it comes to explicit criteria, which involve lists of potentially inappropriate medications, the use of non-validated tools for those with life-limiting illnesses may lead to misclassifying essential medications as unnecessary. This misclassification can occur when medications are mistakenly perceived as lacking indication, effectiveness, or being used for prolonged durations, even though they are appropriate for managing and controlling signs and symptoms.20
In this context, STOPPFrail could offer non-specialist physicians with explicit criteria and time-efficient guidance for deprescribing inappropriate medications for patients with palliative care needs.34 The tool was initially developed based on clinical experience, literature review, and a Delphi consensus process. It consists of 27 medications that are potentially inappropriate for frail older patients with limited life expectancy. To meet the inclusion criteria, patients must fulfill the following conditions: end-stage irreversible pathology, poor 1-year survival prognosis, severe functional or cognitive impairment, and prioritization of symptom control over disease progression prevention.36 Studies have demonstrated that STOPPFrail-guided deprescribing decisions generally align with gold-standard methods, including assessments by geriatricians,37 general physicians, and clinical pharmacologists.34 Additionally, there was no statistically significant difference in deprescribing performance between STOPPFrail and OncPal,38 a tool developed to support deprescribing of inappropriate medications for oncological patients transitioning from curative to palliative care, when applied to an unselected palliative population (beyond the original inclusion criteria, for instance frail and oncologic patients).34 This finding suggests the potential interchangeability of the tools regardless of the life-limiting condition.
Despite the mentioned advantages of STOPPFrail, the study included in our systematic review did not observe improvements in QoL and patient safety.29 The lack of evidence regarding QoL improvement may be influenced by the characteristics of the instruments used in the study, namely QUALIDEM (which was developed for individuals with dementia in residential settings),39 and the ICEpop CAPability measure for Older people (ICECAP-O) (which focuses on overall QoL rather than specific health-related factors and is intended for decision-making across health and social care).40
Moreover, to ensure the deprescribing process is as safe and optimal as possible, without negatively affecting the QoL of the patient, it is preferable to conduct it in agreement with the patient and/or their relatives.41 Older people with limited life expectancy are willing to discuss the risks and benefits associated with deprescribing before making a decision to reduce their number of medications.42 In particular, engaging in discussions with patients or their surrogates about which risks the patient would find acceptable in relation to the potential benefits of medication cessation appears to be an important step. Deprescribing medications raises ethical dilemmas that can be challenging to resolve.43 Therefore, shared decision-making that considers the preferences of the patient, family, and/or caregivers could help achieve better clinical and humanistic outcomes.
To incorporate the values and preferences of patients, family, and/or caregivers into the deprescribing process, an updated version of STOPPFrail was developed.44 STOPPFrail version 2 includes practical methods for identifying older individuals approaching the end of life, such as those with activities of daily living dependency, severe chronic disease, terminal illness, severe irreversible frailty, and a life expectancy of ≤12 months. Additionally, new criteria were added for antihypertensive therapies and vitamins, expanding the validated 27 criteria from version 1 to include 8 additional criteria.44 Further studies are needed to assess the impact of the new version on humanistic outcomes.
Cost assessment following deprescribing is rarely performed, and if conducted, studies often consider the perspective of the healthcare system (i.e. costs for the insitution which provides healthcare assistance), resulting in heterogeneous data.35 Our findings align with existing literature,35 as we found one source of evidence demonstrating that deprescribing reduces the monthly costs of unnecessary pharmacotherapy.29 However, it is important to note that the economic impact is not fully understood, as the authors in this study only considered direct costs associated with medications. Similarly, the impact on patient safety could not be fully analyzed, as the authors were unable to detect withdrawal symptoms or adverse drug reactions. Therefore, these findings emphasize the need for higher-quality studies to assess the impact of deprescribing in older patients with palliative care needs.
Strengths and limitationsOur search strategy may have limitations. For pragmatic reasons, we excluded studies that did not report using non-Roman characters, and we did not extract data from otherwise eligible trial database registries. Furthermore, due to the small sample size and absence of a validated tool for palliative care, we cannot definitively confirm that deprescribing improves clinical, humanist, and economic outcomes.
Reservations for the tool's application in clinical practice is another hypothesis that could explain the lack of evidence found in our systematic review. During the screening and eligibility assessment, most studies were excluded because the authors did not recommend the discontinuation, tapering/reduction, or switching of medications for patients with life-limiting illnesses. Additionally, some authors aimed to develop instruments or protocols for guiding the identification of potentially inappropriate medication in various conditions, including both cancer and non-cancer patients.11,35,42–47
Therefore, it is justified why our systematic review did not include tools and/or evidence-based guidelines in the literature for deprescribing proton pump inhibitors,42 antihyperglycemic agents,43 and antipsychotics,44 as well as instruments developed specifically for patients diagnosed with neoplasm (OncPal),38 dementia,48 advanced disease,49 or those in the end-of-life stage.11,50 These guidelines aimed to identify potentially inappropriate medications; however, deprescribing was not carried out.
Moreover, our findings must be carefully interpreted; it is only possible to infer participants in the included study may have had palliative care needs based on a clinician-reported perception to the 'surprise' question, "Would I be surprised if this patient died in the next 12 months?". A recent systematic review and meta-analyses indicates the 'surprise' question has reasonable accuracy and is an appropriate screening tool to identify patients that could benefit from advance care planning.51 Consequently, this underscores the need for prioritising the validation of desPREScribing tools specifically for populations with palliative care needs.
However, our study has strengths as it employed robust methods to identify relevant studies, extract data, and identify validated deprescribing tools for palliative care patients. Furthermore, our systematic review allowed us to conclude the following: (i) there are several tools for deprescribing inappropriate medications in both oncological and non-oncological patients; (ii) the impact on clinical, humanistic, and economic outcomes has mainly been studied in older people with palliative care needs residing in high-income countries, where patient participation in decision-making was limited; (iii) indirect costs associated with discontinued medications were not analyzed; (iv) QoL was evaluated with the aid of instruments not specifically designed for patients with palliative care needs; and (v) clinical outcomes were not adequately analyzed.
ConclusionDespite the availability of several instruments to support deprescribing in patients with probable palliative care needs, only 1 of them has undergone validation and robust assessment for effectiveness in clinical practice. The STOPPFrail tool appears to reduce the number of inappropriate medications for older people with probable palliative care needs and decrease monthly pharmacotherapy costs. However, the impact on patient safety and humanistic outcomes remain unclear. There is a need for high-quality studies to evaluate the effects of deprescribing of inappropriate medications with the aid of validated instruments for patients with palliative care needs, regardless of their illness, frailty, or age group.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statementFrangie Kallas de Andrade: Investigation, Formal analysis, Writing – original draft, Visualization, Writing – review & editing. Raziel Prado Ignacio Nunes: Investigation, Formal analysis, Writing – original draft, Visualization, Writing – review & editing. Maria Olívia Barboza Zanetti: Conceptualization, Methodology, Formal analysis, Supervision, Writing – original draft, Visualization, Writing – review & editing. Ariane Cristina Barboza Zanetti: Conceptualization, Methodology, Visualization, Writing – review & editing. Márcia dos Santos: Methodology, Visualization, Writing – review & editing. Alan Maicon de Oliveira: Methodology, Visualization, Writing – review & editing. Andrew Carson-Stevens: Conceptualization, Methodology, Visualization, Writing – review & editing. Leonardo Régis Leira Pereira: Conceptualization, Methodology, Supervision, Writing – original draft, Visualization, Writing – review & editing. Fabiana Rossi Varallo: Conceptualization, Methodology, Formal analysis, Supervision, Writing – original draft, Visualization, Project administration, Writing – review & editing.