To identify validated questionnaires to assess medication adherence, and its associated factors, in adult patients with chronic pathologies.
MethodA systematic review of scientific publications that describe validated medication adherence questionnaires in PubMed and Scopus was carried out during May 2022. The search strategy combined the MeSH heading “Medication adherence” with the keywords: “Questionnaire” and “Validation”; adding “Spanish” to rescue questionnaires in our language. Systematic reviews, meta-analyses, or scientific articles with full text available in Spanish or English were selected; published from January 2000 to April 2022; that present the application and validation of a medication adherence questionnaire in adults with chronic pathologies; and publications of the initial validation of a questionnaire, recovered through bibliographic citations of the previously identified publications, even if they are prior to the year 2000.
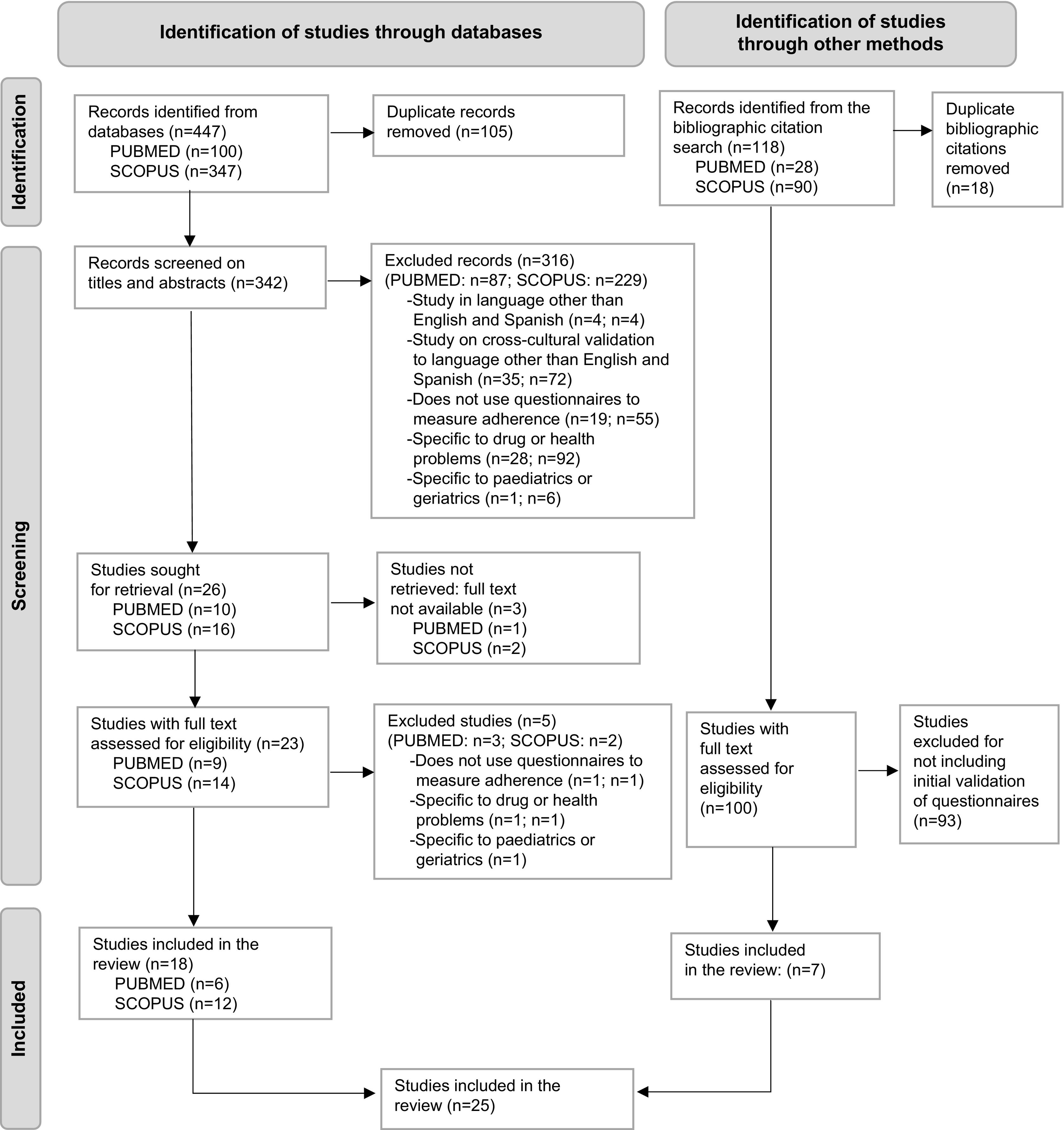
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed to represent the search process, inclusion and exclusion of the retrieved publications.
Results(97) records in PubMed and 3 adding “Spanish” were retrieved; in Scopus, 334 records were retrieved and 13 with “Spanish”. 118 records were retrieved through bibliographic citations identification.
From the analysis of the previous publications, 14 validated questionnaires were identified that assess medication adherence and are applied in English and/or Spanish in adult patients with chronic pathologies. For each questionnaire, the following characteristics were described: name, authors, year of publication, dimensions (barriers and facilitators factors), number and wording of the items, response scale, form of administration, language, and pathologies of the initial validation. Of the subsequent validations, only those carried out in English and/or Spanish were presented. So far, 6 questionnaires were validated in Spanish and only for certain chronic pathologies.
Conclusions(14) validated questionnaires were identified, 6 of them were validated in Spanish. They are designed to evaluate medication adherence in a comprehensive manner, being useful to be applied in hospital and community pharmaceutical services. This review provides health professionals with tools to develop and validate their own questionnaire, adapting the wording to the local language and context of the health system.
Identificar cuestionarios validados para evaluar adherencia a la medicación, y sus factores asociados, en pacientes adultos con patologías crónicas.
MétodoSe realizó una revisión sistemática de publicaciones científicas que describen cuestionarios validados de adherencia a la medicación en PubMed y Scopus, durante mayo 2022. La estrategia de búsqueda combinó el MeSH Heading «Medication adherence» con las palabras claves: «Questionnaire» y «Validation»; sumando «Spanish» para rescatar cuestionarios en nuestro idioma. Se seleccionaron revisiones sistemáticas, metaanálisis o artículos científicos con texto completo disponible en español o inglés; publicados desde enero 2000 a abril 2022; que presentan aplicación y validación de un cuestionario de adherencia a la medicación en adultos con patologías crónicas; y publicaciones de la validación original del cuestionario, recuperadas a través de las citas bibliográficas de las publicaciones antes identificadas, aun cuando sean previas al año 2000.
Se siguieron directrices de las guías PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) para representar el proceso de búsqueda, inclusión y exclusión de las publicaciones recuperadas.
ResultadosSe rescataron 97 registros en PubMed y 3 sumando «Spanish»; en Scopus se rescataron 334 registros y 13 con «Spanish». Se rescataron 118 registros a través de identificación de citas bibliográficas.
A partir de su análisis, se identificaron catorce cuestionarios validados, aplicados en inglés y/o español en pacientes adultos con patologías crónicas. De cada cuestionario se describieron: denominación, autores, año de publicación, dimensiones (factores barreras y facilitadores), cantidad y modo de redacción de los ítems, escala de respuesta, forma de administración, idioma y patologías de la validación inicial. De las validaciones posteriores se presentan sólo las realizadas en inglés y/o español. Hasta el momento, 6 de dichos cuestionarios fueron validados en español y solo para determinadas patologías crónicas.
ConclusionesSe identificaron 14 cuestionarios validados, 6 de ellos cuentan con validación en idioma español. Los mismos están diseñados para evaluar adherencia a la medicación en forma integral, siendo útiles para ser aplicados en servicios farmacéuticos hospitalarios y comunitarios. Esta revisión provee herramientas para desarrollar y validar un cuestionario propio, adecuando la redacción al idioma y al contexto del sistema de salud local.
The World Health Organization (WHO) defines medication adherence as the extent to which a person's behaviour corresponds with agreed recommendations from a healthcare provider.1 Research has focused on medication adherence, understood as the extent to which patients take medicines as prescribed by their healthcare providers and as agreed in the treatment plan.2 This concept has the positive aspect of engagement and reflects the patient's autonomy in selecting and maintaining the therapeutic regimen.3
Adherence in patients with chronic diseases averages only 50% in developed countries and is lower in developing countries.1,4 Non-adherence to medication is recognised as a drug related problem that negatively impacts the achievement of pharmacotherapeutic goals.5 This problem has relevant clinical and economic consequences, as effective treatments may be judged ineffective, costly diagnostic procedures may be ordered, and therapy may be unnecessarily and dangerously intensified.6 The economic costs of non-adherence include wasted drugs, economic losses associated with disability, absenteeism, lost productivity, and higher costs associated with increased of demand for healthcare services.7,8
Improving medication adherence requires understanding its multifactorial causes. The WHO classifies these factors, whether barriers or facilitators, into 5 categories: socioeconomic factors, disease-related factors, treatment-related factors, patient-related factors, and healthcare system/team-related factors.1,9
Addressing non-adherence begins with an exploration of patients' perspectives on drugs and the reasons why they are unwilling or unable to take them.7 Understanding the magnitude of the problem is fundamental to defining effective individualised pharmaceutical interventions to improve adherence in pharmaceutical care practice. Adherence can be assessed with a wide range of tools, which must be proven as valid, reliable, and sensitive to change. A combination of direct and indirect methods is recommended to more accurately collect the information needed to determine medication adherence.8,10 Questionnaires are useful indirect tools to assess adherence in healthcare settings because they are affordable, quick to administer, and are easy to use across a wide range of drug regimens. Any questionnaire in use must be validated, meaning it must fulfil specific psychometric properties, the main ones being reliability (measurements are reproducible) and validity (it measures what it is designed to measure).11,12
Validated questionnaires on medication adherence have been described in the literature. However, there is no ideal standard and no questionnaire is appropriate for all settings.2 Furthermore, very few are available in Spanish.
Comprehensive medication management is one of the priority care functions of pharmacy services. A tool that can assess medication adherence and identify barriers or facilitators is needed to improve the clinical, economic, and human outcomes of patients in all areas of professional practice. It is therefore essential to have available a questionnaire that can identify adherent patients as well as the specific factors that influence medication adherence.9,13
Having a validated questionnaire available saves pharmacists the time and resources needed to develop a new one. If an appropriate questionnaire is unavailable, or is unavailable in Spanish, items with the best statistical performance can be extracted. These items are useful for identifying adherence problems and barriers in the local setting, serving as a first step in designing new questionnaires.
The aim of this study was to identify validated questionnaires to assess medication adherence and its associated factors in adult patients with chronic diseases.
MethodsIn May 2022, we conducted a systematic review of scientific publications describing validated medication adherence questionnaires in adult patients with chronic diseases, using the PubMed and Scopus databases. The search period was January 2000 to April 2022.
The search strategy was designed following the recommendations of the Cochrane Handbook of Systematic Reviews of Health Interventions to use a combination of medical subject headings (MeSH) and free text terms.14 The MeSH Heading “Medication adherence” was combined with the keywords “Questionnaire” and “Validation”. The keyword “Spanish” was added in a second stage to retrieve questionnaires in the Spanish language.
The PubMed search: ([“Medication adherence”{MeSH Terms}] AND [“Questionnaire”{Title/Abstract}]) AND (“Validation”[Title/Abstract]). Pubmed second stage: (([“Medication adherence”{MeSH Terms}] AND [“Questionnaire”{Title/Abstract}]) AND [“Validation”{Title/Abstract}]) AND (“Spanish”[Title/Abstract]).
The Scopus search: “Medication adherence” AND “Questionnaire” AND “Validation” in Article title/abstract/keywords. Scopus second stage: “Medication adherence” AND “Questionnaire” AND “Validation” AND “Spanish”.
We applied the following inclusion criteria: (a) systematic reviews, meta-analyses, or original scientific articles; (b) full text available in Spanish or English; (c) published between January 2000 and April 2022; (d) studies that applied and validated a medication adherence questionnaire in adult patients with chronic diseases; and (e) initial validation studies that were cited in any of the included studies, even if they were published before 2000.
Exclusion criteria were applied in the following order: (a) articles in languages other than English or Spanish; (b) articles regarding cross-cultural adaptation and validation of questionnaires in languages other than English or Spanish; (c) articles without questionnaires that only report on barriers and facilitators of adherence, or only review interventions to improve adherence; (d) articles with questionnaires specifically designed for a disease and/or its associated medication; (e) articles with questionnaires involving specific population groups such as paediatrics and geriatrics; and (f) the full text of the articles was unavailable.
The search, inclusion, and exclusion process were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.15
Validated questionnaires were extracted from the included articles. Each was described in terms of the following aspects: name of the questionnaire, authors, year of publication, number of dimensions, number and wording of the items, response scale, form of administration, language, and diseases addressed in the initial validation. When analysing the dimensions, we identified the adherence factors that each questionnaire focuses on, encompassing both barriers and facilitators.
We only present subsequent validations conducted in English and/or Spanish, the latter being more useful to Spanish-speaking pharmacists.
ResultsThe results of this review provide valuable information for selecting or designing tools to assess medication adherence in adult patients with chronic diseases. In particular, it focuses on the different factors that influence adherence.
In PubMed, 97 records were retrieved and 3 when adding “Spanish”. In Scopus, 334 records were retrieved and 13 when adding “Spanish”. There were 105 duplicates out of 447 records in the databases. We screened the titles and abstracts of the 342 resulting records, excluding 316 articles according to the established criteria. A total of 26 articles were selected for retrieval, but the full text of 3 of them could not be accessed. To decide eligibility, the remaining 23 full-text articles were analysed in detail and 18 were included.
While the initial search was limited to studies published between January 2000 and April 2022, an examination of their citations led to a further 118 records being retrieved. Of these, 7 publications presented validated questionnaires and were included in the review on the basis of their psychometric properties, although the initial validation of some of them had been published before 2000.
Finally, a total of 25 articles that met the pre-established criteria were included in the review.
Fig. 1 shows the procedure followed for selecting articles according to the PRISMA guidelines for systematic reviews.
We analysed these articles and identified 14 validated questionnaires assessing medication adherence in adult patients with chronic diseases, in English and/or Spanish. Some of them appear in several articles due to subsequent validations in different settings, disease settings, or languages. In some cases, the original questionnaires were published before 2000, but were found through the citations of the articles presenting a subsequent adaptation and validation.
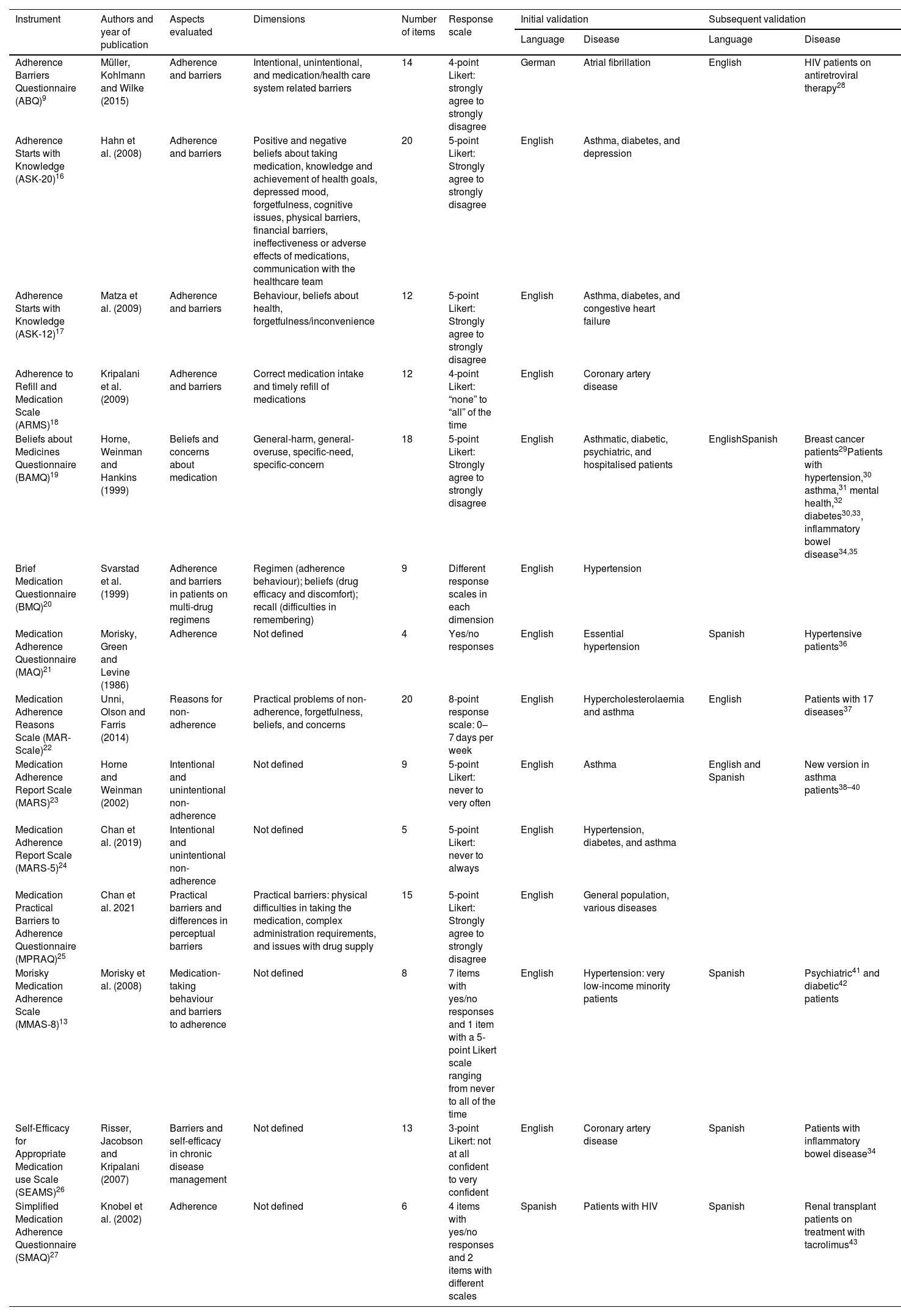
Table 1 describes the questionnaires identified in the systematic review, as detailed in the methods. The names of the questionnaires are as follows: Adherence Barriers Questionnaire (ABQ),9 Adherence Starts with Knowledge (ASK-20),16 Adherence Starts with Knowledge (ASK-12),17 Adherence to Refill and Medication Scale (ARMS),18 Beliefs about Medicines Questionnaire (BAMQ),19 Brief Medication Questionnaire (BMQ),20 Medication Adherence Questionnaire (MAQ),21 Medication Adherence Reasons Scale (MAR-Scale),22 Medication Adherence Report Scale (MARS),23 Medication Adherence Report Scale (MARS-5),24 Medication Practical Barriers to Adherence Questionnaire (MPRAQ),25 Morisky Medication Adherence Scale (MMAS-8),13 Self-Efficacy for Appropriate Medication use Scale (SEAMS),26 and Simplified Medication Adherence Questionnaire (SMAQ).27
Validated medication adherence questionnaires found in the systematic review.
| Instrument | Authors and year of publication | Aspects evaluated | Dimensions | Number of items | Response scale | Initial validation | Subsequent validation | ||
|---|---|---|---|---|---|---|---|---|---|
| Language | Disease | Language | Disease | ||||||
| Adherence Barriers Questionnaire (ABQ)9 | Müller, Kohlmann and Wilke (2015) | Adherence and barriers | Intentional, unintentional, and medication/health care system related barriers | 14 | 4-point Likert: strongly agree to strongly disagree | German | Atrial fibrillation | English | HIV patients on antiretroviral therapy28 |
| Adherence Starts with Knowledge (ASK-20)16 | Hahn et al. (2008) | Adherence and barriers | Positive and negative beliefs about taking medication, knowledge and achievement of health goals, depressed mood, forgetfulness, cognitive issues, physical barriers, financial barriers, ineffectiveness or adverse effects of medications, communication with the healthcare team | 20 | 5-point Likert: Strongly agree to strongly disagree | English | Asthma, diabetes, and depression | ||
| Adherence Starts with Knowledge (ASK-12)17 | Matza et al. (2009) | Adherence and barriers | Behaviour, beliefs about health, forgetfulness/inconvenience | 12 | 5-point Likert: Strongly agree to strongly disagree | English | Asthma, diabetes, and congestive heart failure | ||
| Adherence to Refill and Medication Scale (ARMS)18 | Kripalani et al. (2009) | Adherence and barriers | Correct medication intake and timely refill of medications | 12 | 4-point Likert: “none” to “all” of the time | English | Coronary artery disease | ||
| Beliefs about Medicines Questionnaire (BAMQ)19 | Horne, Weinman and Hankins (1999) | Beliefs and concerns about medication | General-harm, general-overuse, specific-need, specific-concern | 18 | 5-point Likert: Strongly agree to strongly disagree | English | Asthmatic, diabetic, psychiatric, and hospitalised patients | EnglishSpanish | Breast cancer patients29Patients with hypertension,30 asthma,31 mental health,32 diabetes30,33, inflammatory bowel disease34,35 |
| Brief Medication Questionnaire (BMQ)20 | Svarstad et al. (1999) | Adherence and barriers in patients on multi-drug regimens | Regimen (adherence behaviour); beliefs (drug efficacy and discomfort); recall (difficulties in remembering) | 9 | Different response scales in each dimension | English | Hypertension | ||
| Medication Adherence Questionnaire (MAQ)21 | Morisky, Green and Levine (1986) | Adherence | Not defined | 4 | Yes/no responses | English | Essential hypertension | Spanish | Hypertensive patients36 |
| Medication Adherence Reasons Scale (MAR-Scale)22 | Unni, Olson and Farris (2014) | Reasons for non-adherence | Practical problems of non-adherence, forgetfulness, beliefs, and concerns | 20 | 8-point response scale: 0–7 days per week | English | Hypercholesterolaemia and asthma | English | Patients with 17 diseases37 |
| Medication Adherence Report Scale (MARS)23 | Horne and Weinman (2002) | Intentional and unintentional non-adherence | Not defined | 9 | 5-point Likert: never to very often | English | Asthma | English and Spanish | New version in asthma patients38–40 |
| Medication Adherence Report Scale (MARS-5)24 | Chan et al. (2019) | Intentional and unintentional non-adherence | Not defined | 5 | 5-point Likert: never to always | English | Hypertension, diabetes, and asthma | ||
| Medication Practical Barriers to Adherence Questionnaire (MPRAQ)25 | Chan et al. 2021 | Practical barriers and differences in perceptual barriers | Practical barriers: physical difficulties in taking the medication, complex administration requirements, and issues with drug supply | 15 | 5-point Likert: Strongly agree to strongly disagree | English | General population, various diseases | ||
| Morisky Medication Adherence Scale (MMAS-8)13 | Morisky et al. (2008) | Medication-taking behaviour and barriers to adherence | Not defined | 8 | 7 items with yes/no responses and 1 item with a 5-point Likert scale ranging from never to all of the time | English | Hypertension: very low-income minority patients | Spanish | Psychiatric41 and diabetic42 patients |
| Self-Efficacy for Appropriate Medication use Scale (SEAMS)26 | Risser, Jacobson and Kripalani (2007) | Barriers and self-efficacy in chronic disease management | Not defined | 13 | 3-point Likert: not at all confident to very confident | English | Coronary artery disease | Spanish | Patients with inflammatory bowel disease34 |
| Simplified Medication Adherence Questionnaire (SMAQ)27 | Knobel et al. (2002) | Adherence | Not defined | 6 | 4 items with yes/no responses and 2 items with different scales | Spanish | Patients with HIV | Spanish | Renal transplant patients on treatment with tacrolimus43 |
HIV: Human Immunodeficiency Virus.
The initial validations for ARMS, BAMQ, BMQ, MAQ, MARS, MMAS-8, and SEAMS were found through the citations of other articles.
The questionnaires identified have different characteristics in terms of structure, item wording, length, and form of administration.
The ABQ, ASK-20, ASK-12, BAMQ, MARS, MARS-5, Mar-Scale, and MPRAQ have items written as first-person statements, whereas the ARMS, BMQ, MAQ, MMAS-8, SEAMS, and SMAQ present the items as questions.
Morisky's questionnaires, both the MAQ and MMAS-8, have widespread use across different diseases, populations, and countries. The questions are formulated to avoid agreement bias and have fewer items. However, they only partially address barriers and do not provide a comprehensive assessment of medication adherence.44
Both the BMQ and MMAS-8 contain parts that use different scales to measure adherence, which could lead to some difficulty in quantification and limit their applicability.
The ABQ, ASK-20, BAMQ, BMQ, MPRAQ, and SEAMS questionnaires may be difficult to administer in pharmacy service settings as they require more time to complete due to their length. However, the shorter versions of the ASK-20 and MARS questionnaires are available with adequate reliability and validity.
In contrast, the MAQ, MARS, MMAS-8, and SMAQ questionnaires are quicker to complete, which is practical in clinical situations. However, they only focus on adherence behaviour and lack in-depth analysis of barriers. For this reason, the results of these questionnaires are of limited use for defining pharmaceutical interventions aimed at overcoming adherence barriers.
The following questionnaires are administered by health professionals during an interview: ARMS, BMQ, MAQ, MMAS, and SMAQ. The remaining 9 questionnaires are self-administered, making it the most common method. This observation is in line with those of previous reviews. Self-administered tools have the advantages of being affordable, brief, acceptable to patients, easy to administer, valid, non-intrusive, and able to provide information on attitudes and beliefs about medication.10 However, potential limitations of these tools include the patients' ability to understand the items and willingness to disclose information, which may affect the accuracy of responses, and reported adherence may not reflect actual medication adherence.45,46
The factors identified in these questionnaires, whether facilitators or barriers, cover the 5 dimensions recognised by the World Health Organisation as affecting adherence.1,45 Different questionnaires contain items that address the following aspects: access to medicines, financial barriers, lack of family or social support, impact of lifestyle and routine, social embarrassment, knowledge and achievement of health goals, knowledge of pharmacotherapy, polymedication, complex administration requirements, perceived ineffectiveness or adverse effects of medicines, physical difficulties in using the medicine, cognitive and memory problems, daily forgetfulness, depressed mood, attitudes towards illness, positive and negative beliefs about taking medicines, concerns, supply of medication, communication and advice from healthcare providers. However, we were unable to identify a single validated questionnaire involving all the dimensions proposed by the WHO.
We identified 6 questionnaires that measure medication adherence and have been validated in Spanish: the BAMQ, MARS, MAQ, MMAS-8, SEAMS, and SMAQ.
The BAMQ assesses beliefs and concerns about medication and considers their impact on adherence. It comprises 2 subscales: A general subscale, designed to broadly assess beliefs about medications, focusing on overuse and harm; and a specific subscale, which assesses beliefs about specific medications administered to treat a disease, focusing on need and concerns.19 It has been used and validated in different populations, and in different diseases, most of which are chronic. Its validation in the Spanish language involved its translation and application in a range of settings: diabetic and hypertensive patients in 2 communities in Spain,30 outpatients with asthma in the city of Valencia,31 patients with mental health problems taking psychiatric medication in Spain,32 Spanish-speaking diabetic patients in the USA,33 and patients with inflammatory bowel disease in Mexico34 and Spain.35
The MARS is a 9-item questionnaire initially designed and validated in patients with asthma and other respiratory diseases.23 It has been applied to other diseases and validated in different languages. Regarding Spanish versions, Cohen et al. (2009) added one extra item and validated it in English- and Spanish-speaking asthma patients in the USA.38 Mora et al. (2011) applied it to Spanish-speaking asthma patients in the USA,39 and Lugo González and Vega Valero (2020) applied and validated it in an adult population with asthma in Mexico.40
The MMAS-8 has been applied and validated in various settings and chronic diseases. It is cited by 42 of 447 articles in our review, including studies conducted across different populations, diseases, and languages, and shows good psychometric properties.36,41,44 The initial 4-item version (MAQ) was validated in Spanish in a cohort of hypertensive patients in 1992.36 Additional items were added to create the MMAS-8. These items focus on medication-taking behaviour, especially those related to underuse (i.e. forgetfulness), such that barriers to adherence can be more clearly identified.10 It was initially validated in English in hypertensive patients and then applied to other conditions and languages. Those in the Spanish language found good correlations with 4 other questionnaires in psychiatric patients41 and in diabetic patients.42
The SEAMS measures barriers and focuses on self-efficacy in chronic disease management, recognising that the patients' ability to perform a given task, such as taking medication, is an important determinant of medication adherence.26 It was initially validated in patients with coronary heart disease, then validated in various chronic conditions. It was translated into Spanish and validated in patients with inflammatory bowel disease in Mexico.34
The SMAQ is a short simple instrument which includes 6 questions on the patient's medication-taking habits; it was initially validated to measure adherence in patients receiving antiretroviral treatment.27 It was then applied in different settings, including among Spanish patients. Ortega Suárez et al. (2011) adapted and validated a Spanish version for use in transplant patients and obtained adequate psychometric properties. The MAQ questionnaire was used to define the convergent validity of this version.43
DiscussionOur scientific literature search strategy identified 14 validated questionnaires on medication adherence, 6 of which were validated in Spanish.
A limitation of this review is that it was impossible to access the 3 articles marked as “full text unavailable”. These articles could not be retrieved from databases or by direct payment to the publisher.
By reading the articles retrieved in the search with the keyword “Spanish”, it was possible to identify questionnaires that had been validated in the Spanish language. In addition, the citations of these articles led to finding previous validations of these questionnaires in Spanish. Our initial search may not have retrieved these previous validations because of the keywords used; that is, the keywords used in these articles did not match the keywords we used (e.g. “scale” or “tool” instead of “questionnaire”).
When selecting a questionnaire to assess medication adherence and its barriers, it is essential that the questionnaire is validated. However, the structure of the questionnaire should also be considered.
Previous reviews of adherence questionnaires, which have already been discussed in this paper, have varied in scope. Lam and Fresco10 described the strengths and weaknesses of direct and indirect methods, and analyse 6 validated questionnaires; Pagès-Puigdemont and Valverde-Merino36 took a similar approach towards questionnaires in Spanish; Tan et al.44 examined Morisky's questionnaires in depth;13,21 and Culig and Leppée46 described 6 extensively validated questionnaires and proposed one developed in Croatia. Finally, Nguyen et al.47 proposed a systematic review of adherence questionnaires and associated factors, but only included articles in English up to 2012; the authors acknowledged the lack of a comprehensive analysis of questionnaire validation. We suggest that our article provides information over and above that of the aforementioned review by considering the factors, barriers, and facilitators of medication adherence included in the questionnaires, by including publications up to April 2022, and by focusing on questionnaires that have not only been translated but also validated in Spanish.
The structure of the questionnaires described in this review varies considerably in terms of the number and wording of the items, whether they are questions or statements, the response scale used, and the period of time the patient has to recall in order to provide a response.
The proposed items cover all recognised factors regarding adherence barriers. This is relevant since, from the philosophy of pharmaceutical care, to effectively improve medication adherence it is important to implement personalized interventions. These are defined as strategies to optimize professional practice that are planned considering the identified barriers to change prospectively.48 Future interventions should be developed within a theoretical framework that can address the complexity of adherence behaviour, take into account individual patient preferences and needs, and incorporate an approach that identifies patient-specific factors for adherence.48,49 Evidence shows that personalised interventions implemented by pharmacists in both community and hospital pharmacies increase medication adherence, improve health outcomes, and reduce resource use, and are cost-effective.45,50–52
The following 6 questionnaires were identified as validated in Spanish, out of the 14 presented in this review: the BAMQ, MARS, MAQ, MMAS-8, SEAMS, and SMAQ. Successive applications and evaluations of the psychometric properties of these questionnaires in Spanish contributed to their validation in this language.
Taking into account that there are approximately 590 million Spanish-speaking people worldwide, it is useful to have questionnaires in Spanish to assess medication adherence in a comprehensive way, applicable to chronic diseases in hospital and community pharmacy services. However, these tools are sometimes poorly adapted to the types of Spanish spoken around the world and to the context of different healthcare systems. We therefore hope that this review will provide future researchers with tools to facilitate the design and validation of their own questionnaires, identifying the barriers to medication adherence in the local setting, and adapting their wording to the local language and healthcare system.
Contribution to the scientific literatureThis review identifies and describes validated questionnaires to assess medication adherence in adult patients with chronic diseases.
It can help pharmacists select the tools that best fit the needs of the pharmaceutical services, patient characteristics, and healthcare system.
Ethical responsibilitiesThis systematic review does not involve confidential information or patient data.
The Research and Development Project on which this systematic review is based was endorsed by the Bioethics Commission of the Faculty of Biochemical and Pharmaceutical Sciences of the National University of Rosario, by Resolution N°398/2022.
FundingThe systematic review was written within the framework of a Research and Development Project (2022–2025) accredited by the Secretariat of Science, Technology and Innovation (CTeI) of the National University of Rosario, by Resolution of the Higher Council N° 913/22. The CTeI Secretariat grants an annual subsidy to each accredited project.
- •
Director: Dr. María Luz Traverso, Assistance Pharmacy Area, Faculty of Biochemical and Pharmaceutical Sciences, Natinal University of Rosario, Argentina.
- •
Project title: Development of an instrument to assess medication adherence in the practice of Pharmaceutical Care.
All authors accept responsibility as defined by the International Committee of Medical Journal Editors (Available at: http://www.icmje.org/).
In the event of publication, the authors grant exclusive rights of reproduction, distribution, translation, and public communication (by any media or sound, audiovisual, or electronic support) of their work to Farmacia Hospitalaria and, by extension, to the SEFH. To this end, a letter of assignment of rights will be signed at the time of submission of the paper through the online manuscript management system.
CRediT authorship contribution statementAldana Intilangelo: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. Sofía Majic: Writing – original draft, Methodology, Investigation, Data curation. Valeria Palchik: Writing – original draft, Methodology, Investigation. María Luz Traverso: Writing – review & editing, Writing – original draft, Investigation, Data curation.