Deprescribing is considered one of the main strategies available for preparing an individualized therapeutic plan in patients with multiple pathologies or complex chronic conditions. However, despite the practice has been in place for some years, there is a need for studies that support the achievement of satisfactory health outcomes, as well as tools that help implement deprescribing in routine clinical practice. The objective of this project is to analyze the impact of reducing polypharmacy, through the LESS-CHRON deprescribing tool, on a population of elderly polymedicated patients with multiple conditions.
MethodThis will be a quasi-experimental, pre-and-post intervention multicenter cohort study. The variables to be analyzed will be divided into two large groups: those related to pharmacology and those related to health outcomes. They will be collected at baseline, at 3 and at 6-12 months. A first screening phase will be carried out to recruit candidate patients and obtain information about the identified deprescribing opportunities. The second phase will constitute the intervention phase, where the LESS-CHRON tool will be applied and the actual deprescribing of the drugs will be carried out.
DiscussionLESS-CHRON has been proclaimed as a suitable deprescribing tool in clinical practice. Validation of LESS-CHRON will seek to show the results that can be achieved following the deprescribing of drugs, in addition to demonstrating that the tool can reliably be used by clinicians in their routine practice. On the other hand, the results of this project may provide information leading to improving or adapting the tool itself, giving rise to a second generation of more efficient versions.
La desprescripción se considera una de las principales estrategias disponibles para el abordaje del plan terapéutico individualizado en pacientes pluripatológicos o crónicos complejos. Sin embargo, a pesar de ser una práctica ya instaurada desde hace algunos años, se reclama la necesidad de estudios que avalen la obtención de buenos resultados en salud, además de herramientas que ayuden a su desarrollo en la práctica clínica habitual. El objetivo de este proyecto es analizar el impacto en la disminución de la farmacoterapia mediante la desprescripción de fármacos, aplicando la herramienta LESS-CHRON, en una población de pacientes de edad avanzada, polimedicada y con múltiples patologías.
MétodoEstudio cuasiexperimental, pre-post, multicéntrico. Las variables a analizar se dividirán en dos grandes grupos: referidas a la esfera farmacológica y referidas a resultados en salud. Se recogerán en el momento basal, a los 3 y a los 6-12 meses. Se realizará una primera fase de screening para localizar a los pacientes candidatos, que permitirá obtener la información relativa a las oportunidades de desprescripción identificadas. La segunda constituirá la fase de intervención, en la que se aplicará la herramienta LESS-CHRON y se llevará a cabo la desprescripción real de los fármacos.
DiscusiónLESS-CHRON se ha proclamado como una herramienta adecuada para llevar a cabo la desprescripción de fármacos en la práctica clínica. La validación de la herramienta LESS-CHRON tratará de poner de manifiesto los resultados que se logran tras efectuar la desprescripción, además de demostrar su fiabilidad como herramienta, de modo que los clínicos puedan usarla como parte de su actividad asistencial con total confianza. Por otro lado, los resultados de este proyecto podrán arrojar información para la mejora o adaptación de la propia herramienta, dando lugar a una segunda generación o futuras versiones mejoradas y más eficientes.
Patients with multimorbidity (MMPs) may be defined as those suffering from two or more conditions pertaining to two or more of a series of distinctive clinical categories, lasting more than one year and requiring ongoing medical care and/or limiting the performance of activities of daily living1. Four percent of MMPs account for the consumption of 30% of hospital and primary-care resources1. MMPs are a heterogeneous population in terms of their age, complexity, mortality rate, limited function and vulnerability2. The term patient with complex health needs (or chronic complex patient) has recently been adopted to refer to patients who meet at least one of the criteria in the MMP definition and who fall in at least one of the following categories: severe mental disorder, extreme polypharmacy, social/family-related risk, stage II or higher pressure ulcers, delirium (currently or at a previous admission to hospital), malnutrition, long-term enteral nutrition, two or more hospital admissions in the previous 12 months or alcohol abuse1.
Current international trends for the management of these patients are based on an individualized care plans where pharmacological treatment plays an important role3. These plans require the participation of pharmacists in the multidisciplinary to ensure appropriate follow-up of MMPs. Indeed, the pharmacists have succeeded in successfully resolving issues related with these patients’ drug therapy, preventing the health problems they may cause and optimizing the cost of healthcare4,5.
Deprescribing has already become part and parcel of most pharmacotherapeutic optimization strategies. The concept has always been understood as a process whereby a patient's drug treatment is reviewed in order to discontinue, reduce or replace inappropriate medicines in elderly patients6. Although deprescribing is currently part of good prescribing practice, specialists are clamoring for more robust health outcomes-based evidence that may warrant its application, and for tools that may facilitate its widespread use in clinical practice6. A new deprescribing concept has emerged in the last few years as a counterpoint to the term adequacy whereby deprescribing is defined as the long-term review and evaluation of a patient's therapeutic plan with a view to discontinuing, replacing or modifying a previously prescribed drug regimen which, under certain clinical conditions, may be considered unnecessary or ill-advised from a risk/benefit perspective7. Moreover, a series of features have been established as characteristic of the deprescribing process, including monitoring of health variables (intended to prevent negative effects following discontinuation of a drug), and consideration of the patient's life expectancy, as an important criterion for deprescribing certain treatments.
Based on the definition above, the LESS-CHRON tool8 was developed in order to contribute to systematizing and developing the deprescribing process. The LESS-CHRON can be found in drug portals (Centro Andaluz de Documentación e Información de Medicamentos [CADIME], Comisión Asesora en Farmacoterapia de Castilla y León [CAFCYL]) and the websites of scientific societies (Sociedad Española de Farmacéuticos de Atención Primaria [SEFAP], Sociedad Española de Medicina de Familia y Comunitaria [SEMFYC]) from all over Spain. They have been used in different research projects and their usefulness has been demonstrated at different levels of healthcare9. Internationally, it is gaining popularity as one of the most useful and easily applicable deprescribing tools for elderly patients10.
The purpose of this project was to analyze the impact of the LESS-CHRON tool on the reduction of therapeutic load by discontinuation of at least 10% of the drugs prescribed at baseline in a population of MMPs and patients with complex healthcare needs in two clinical settings.
MethodsStudy designThis protocol was developed in accordance with the recommendations of the SPIRIT guidelines for reporting clinical trial protocols11. It will be a quasi-experimental, pre-and-post intervention multicenter cohort (ambulatory and institutionalized patients) study. There will be a researcher in charge of coordinating the overall project and a principal investigator for each participating center. Patients will be recruited in a competitive way until the target sample size is reached, over a period of no more than 4 months from baseline. The study period will extend from patient recruitment, which is when the intervention (deprescribing) will be performed, to 6 or 12 months post-intervention, with a follow-up at 3 months.
Participation of the patients and the general publicThe studied population will be made up of institutionalized (in extended care facilities or nursing homes) or ambulatory (treated in subacute care facilities or internal medicine or geriatrics outpatient units in hospitals or primary care centers) patients.
Eligibility criteriaPatients will be selected in accordance with the following inclusion criteria: age > 65 years, polypharmacy (>5 drugs), complex healthcare needs (multimorbid or chronic complex patients), institutionalized or ambulatory patients under the supervision of a multidisciplinary care team that includes a pharmacist, treated with at least one drug included in the LESS-CHRON tool, compliance with at least one of the criteria proposed; and having given their informed consent to participate in the study.
Patients who meet one or more of the following criteria will be excluded from the study: having been prescribed drugs that do not comply with the specific indication included in the tool; presence of a non-stabilized active malignant neoplasm or a disseminated metastasis, neurological or mental impairment (without a guardian or legal representative, patients in their death throes [according to the Palliative-Prognostic Index, PPI])12.
Phases of the studyExploratory (screening)All patients who meet the inclusion criteria will be asked whether they wish to participate in the study, they will be explain what the study involves and will be requested to signed an informed consent form. Subsequently, they will be administered the LESS-CHRON tool, and their descriptive and outcome-related variables corresponding to the baseline will be recorded.
Intervention phaseThe pharmacists in charge will identify deprescribing opportunities for each patient and prepare a deprescription report to be discussed by the multidisciplinary team, made up of physicians, nurses (in those centers where they are on staff) and pharmacists. Deprescribing decisions will be made based on the team's subjective evaluation and on the patients’ clinical situation and preferences. If the decision is not to deprescribe, the drugs involved will be classified as unimplemented deprescribing opportunities. The proposed intervention will be recorded in the patients’ medical record.
At 3 months from the intervention, patients will be called for a follow-up visit to determine whether the deprescribed drugs need to be reintroduced. This decision will be made based on their clinical situation and the results of the analytical tests requested by the clinician, specified in the tool (health-related variables to be monitored). All treatment-related variables will be calculated at this appointment.
Subsequent follow-up visits will be scheduled at 6 and 12 months post-intervention to obtain health variables and hospital attendance patterns. Patients where no drug has been deprescribed at the first follow-up visit will be reassessed at 6 months through a telephone appointment to determine whether there have been any significant changes in their clinical situation.
All the results obtained will be subjected to a statistical analysis.
VariablesDescriptive variables (gathered at baselineI
- –
Sociodemographic variables: date of birth, sex, date of inclusion, main caregiver (yes/no) and type of caregiver (external/family member), current address, recruitment site.
- –
Pharmacological treatment: number of drugs prescribed at the time of inclusion, drug burden index (DBI) calculated using he anticholinergic load calculator)13, number of drugs that could be deprescribed (deprescribing opportunities) and pharmacological group they belong to, number and identification of all potentially applicable LESS-CHRON criteria.
- –
Clinical variables: number and subtypes of diagnoses, most common comorbidities, prognosis (PROFUND index)14, degree of dependence for the performance of activities of daily living (Barthel index)15, cognitive impairment (Pfeiffer's index)16, self-perceived health status17, adherence to treatment (ARMSe questionnaire)18, degree of frailty (measured using the Comprehensive Geriatric Assessment [CGA] tool)19, number of falls 6-12 months before inclusion.
- –
Hospital attendance patterns (during the 6-12 months before inclusion): number of hospital admissions and days of hospitalization, number of visits to the emergency room (primary/specialized care), unplanned home visits made to the patient.
Outcome-related variables
- –
Main efficacy variable: drug reduction rate; it is obtained by dividing the total number of drugs deprescribed by the total number of drugs that the patient was taking at the time of inclusion, multiplied by 100.
- –
Secondary efficiency variables related to the treatment and to the tool:
- •
Acceptance/feasibility percentage: number of drugs deprescribed divided by the number of drugs liable to deprescribing, multiplied by 100.
- •
Number of drugs withdrawn per patient: mean and range of the total number of drugs eventually deprescribed.
- •
Number and identification of the LESS-CHRON criteria applied following clinical assessment.
- •
Pharmacological class withdrawn drugs from the same pharmacological class withdrawn as a percentage of the total of drugs withdrawn.
- •
Percentage of success: number of drugs that do not need to be reintroduced at 3 months from deprescribing divided by the total of drugs deprescribed, multiplied by 100.
- •
Number of criteria and their identification, related to the drugs that must be reintroduced at 3 months from withdrawal.
- •
Reasons for reintroducing a drug: reasons why a deprescribed drug must be reintroduced.
- •
Adverse events caused by withdrawal: potential withdrawal syndrome, rebound effect, reappearance of symptoms or reactivation of the disease.
- •
- –
Secondary efficiency variables related to health outcomes: they are compared with their baseline value.
- •
Clinical variables: self-perception of health status, dependence on others for the performance of activities of daily living, cognitive impairment, one-year prognosis, number of falls, and mortality.
- •
Hospital attendance: number of hospital admissions, days of hospitalization and visits to the emergency department within 6-12 months from inclusion.
- •
- –
Subgroup analysis: an exploratory subgroup analysis will be conducted based on the patients’ home address (ambulatory/institutionalized), their life expectancy (according to the PROFUND index) and their level of polypharmacy.
The sample size was determined using the GRANMO v. 7.12 calculator. A random sample of 231 individuals is sufficient to estimate for each hospital, with a 95% confidence Interval and an accuracy of ± 5 percentage units, the percentage of the population where deprescribing will be required (around 10%). The estimated percentage of reintroductions will be 40%.
Data collection and managementThe data will be collected from the patients’ electronic medical records and the e-prescribing modules of each participating hospital. The data will be included in a data collection logbook.
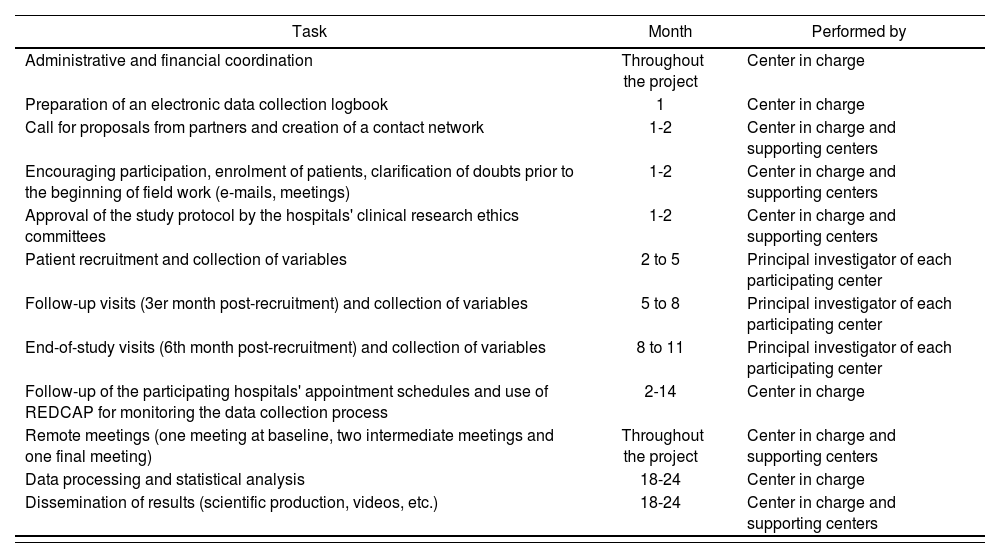
Work planThe schedule of work for the study is presented in table 1.
Schedule of work
| Task | Month | Performed by |
|---|---|---|
| Administrative and financial coordination | Throughout the project | Center in charge |
| Preparation of an electronic data collection logbook | 1 | Center in charge |
| Call for proposals from partners and creation of a contact network | 1-2 | Center in charge and supporting centers |
| Encouraging participation, enrolment of patients, clarification of doubts prior to the beginning of field work (e-mails, meetings) | 1-2 | Center in charge and supporting centers |
| Approval of the study protocol by the hospitals' clinical research ethics committees | 1-2 | Center in charge and supporting centers |
| Patient recruitment and collection of variables | 2 to 5 | Principal investigator of each participating center |
| Follow-up visits (3er month post-recruitment) and collection of variables | 5 to 8 | Principal investigator of each participating center |
| End-of-study visits (6th month post-recruitment) and collection of variables | 8 to 11 | Principal investigator of each participating center |
| Follow-up of the participating hospitals' appointment schedules and use of REDCAP for monitoring the data collection process | 2-14 | Center in charge |
| Remote meetings (one meeting at baseline, two intermediate meetings and one final meeting) | Throughout the project | Center in charge and supporting centers |
| Data processing and statistical analysis | 18-24 | Center in charge |
| Dissemination of results (scientific production, videos, etc.) | 18-24 | Center in charge and supporting centers |
Quantitative variables will be described using means and standard deviations, followed by ranges and median values, in addition to the interquartile range for asymmetrical distributions. Comparisons between groups will be made by applying Student's t test or the nonparametric Mann-Whitney U test, and correlations will be analyzed by calculating Pearson's or Spearman's linear correlation coefficients. Qualitative variables will be described by frequencies and percentages, while associations between groups will be studied by means of contingency tables and applying the chi-squared test or non-asymptotic methods. The patients’ evolution over time will be analyzed using Wilcoxon's nonparametric test quantitative or ordinal variables, or McNemar's test for categorical variables.
Statistical significance will be set at a p value < 0.05.
The data will be analyzed using the IBM SPSS 28 and R statistical software programs.
Ethical and legal aspectsThe study has been approved by the Clinical Research Ethics Committee of the Virgen Macarena and Virgen del Rocío hospitals in Seville, Spain. All relevant clinical best practice criteria and the principles of the Helsinki Declaration will be followed. The authors undertake to abide by such principles and all participants will be informed about the nature of the study and of its goals and will formalize their participation by signing an informed consent document.
DiscussionThe LESS-CHRON criteria constitute a highly appropriate tool for deprescribing drugs in elderly patients given their methodical approach and high methodological quality, and thanks to having been validated by an inter- intraobserver reliability study20. They are also characterized by including specific implementation situations for each deprescribing proposal, which are always validated by a multidisciplinary expert panel made up of clinicians specialized in the treatment of chronic complex patients. The tool includes follow-up parameters intended to ensure a successful deprescribing process.
Against this background, validation of the LESS-CHRON tool in two different hospitals will be a crucial test of its utility. If the test is successful, clinicians will be able to use the tool in clinical practice in a reliable way. The multicenter nature of this project, which is intended to bring together clinical resources from different Spanish autonomous regions and different kinds of health centers (large third-level hospitals, smaller rural centers and extended care facilities), and which will analyze patients treated in outpatient clinics, emergency rooms and in the home setting, will ensure a robust validation of deprescribing practices. It must be noted that the tool will be tested at sites with disparate IT systems, where deprescribing activities may be led alternatively by internal medicine physicians, family doctors or geriatricians, and where the support of pharmacists may be more or less available. It must also be mentioned that the LESS-CHRON tool will be integrated into CHRONIC-PHARMA's13 web and app, which will facilitate its application.
The information provided by this project may contribute to improving the LESS-CHRON tool or adapting it to the routine clinical procedures that this study may reveal as particularly complex. Moreover, the results of this study will make it possible to introduce changes to the tool, giving rise to a second-generation LESS-CHRON system where scenarios characterized by low deprescribing opportunities, low acceptance by clinicians, low lates of therapeutic burden simplification or an urgent need of drug reintroduction are suppressed or radically modified.
FundingSpanish Hospital Pharmacy Foundation (FEFH). 2021/2022. Call for proposals. GT CRONOS (2021-02).
AcknowledgementsList of participating centers:
- –
Centre Forum, Hospital del Mar, Barcelona. Mónica Marín Casino.
- –
Corporació de Salut del Maresme i la Selva, Barcelona. Julia González.
- –
Hospital Bermingham, Donosti. Idoia Beobide Tellería.
- –
Hospital Clinic, sede Platón Barcelona. Ana Rizo Gómez.
- –
Hospital de la Santa Creu i Sant Pau, Barcelona. Ana Juanes Borrego.
- –
Hospital Galdakao-Usansolo, Vizcaya. Pedro Gemio Zumalave.
- –
Hospital San Juan de Dios, Zaragoza. Alejandro J. Sastre Heres.
- –
Hospital Universitario de Fuenlabrada, Madrid. Carolina Mariño Martínez.
- –
Hospital Universitario Joan XXIII, Tarragona. Carlos Cortés Sánchez.
- –
Hospital Universitario Puerta de Hierro Majadahonda, Madrid. Virginia Saavedra Quirós.
- –
Hospital Universitario Ramón y Cajal, Madrid. Eva Delgado.
- –
Hospital Universitario Virgen del Rocío, Sevilla. Aitana Rodríguez Pérez, Marta Mejías Trueba y Lupe Rodríguez de Francisco.
- –
Hospital Virgen de la Victoria, Málaga. Nuria Martínez Casanova.
- –
Pius Hospital de Valls, Barcelona. Silvia Conde Giner.
No conflict of interest.
Contribution to the scientific literatureThe results from this study will provide greater scientific evidence on the actual benefits of deprescribing for patients with multimorbidity in different healthcare scenarios.
Early Access date (07/06/2022).