Cobalt (Co) released by some types of prosthesis causes arthroprosthetic cobaltism. Manifestations of this complication include polyneuropathy, thyroid hypofunction, polyglobulia, diabetes, and cardiomyopathy, which may be life-threatening. Plasma cobalt concentrations (CpCo) exceeding 5 μg/L indicate cobalt poisoning.
Treatment involves removal of the prosthesis and the administration of antidotes: Ethylenediaminetetraacetic acid (EDTA); 2,3-dimercaptopropane-1-sulfonate (DMPS); and dimercaprol.1
Despite the vast number of prosthesis implanted worldwide and the volume of cases of cobaltism reported, mortality is extremely rare.1 We report a case of prosthetic cobaltism that resulted in the death of the patient.
Materials and methodsA case reportA 58-year-old man who presented with ceramic hip prosthesis wear, which was solved with a metal polyethylene-containing revision. The patient had a history of subclinical hypothyroidism treated with Levothyroxine 25 μg/day.
One year after revision surgery, the patient developed poor hypothyroidism control, hearing loss and amaurosis, proximal muscle weakness, upper-limb hyporeflexia, and lower-limb areflexia. X-ray of the hip revealed the presence of a mass lesion with calcifiications, which led to a diagnosis of Co poisoning.
Empirical treatment with 6-h EDTA infusion at 30 mg/kg/12 h was administered for 5 days. The patient developed a pericardial effusion. After Co poisoning was confirmed, with CpCo being 114.5 μg/L, the prosthesis was removed. Later, treatment was initiated with: DMPS 14 mg/kg, 6 days; followed by 4 mg/kg for 5 further days; dimercaprol 3 mg/kg/4 h on days 1–2; every 6 h on Day 3, and every 12 h from Day 4 to 10. Following treatment completion, CpCo decreased by 60% (CpCo = 45.7 μg/L).
Despite the treatment, the patient developed severe hemodynamic instability resulting in death.
The Pierson, Bradford Hill and Newcastle-Ottawa tool was used to determine bias and the quality of evidence in the description of the case, which demonstrated that this report meets selection, ascertainment, causality, and reporting criteria.2
Discussion and conclusionCeramic prosthesis that wear out are replaced with a metal polyethylene prosthesis. Microscopic residual ceramic fragments may cause abrasion to the new prosthesis and result in the release of Co and metals into the bloodstream, thereby causing systemic cobaltism.3
Normal CpCo includes <5 μg/L (1–2 μg/L), both in blood and urine. Levels >5 μg/L are considered toxic and >50 μg/L (300–1000 μg/L) originate systemic cobaltism. In carriers of cobalt prosthesis, the cut-off point that defines CpCo risk is >10 μg/L.4,5 When ingested orally, Co is absorbed between 5-45% and circulates in the bloodstream bound to albumin and transferrin until 85% is excreted via the kidneys, with the remaining amount eliminated through the bile and feces.5
Co blocks mitochondrial metabolism, thereby causing damage to multiple organs. In patients with worn prostheses, toxicity manifests at 2–3 weeks in the form of local pain (complaints, edema, palpable mass, and limping), followed by unspecific symptoms (malaise, fatigue, and anorexia). At 2–3 months, exposed patients may develop neurotoxicity in the form of mixed polyneuropathy, visual and hearing loss (blindness and deafness), and hypothyroidism. In severe cases, patients may develop congestive cardiomyopathy, lactic acidosis, and death.5,6
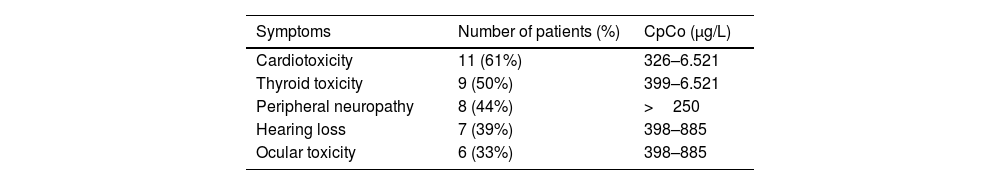
The most frequent symptoms and their association with CpCo have been examined in several studies. A study conducted on 25 patients with hip prosthesis revealed that 84% of symptoms were related to the prosthesis; 60% to the cardiovascular system; 52% to the audiovestibular system, and 48% to the thyroid gland and the peripheral nervous system. CpCo was most significantly associated with abnormal thyroid function.7. Similar results were reported in a study involving 18 patients. The correlation between CpCo and symptoms is shown in Table 1.8
Description of symptoms and mean plasma cobalt concentrations in 18 patients.
| Symptoms | Number of patients (%) | CpCo (μg/L) |
|---|---|---|
| Cardiotoxicity | 11 (61%) | 326–6.521 |
| Thyroid toxicity | 9 (50%) | 399–6.521 |
| Peripheral neuropathy | 8 (44%) | >250 |
| Hearing loss | 7 (39%) | 398–885 |
| Ocular toxicity | 6 (33%) | 398–885 |
From Bradberry et al.
CpCo: Plasma cobalt concentration.
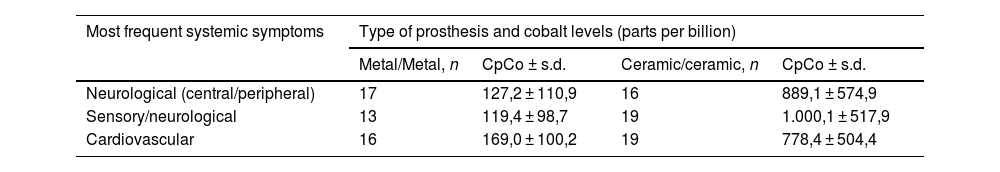
A systematic review was recently conducted to determine the correlation between symptoms, the type of hip prosthesis (metal-on-metal vs fractured ceramic-on-ceramic where the head was replaced with a metal head), and associated CpCo. Neurological symptoms were the most frequent, and CpCo were higher in patients using a ceramic prosthesis (6623 μg/L) as compared to a metal prosthesis (755 μg/L) (Table 2). Metal release is slower and more gradual in metal-on-metal prostheses, as compared to fractured ceramic–ceramic prostheses replaced with a metal one. The reason is that microscopic residual ceramic debris causes heavier friction on the metal, thereby resulting in the massive release of metal into the body.9
Total number of the 3 main systemic symptoms related to toxic Co concentrations in the 2 types of prostheses.
| Most frequent systemic symptoms | Type of prosthesis and cobalt levels (parts per billion) | |||
|---|---|---|---|---|
| Metal/Metal, n | CpCo ± s.d. | Ceramic/ceramic, n | CpCo ± s.d. | |
| Neurological (central/peripheral) | 17 | 127,2 ± 110,9 | 16 | 889,1 ± 574,9 |
| Sensory/neurological | 13 | 119,4 ± 98,7 | 19 | 1.000,1 ± 517,9 |
| Cardiovascular | 16 | 169,0 ± 100,2 | 19 | 778,4 ± 504,4 |
After a diagnosis of Co poisoning has been established, the first step is to remove the prosthesis and clean the bed, since this is the most effective way of removing residual Co. On another note, given that Co is primarily excreted in urine, inducing or optimizing diuresis is recommend. In case of kidney failure, hemodialysis is recommended.5
There is not any specific chelating agent available for Co poisoning as effective treatment. EDTA is the most widely used chelating agent, as it induces ion excretion. Administration for 5 days is recommended to achieve that concentrations decrease below 300 μg/L.10
DMPS at a dose of 4 mg/kg for 5 days for 2–3 weeks reduces CpCo by 25% at 1 month of treatment.11 Dimercaprol reduces CpCo by 30% within the first 3 days of treatment.12
The Spanish Agency for Medicines and Medical Devices (AEMPS) released some recommendations for the radiological and clinical follow-up of patients using implants manufactured by DePuy International Ltd., marketed by Johnson & Johnson Medical Iberia. These prostheses were withdrawn from the market because the bearing system loosened at 5 years from implantation, and the potential release of metallic debris into tissues from the chromium-cobalt prosthesis. In symptomatic patients, measuring levels of chrome and cobalt is recommended.13 Then, in 2012, the AEMPS warned about adverse reactions associated with the release of metal from friction of bearing surfaces of metal-on-metal hip prosthesis.
Sometime later, the medical societies published follow-up guidelines for these patients. Obtaining CpCo and radiological images of the hip is indicated by the American Academy of Orthopedic Surgeons if the patient experienced pain, swelling, or hip sounds. In addition, the Spanish Society of Hip Surgery recommends surgery when CpCo >10 μg/mL.14
Authorship and originalityAll authors contributed equally to the preparation, correction and approval of the final version of this manuscript.
FundingNo funding.