Latest MASCC/ESMO guidelines of the recommendations for the prophylaxis of acute and delayed emesis induced by moderately emetogenic chemotherapy was published in 2016 incorporating anthracycline schemes as highly emetogenic chemotherapy (HEC), proposing triple antiemetic therapy to control nausea and vomiting. Likewise, they recommend triple therapy for carboplatin.
The objectives of this study were to analyze the degree of concordance between guidelines and antiemetic prophylaxis used in the Chemotherapy Outpatient Unit in patients undergoing treatment with HEC and carboplatin, to evaluate its effectiveness and to determine the savings due to the use of netupitant/palonosetron (NEPA) oral (or) with intravenous (iv) dexamethasone (NEPAd) compared to iv Fosaprepitant with ondansetron and dexamethasone (FOD iv).
MethodsProspective observational study recording demographic variables, chemotherapy protocol, tumor location, patient emetogenic risk, antiemetic regimen prescribed, concordance with the MASCC/ESMO guideline, and effectiveness, evaluated by MASCC survey, use of rescue medication and visits to the Emergency Department or hospitalization due to emesis.
A cost minimization pharmacoeconomic study was carried out.
Results61 patients were included; 70% women; median age 60.5.
Platinum schemes were more frequent in period 1, being 87.5% compared to 67.6% in period 2. Anthracycline schemes were 21.6% and 10% respectively in each period.
A 21.1% of the antiemetic regimens did not coincide with the MASCC/ESMO recommendations, being entirely in period 1. The score of the effectiveness questionnaires was total protection in 90.9% in acute nausea, from 100% in acute vomiting and delayed nausea, and 72.7% in delayed vomiting.
The frequency of use of rescue medication was 18.7% in period 1 and was not necessary in period 2.
No visits to the emergency room or admissions were detected in any of the periods.
ConclusionsUse of NEPAd led to a 28% reduction in costs with respect to the use of FOD.
A high level of concordance was obtained in both periods between the latest published guideline and healthcare practice in our field. Surveys carried out on patients seem to suggest that both antiemetic therapies have similar effectiveness in clinical practice. The inclusion of NEPAd has led to a reduction in costs, positioning itself as an efficient option.
En 2016 se publicaron las guías de la MASCC/ESMO que incorporaron los esquemas de antraciclinas como quimioterapia altamente emétogena (QAE) proponiendo la triple terapia antiemética, así como para los esquemas de carboplatino.
Los objetivos fueron analizar el nivel de concordancia entre las guías y la profilaxis antiemética utilizada en el hospital de día de hemato-oncología, evaluar su efectividad y determinar el ahorro de la inclusión de netupitant/palonosetrón (NEPA) oral (vo) con dexametasona intravenosa (iv) (NEPAd) respecto a Fosaprepitant con ondansetrón y dexametasona (FOD iv).
MétodoEstudio observacional prospectivo registrando variables demográficas, esquema de quimioterapia recibido, localización tumoral, riesgo emetógeno del paciente, pauta antiemética prescrita, concordancia con guía MASCC/ESMO y su efectividad, utilización de medicación de rescate y registro de visitas a urgencias o ingresos por emesis.
Se llevó a cabo un estudio farmacoeconómico de minimización de costes.
ResultadosSe incluyeron 61 pacientes; 70% mujeres; mediana edad 60,5.
Los esquemas de platino fueron más frecuentes en el periodo 1 siendo el 87,5% respecto al 67,6% en el periodo 2, Los esquemas con antraciclinas fueron del 21,6% y 10% respectivamente en cada periodo.
Un 21,1% de las pautas antieméticas no coincidían con las recomendaciones MASCC/ESMO, siendo en su totalidad en el periodo 1. La puntuación de los cuestionarios de efectividad fue de protección total en el 90,9% en las náuseas agudas, del 100% en los vómitos agudos y en las náuseas retardadas, y del 72,7% en los vómitos retardados.
La frecuencia de uso de medicación de rescate fue del 18,7% en el periodo 1 y no fue necesaria en el periodo 2.
No se detectaron visitas a urgencias ni ingresos en ninguno de los periodos.
El uso de NEPAd comportó una reducción del 28% de los costes con respecto al empleo de FOD.
ConclusionesSe obtuvo un alto nivel de concordancia en ambos periodos entre la última guía publicada y la práctica asistencial de nuestro ámbito. Las encuestas llevadas a cabo en los pacientes parecen sugerir que ambas terapias antieméticas presentan una efectividad similar en la práctica clínica. La inclusión de NEPAd ha comportado una disminución de costes, posicionándose como una opción eficiente.
Preventing and controlling nausea and vomiting (NV) is crucial in the management of oncology patients. Chemotherapy-induced nausea and vomiting (CINV) are one of the most relevant adverse events of anticancer therapies. More than 80% of patients experience CINV, which impairs the quality of life of patients1. Nausea is defined as the subjective feeling of epigastric distress associated with a need to vomit. Vomiting is defined as the oral ejection of gastrointestinal contents by an emetic reflex coordinated by the central nervous system1.
CINV are categorized into acute, when they occur within 24 h following the last dose, or delayed, when they begin 24 h after the last dose of chemotherapy. Acute CINV are mostly mediated by the binding of serotonin to 5-HT3 receptors in peripheral vagal afferents in the intestine. The activation of neurokinin-1 receptors (NK1) by substance P occurs in central locations, primarily in the area postrema and nucleus tractus solitarius (NTS), and is the primary mechanism underlying delayed CINV. These different pathophysiologies of acute and delayed CINV necessitate diverse pharmacologic approaches2.
Emetogenicity is determined by the agent used and the characteristics of the patient (age, sex or alcohol use) and tumor. The level of emetogenicity of an agent is classified as high, moderate, low and minimal3–8.
There is a diversity of tools available to assess the individual emetic risk of a patient, including the algorithm designed by Junker & Wiedemann in the ‘90s9, or the more recent online CINV Risk Assessment system10. The latter is an intuitive 10-item platform that collects information on the cytostatic agent that is being used, along with some patient-related risk factors. This tool provides an individualized recommendation of antiemetic prophylaxis for each patient. Fig. 1 displays a screenshot of the first screen of the application.
MASCC application for assessing emetic risk in patients (first screen)10.
Dopamin receptor antagonists, such as metoclopramide, used to be administered as a prophylactic treatment in highly emetogenic chemotherapy (HEC). However, their efficacy was low at standard doses and they were associated with a broader range of adverse events.
This therapy was later replaced with serotonin receptor antagonists (ondansetron, granisetron) administered either, alone or in combination with corticosteroids. Some years later, other agents with similar mechanisms of action were developed, including dolasetron and palonosetron1,3,8. Palonosetron has higher affinity for serotonin receptors and a longer clearance time, which increases its antiemetic potential, especially in the late phase, as shown in several studies comparing palonosetron against other antiserotoninergic agents11,12.
The most innovative agents are selective antagonists of NK1 receptors (aprepitant, fosaprepitant, netupitant). These agents, used in combination with a standard combination therapy of corticosteroids and serotonin receptor antagonists, increase antiemetic response in patients receiving HEC, especially the combinations containing palonosetron2.
The combination of netupitant and palonosetron (NEPA) was recently approved. In clinical trials, the patients who received NEPA exhibited non-inferior rates of complete response. Otherwise said, patients did not experience emesis, and rescue medication was not required, as compared to palonosetron in highly/moderately emetogenic chemotherapy2,13,14. These rates were observed both, in acute and delayed emesis.
The availability of different pharmacological groups and routes of administration facilitates the escalation of antiemetic regimens and guarantees prophylaxis. Indeed, suboptimal prophylaxis against emesis is the main risk factor for poor emesis control in successive cycles1–3,5,7,8.
The National Comprehensive Cancer Network (NCCN)15 and the American Society of Clinical Oncology (ASCO)16 published new antiemetic guidelines in 2017, whereas the Multinational Association of Supportive Care in Cancer (MASCC) in association with the European Society for Medical Oncology (ESMO)4 updated their recommendations in 2016. These new guidelines incorporated the AC scheme (anthracycline-cyclophosphamide) as HEC and the triple therapy as a prophylactic antiemetic for carboplatin chemotherapy. Thus, the most widely recommended treatment for the prevention of NV in HEC and carboplatin is the triple antiemetic therapy with serotonin receptor antagonists, selective NK1 receptor antagonists, and corticosteroids4,15,16. In cases of poor emesis control, quadruple antiemetic therapy can be administered in combination with a 5-day olanzapin regimen4,15,16. Adherence to clinical antiemetic guidelines guarantees good NV control and improves the quality of life of patients17.
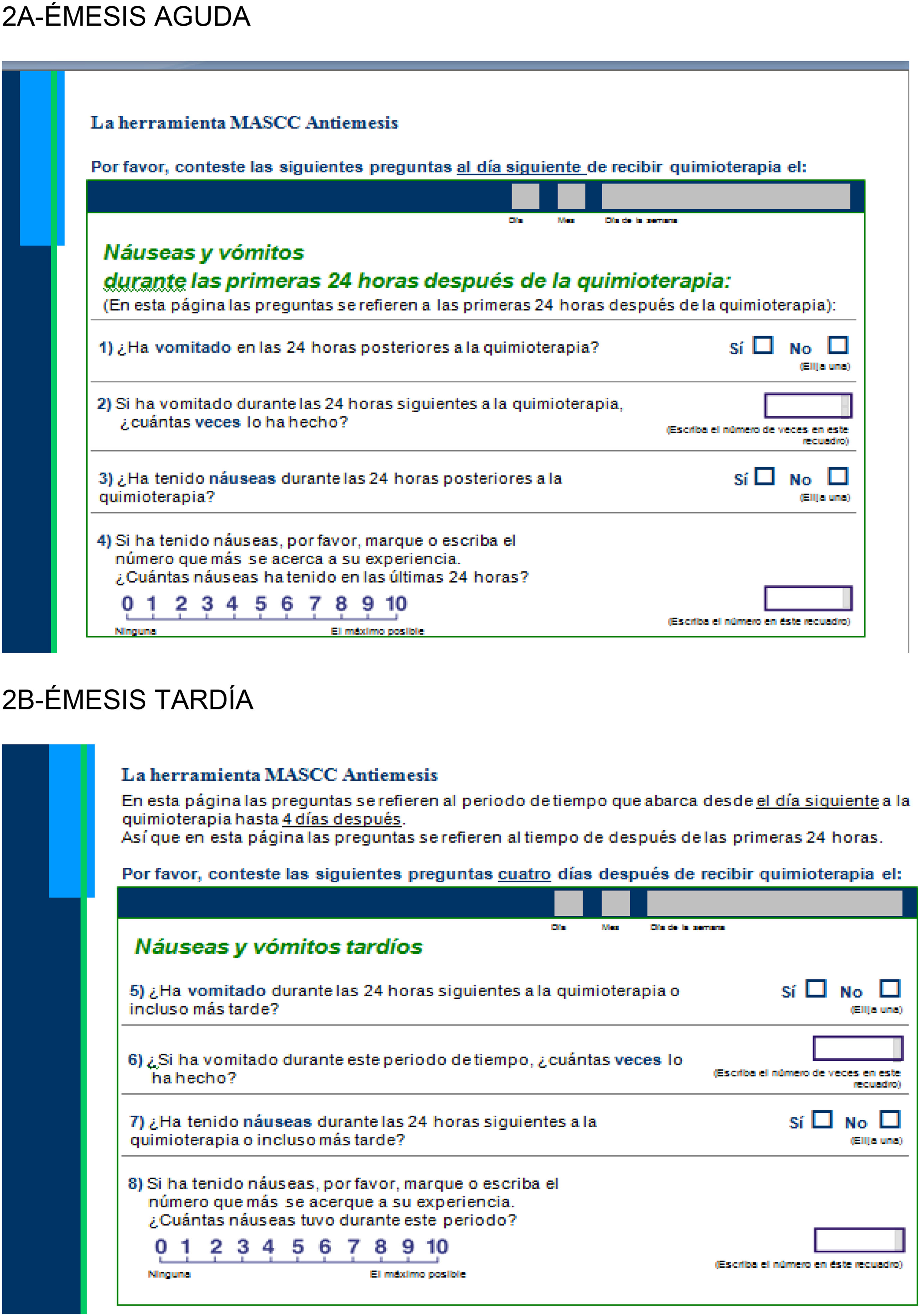
There is a variety of questionnaires available to measure the effectiveness of antiemetic therapies, such as the MASCC Antiemesis Tool18, which assesses acute (Fig. 2A) and delayed (Fig. 2B) emesis.
As shown in Fig. 2A and B, the self-administered questionnaires include four items for each type of emesis,
As a result of the incorporation of NEPA into the Pharmacology Guide of our hospital, antiemetic regimens were modified. In addition, prescribing physicians received specific training in antiemetic strategies.
The primary objective of this study were to assess adherence to MASCC/ESMO guidelines regarding the use of prophylactic regimens in HEC (including AC schemes and triple therapy for carboplatin in our environment) before and after the incorporation of NEPA to the Pharmacotherapy Guide of our hospital and the provision of specific training to prescribing physicians. Other objectives included to assess the effectiveness of antiemetic regimens; and estimate cost savings resulting from the inclusion of oral NEPA and intravenous dexamethasone (NEPAd), as compared to the previous regimen of fosaprepitant (F) plus ondansetron (O) and dexamethasone (DXM) (i.v. FOD).
Materials and methodsA prospective, observational, four-week study was conducted in patients older than 18 years who started HEC and voluntarily agreed to take part in the study.
Prior to the incorporation of oral NEPA (Period 1), two-month follow-up was performed in patients undergoing HEC who received antiemetic therapy with i.v. FOD. Following the incorporation of oral NEPA to routine practice (Period 1), a two-month inclusion period was opened to include patients receiving HEC treated with NEPAd.
MASCC/ESMO guidelines4 were used to classify the emetogenic risk of chemotherapy. Carboplatin and AC schemes were included in the analysis, as established by guidelines4.
Adherence to guidelines was determined based on 2016 MASCC/ESMO antiemetic recommendations4, which was the most recent version available when our review of the use of antiemetic regimens was performed.
The effectiveness of antiemetic regimens before and after the incorporation of NEPA in local routine practice was assessed using the validated Spanish version of the MASCC scale18. Based on a 0–10 scale, MASCC assesses the occurrence of NV within the first 24 and 96 h following the last dose of chemotherapy. Medical records were reviewed for prescriptions of rescue medication, admissions to emergency care, or hospitalizations for episodes of NV. Informed consent was obtained from all patients prior to the administration of the MASCC scale.
Demographic data was collected. Other data included the chemotherapy prescribed (CT); tumor location; individual emetogenic risk as assessed by the CINV Risk Assessment tool10; the antiemetic regimen prescribed; level of adherence to MASCC guidelines4, and their effectiveness.
The pharmacoeconomic study involved an analysis of cost minimization based on direct acquisition costs. The results of the pharmacoeconomic study are presented as percentages of cost reduction achieved with a medication with respect to the other.
The study was approved by the Institutional Review Board of the center.
Statistical analysis: a descriptive statistical analysis of quantitative variables was carried out, with results expressed as means and standard deviations. Median values, and 25th–75th interquartile ranges and distance (IQR 25–75) were determined for normally-distributed variables. Qualitative variables are expressed as absolute values and percentages.
For non-parametric data confirmed by the Kolmogorov–Smirnov test, the efficacy of the two options was analyzed using Mann–Whitney U test for independent variables, considering similar effectiveness and equality of variances on Levene's test. A p value < 0.05 was considered statistically significant.
All statistical analyses were performed using the Minitab-18.1.software package.
ResultsThe sample was composed of 61 patients, of whom 62% were women, with a median age of 60.16 (range ± 11.3).
During Period 1, a total of 24 patients were included, with a median age of 61 (±10.62) of whom 50% were women. During Period 2, a total of 37 patients were included, with a median age of 59 (±13.54), of whom 70.3% were women.
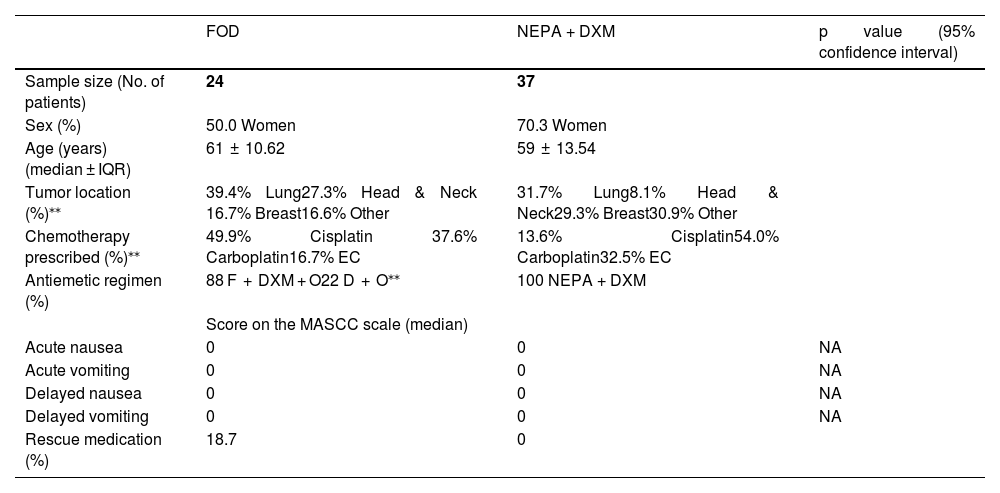
Table 1 displays the main variables analyzed stratified by study period.
Comparison of demographic data and score on the MASCC scale of F + O + DXM with NEPA + DXM.
| FOD | NEPA + DXM | p value (95% confidence interval) | |
|---|---|---|---|
| Sample size (No. of patients) | 24 | 37 | |
| Sex (%) | 50.0 Women | 70.3 Women | |
| Age (years) (median ± IQR) | 61 ± 10.62 | 59 ± 13.54 | |
| Tumor location (%)⁎⁎ | 39.4% Lung27.3% Head & Neck 16.7% Breast16.6% Other | 31.7% Lung8.1% Head & Neck29.3% Breast30.9% Other | |
| Chemotherapy prescribed (%)⁎⁎ | 49.9% Cisplatin 37.6% Carboplatin16.7% EC | 13.6% Cisplatin54.0% Carboplatin32.5% EC | |
| Antiemetic regimen (%) | 88 F + DXM + O22 D + O⁎⁎ | 100 NEPA + DXM | |
| Score on the MASCC scale (median) | |||
| Acute nausea | 0 | 0 | NA |
| Acute vomiting | 0 | 0 | NA |
| Delayed nausea | 0 | 0 | NA |
| Delayed vomiting | 0 | 0 | NA |
| Rescue medication (%) | 18.7 | 0 |
Although percentages differed between the two study periods, the most frequently prescribed schemes in the two study periods were platinum-based regimens, with 78.4% and 90% for Period 1 and Period 2, respectively (Table 1).
Based on the CINV Risk Assessment tool10, 54.1% and 60.6% of patients had a high emetic risk in Periods 1 and 2, respectively.
In Period 1, 22% of antiemetic regimens did not comply with MASCC recommendations. Two patients underwent carboplatin CT, whereas six received AC schemes, more specifically, epirubicin-cyclophosphamide. In Period 2, 100% of regimes coincided.
According to the effectiveness evaluation questionnaires, protection against acute nausea was total in 90.9% of cases in Period 1. Total protection was not achieved in three patients (9.1%), who obtained scores of 5, 2 and 1, respectively. A 100% protection against acute vomiting and delayed nausea was observed, with delayed vomiting having been prevented in 72.7% of cases.
In Period 2, acute nausea was prevented in 94.6% of patients. Total protection was not achieved in two patients (5.4%), who obtained scores of 4 and 3, respectively. Acute vomiting, and delayed nausea and vomiting were absent in 100% of cases.
The frequency of use of rescue medication was 18.7% in Period 1 and 0% in Period 2.
Prophylactic antiemetic therapy was administered in all cases.
No admissions to Emergency Care or hospitalizations for CINV were observed in any of the two study periods.
Considering the triple FOD therapy as 100% of costs, the use of NEPAd accounted for a 28% cost reduction.
DiscussionThere are different antiemetic therapies currently available and recommended in international antiemetic guidelines. However, 61% of patients receiving antiemetic therapy suffer CINV, which suggests poor control19.
We analyzed the level of compliance with the 2016 MASCC/ASCO antiemetic guidelines when antiemetic schemes are used for HEC in our environment.
International antiemetic guidelines consistently identify CINV prophylaxis as a priority of antiemetic therapies. The type of antiemetic regimen will be determined by the emetogenicity of chemotherapy, patient's history of CINV and individual risk factors. Therefore, prophylaxis should be administered to patients with a risk for CINV of 10% or higher. Therapy should be extended throughout the entire period of risk19.
The antiemetic protocols used in our center demonstrate a high adhesion to MASCC/ESMO guidelines during Period 1, and reached 100% in Period 2.
The inconsistencies detected in Period 1 corresponded to carboplatin schemes, particularly, AC schemes, although a poorer NV control was not observed. These findings is significant, as it may indicate that triple therapy is not required in some patients, as opposed to recent recommendations. Therefore, it is necessary to administer a personalized treatment based on individual risks, regardless of the initial risk of the CT being administered. Close monitoring should be performed during the first CT infusions to adjust antiemetic medication, as a function of the individual needs of the patient.
The use of rescue medication was anecdotal and was not associated with any hospital admission during the study periods.
This result contradicts a recent Spanish study reviewing antiemetic regimens for HEC, which showed that a low percentage of patients (29%) received antiemetic prophylaxis with NK1 antagonists, and only 8% of patients19 received the triple therapy recommended by MASC/ESMO guidelines4. The study also reported that 8.46% of patients receiving HEC did not receive any prophylactic antiemetic therapy to prevent CINV.
The authors find these results important, considering that NK1 antagonists administered in combination with 5-HT3 receptor antagonists and corticosteroids have been included in MASCC/ESMO guidelines for the management of CINV in patients receiving HEC4.
According to the authors, the low level of adherence observed in the Spanish study could be explained by the short time elapsed between the incorporation of new recommendations into the 2016 and 2017 guidelines and the time when the study was performed. Thus, there may have not been enough time for these recommendations to be integrated and implemented in clinical practice.
Other studies conducted in Spain consistently reveal a low adherence to the 2004 guidelines and subsequent updates20,21. These results are consistent with those of other observational studies carried out in Europe and U.S.A22,23.
Our study was performed some years after the new recommendations were released, and after specific training had been provided to our team of medical oncologists and oncology pharmacy. This may explain the higher level of adherence, which improved over the months, as new recommendations were progressively incorporated into routine practice in our hospital.
Some authors presume that one of the main causes of low adherence to guidelines may be that a high number of physicians underestimate chemotherapy-induced emetogenic risk19,24.
A European study concluded that CINV control was significantly better in patients who had received antiemetic prophylaxis as recommended in recent guidelines, as compared to patients who had not received prophylaxis17. Similar results were reported in two studies undertaken in U.SA.24 and the United Kingdom25, respectively.
These results consistently revealed a low adherence to antiemetic prophylaxis recommendations. In the study conducted in UK, adherence to guidelines was as low as 29%. In the USA study, adherence was 29% for patients who received HEC, and 73% for those who received moderately emetogenic CT. It is striking that the incorporation of NEPA was not associated with a higher adherence to guidelines, as it was 70% in the two study periods.
The most frequent tumor location in the Spanish study was breast and colorectal cancer, whereas lung and colorectal cancer were most frequently found in our study, where osaliplatinum was in no case considered HEC. Notably, since MASCC guidelines do not consider oxaliplatinum a highly emetogenic medication, triple medication is not recommended for these schemes.
In contrast, most of the HECs analyzed in the two studies were cisplatin-based HECs.
Cisplatin, alone or in combination with gemcitabin, was the most frequent treatment in the study assessing national clinical practice. This is consistent with our environment, where the most frequently prescribed chemotherapy is platinum-based chemotherapy.
The CINV Risk Assessment tool was not useful for assessing individual risk, since the risk ascribed to carboplatin and AC has not been updated based on new guidelines. In 39.4% of patients, high emetogenic risk was excluded based on the type of CT (carboplatin or EC), rather than on the individual risk factors of the patient.
Based on questionnaire results, triple antiemetic therapies may have a similar effectiveness in clinical practice.
This is consistent with the results of a randomized phase-3 clinical trial conducted in Asia comparing oral NEPA plus DXM versus oral aprepitant-granisetron plus DXM in patients receiving HEC26. It is worth mentioning that the study published in 2018 was the first study to directly compare two regimens including the combination of triple antiemetic therapy. The study demonstrated the non-inferiority of a single oral dose of NEPA plus DXM, as compared to the oral three-day aprepitant-granisetrón-DXM regimen, in terms of complete protection against acute and delayed emesis.
Although our regimens were slightly different, in the absence of other comparative studies, the results of that study could be generalized and validated based on the results obtained in our study.
The advantage of NEPA is that it is composed of two agents targeted against two different emetognic pathways. Additionally, NEPA has a long half life, which enables the administration of a single dose prior to chemotherapy. Its effects are maintained for five days, which facilitates adherence to the antiemetic treatment2. For the treatment to be effective, it must be administered in combination with corticosteroids. It is a well-tolerated medication with a favorable safety profile. Moreover, the literature reviewed does not report any unexpected adverse events, with this therapy having a similar profile to that of palonosetron and aprepitant regimens2.
On another note, NEPA is the only fixed combination of NK1 and HT3 receptor antagonists. As some patients are reluctant to take medication, they may found this simple formulation, which involves a lower number of tablets, more convenient.
In the case of patients with poor oral tolerance, it is necessary to use the intravenous formulation of fosaprepitant, anti5HT3 and corticosteroids. However, no cases of poor oral tolerance were recorded in Period 2.
The inclusion of NEPA in our Pharmacotherapy Guide resulted in a reduction of costs associated with NV control, with efficacy remaining the same. Therefore, the use of NEPA is an effective option.
It should be noted that the sample size was significantly smaller than planned, especially in Period 1, due to the restrictions imposed during the COVID-19 pandemic.
In conclusion, although compliance with MASCC/ESMO guidelines was satisfactory, changes related to the incorporation of carboplatin and AC have not yet been completely introduced into routine practice. It is necessary to keep up to date with recent antiemetic recommendations, provide lifelong training to the health professionals involved, and perform a close follow-up of patients, where the Service of Hospital Pharmacy could play a major role.
Antiemetic regimens based on i.v. FOD and NEPAd have demonstrated a similar effectiveness in our environment, with NEPAd emerging as the most efficient option.
Contribution to the scientific literatureDespite the advances in the management of symptoms, chemotherapy-induced nausea and vomiting persist as the adverse events with the highest impact on the quality of life of patients undergoing systemic chemotherapy1.
Some studies assessing clinical practice demonstrate an inconsistent adherence to guidelines2,3. Both, physicians and nurses recognize that antiemetic prophylaxis is not prescribed in accordance with recent recommendations, especially in highly emetogenic chemotherapy2,3.
This study was carried out in a university 800-bed hospital to assess clinical practice in relation to antiemetic prophylaxis in highly emetogenic schemes in our context.
AuthorshipSusana Redondo Capafons: conception and design, data collection, analysis and interpretation, drafting of the manuscript, and approval of the final version prior to submission.
Laura Soriano Gutierrez: conception and design, data collection, analysis and interpretation, drafting of the manuscript, and approval of the final version prior to submission.
Elsa Dalmau Portulas: conception and design and approved the final version of the manuscript prior to submission.
Àlex Barragán Muñoz: data collection, analysis and interpretation, and approved the final version prior to submission.
Sergio Martínez Robles: data collection and approved the final version prior to submission.
Mònica Gómez-Valent approved the final version of the manuscript prior to submission.