To provide evidence of the effectiveness of certolizumab pegol (CZP) in real clinical practice in adult patients with moderate-to-severe plaque psoriasis (PsO) in the context of a risk-sharing agreement (RSA).
MethodsRetrospective observational study based on variables collected in the RSA for treatment with CZP of adult patients with moderate–severe plaque PsO. Ten Spanish hospitals where the RSA was implemented participated. The percentage of patients who achieved the target clinical response of the RSA at the follow-up visit (week 16) was evaluated: absolute Psoriasis Area and Severity Index (PASI) value ≤3 for biologic naïve population, and ≤5 in case of previous failure to a single biologic drug. In addition, the improvement in the scores of other scales included in the study was analysed: Body Surface Area (BSA), Dermatology Life Quality Index (DLQI), Physician's Global Assessment (PGA), and Nail Psoriasis Severity Index (NAPSI). A descriptive analysis was performed for the total population and by patient subgroups (naive vs. non-naive to biologic, male vs. female, and with vs. without discontinuation).
ResultsSixty-six patients were included, 12 men and 54 women. 90.9% achieved the target clinical response, with a mean reduction of 8 (−78.4%) absolute PASI points. Improvement was observed in BSA, PGA, NAPSI, and DLQI, with a reduction of 11.3 (−80.6%), 1.9 (−65.5%), 3.3 (−30.7%), and 9.0 (−66.4%) absolute value points, respectively. Despite not achieving the therapeutic target set in the RSA in 6 patients (9%) (the cost of the drug was assumed by the laboratory), only 2 (3%) discontinued treatment.
ConclusionOur study shows that CZP is effective in real clinical practice in patients with moderate–severe plaque PsO, with an improvement in absolute PASI and DLQI, as well as other scales, both for the total population and in the subgroups analysed. Nearly 91% of patients reached the therapeutic target fixed in the RSA. Implementing this type of agreement can provide a direct or indirect benefit for all the agents involved in the process, providing valuable information for decision-making.
Aportar evidencia de la efectividad de certolizumab pegol (CZP) en la práctica clínica real en pacientes adultos afectados por psoriasis (PsO) en placas moderada-grave, dentro del contexto de un Acuerdo de Riesgo Compartido (ARC).
MétodosEstudio observacional retrospectivo a partir de variables recogidas en un ARC en pacientes adultos con PsO en placas moderada-grave tratados con CZP. Participaron diez hospitales españoles donde se estableció el ARC. Se evaluó el porcentaje de pacientes que alcanzaron la respuesta clínica objetivo del ARC en la visita de seguimiento (semana 16): valour de Psoriasis Area and Severity Index (PASI) absoluto ≤3 para población naive a biológicos, y ≤ 5 ante fracaso previo a un único fármaco biológico. Además, se analizó la mejora en la puntuación de otras escalas: Body Surface Area (BSA), Dermatology Life Quality Index (DLQI), Physician's Global Assesment (PGA) y Nail Psoriasis Severity Index (NAPSI). Se realizó un análisis descriptivo del total de la población y por subgrupos de pacientes (naive vs no naive a biológico, hombre vs mujer, y con vs sin discontinuación).
ResultadosSe incluyeron 66 pacientes, 12 hombres y 54 mujeres. El 90,9% alcanzaron la respuesta clínica objetivo, con una reducción media de 8 (−78,4%) puntos de PASI absoluto. Se observó una mejora en BSA, PGA, NAPSI y DLQI, con una reducción de 11,3 (−80,6%), 1,9 (−65,5%), 3,3 (−30,7%) y 9,0 (−66,4%) puntos del valour absoluto, respectivamente. Pese a no alcanzar el objetivo terapéutico establecido en el ARC en seis pacientes (9%) (el coste del fármaco fue asumido por el laboratorio), sólo dos (3%) discontinuaron el tratamiento.
ConclusiónNuestro estudio muestra que CZP resulta efectivo en la práctica clínica real en pacientes con PsO en placas moderada-grave con una mejora de PASI absoluto y DLQI, así como de otras escalas, tanto para el total de la población como en los subgrupos analizados. Cerca del 91% de los pacientes cumplieron con el objetivo terapéutico establecido en el ARC. La implantación de este tipo de acuerdos puede proporcionar un beneficio directo o indirecto para todos los agentes implicados en el proceso, aportando información de valour para la toma de decisiones.
While therapeutic innovation is progressing rapidly, it often has substantial economic impacts on the Spanish health system.1 The incorporation and regulation of new drugs is a complex process that should ensure patient access to them while maintaining the economic sustainability of the health system in Spain.2 Furthermore, since drug efficacy is based on evidence obtained from clinical trials, the aforementioned health system is at risk of incorporating new drugs that may be ineffective in clinical practice or non-superior to those already available.2,3 Consequently, data derived from real-world clinical practice play a pivotal role in effectively addressing the uncertainty arising in this context.
Several financial instruments are available to mitigate this significant uncertainty, and among them, risk-sharing agreements (RSAs) are some of the most relevant. These agreements are established between drug providers and payers with the aim of linking the drug's price to a set of variables associated with outcomes.4
There are several types of RSAs, with performance-based RSAs commanding great interest.4,5 In these agreements, drug providers negotiate reimbursement with payers in the event of failure to achieve the expected clinical outcomes.5 These agreements are usually encountered in therapeutic areas with multiple treatment options and well-defined outcomes,5 such as cancer, infectious diseases, rare conditions, and autoimmune diseases.6,7 Regarding the latter group, biologic therapy has been positioned as an appropriate strategy for their management.8 However, the high cost and lack of real-world clinical experience with recently approved biologics necessitate the implementation of mechanisms that enable access while concurrently evaluating their use through evidence-based practice.9,10
Specifically, in the treatment of moderate-to-severe plaque psoriasis (msPs), the use of biologics and their biosimilars has broadened the therapeutic options.10,11 In total, 2.3% of the Spanish population is affected by msPs,12 a chronic immune-mediated inflammatory disease for which there is no curative treatment.13 When traditional topical and systemic treatments are ineffective or unsafe, several biological alternatives are available.11,13 In this setting, 2 categories of biologic agents are currently available, which can be classified based on their mechanism of action: tumour necrosis factor-α inhibitors (anti-TNF-α) and interleukin inhibitors.
The Psoriasis Area and Severity Index (PASI) is the standard tool for assessing the severity of skin involvement before and during treatment with these drugs.14 In most clinical trials with biologic therapies, the relative PASI score serves as the primary endpoint. The specific target is PASI 75, representing a 75% reduction in the PASI score from baseline; however, in routine clinical practice, the absolute PASI score is the more commonly used measure of effectiveness.15
In Spain, certolizumab pegol (CZP) is one of the approved biologic agents for treating msPs in adults eligible for systemic treatment. This anti-TNF-α agent was approved in Spain in 2018, and is dispensed under the trade name Cimzia.16 This agent has exhibited high efficacy in clinical trials, with 67%–83% of patients achieving PASI 75 at 16 weeks17–19; however, few studies have evaluated its effectiveness in real-world clinical practice, and are limited by the small size of the study sample.20,21
In 2020, an RSA was established between the pharmaceutical laboratory responsible for its commercialisation and 10 Spanish hospitals to facilitate its acquisition and dispensation in adult patients with msPs. These agreements set the absolute PASI score at 16 weeks as an objective outcome measure. This study contributes new evidence in real-world clinical practice of the effectiveness of CZP in adult patients with msPs, as well as by subgroup, within the framework of the agreement. We also investigate the effectiveness of CZP by using other scales commonly used for therapeutic decision-making in patients with msPs.
MethodsStudy designA retrospective observational study was conducted, using variables included in the RSA, in adult patients with indications for CZP treatment for msPs.
Data were analysed from patients in 10 Spanish hospitals which, in 2020, established an RSA with the pharmaceutical laboratory that manufactures CZP (UCB, Belgium) in order to facilitate its acquisition and dispensing. As part of the RSA, the pharmaceutical laboratory covered the cost of the drugs for patients who discontinued treatment within the first 16 weeks due to not achieving the predefined efficacy variables or for safety reasons. An optimal clinical response was defined as an absolute PASI score of ≤3 in biologic naïve patients and ≤5 in those who had experienced primary or secondary failure with a previous biologic drug, in line with the current recommendations of the Psoriasis Group of the Spanish Academy of Dermatology and Venereology for psoriasis treatment with biologic therapy.9
In order to conduct a protocolized and standardised evaluation and determine therapeutic success or failure, the treating dermatologists were required to input patient data into an electronic data collection platform as part of the RSA. The drug was prescribed following the Summary of Product Characteristics, beginning with an initial dose of 400 mg at weeks 0, 2, and 4, and then a maintenance dose of either 200 mg or 400 mg every 2 weeks, depending on clinical response.16
Patients eligible for treatment under the RSA were selected according to the following inclusion criteria: (1) adults with msPs who had been prescribed CZP according to the Summary of Product Characteristics; (2) biologic naïve patients or those who had experienced primary or secondary failure with a previous biologic drug; and (3) those who had attended a first follow-up visit as part of routine clinical practice. We extracted the anonymised patient data that had been entered in the electronic platform by the participating centres during the period 2020–2021.
The optimal sample size was set at 75 patients (level of precision: 10%; 95% confidence interval) based on the prevalence of the disease in the Spanish population and systemic treatment of the disease.18,22,23
The study was approved by the Clinical Research Ethics Committee of the Hospital General Universitario de Elche, Spain (approved: 7 October 2021; code 12/2021).
Study variablesThe analysis included data collected at the baseline and first follow-up visits. During the baseline visit, dermatologists collected the following data: date of visit, age, sex, reproductive status (in women), body mass index (BMI), whether the patient was biologic treatment naïve or had received any previous biologic treatment, the dose and administration frequency of CZP, and other current treatments for msPs. During follow-up, the following data were collected: date of visit, date and reason for discontinuation (if any), and other current treatments for msPs. To assess treatment response, the following data were collected at both the baseline and follow-up visits: absolute PASI score, Body Surface Area (BSA) score, Dermatology Life Quality Index (DLQI) score, Physician's Global Assessment (PGA), and Nail Psoriasis Severity Index (NAPSI) score (Supplementary Table 1).
Statistical analysisA descriptive analysis was conducted of the sociodemographic and clinical characteristics of the study sample. Qualitative variables are expressed as absolute and relative frequencies, and quantitative variables are expressed as mean and standard deviation (SD).
Prior to analysis, a descriptive study of the study sample was performed. The absolute PASI score defined the primary endpoint: that is, the percentage of the total sample who achieved the RSA target clinical response. We also analysed this outcome by patient subgroup (biologic naïve vs. biologic non-naïve; and male vs. female). The number of patients who did not reach the target clinical response defined the percentage of patients whose treatment cost up to week 16 was covered by the pharmaceutical laboratory. To analyse the effectiveness of treatment, secondary endpoints were improvements between the baseline and follow-up visit scores on the other scales (BSA, DLQI, PGA, and NAPSI) in the total sample and by patient subgroup (biologic naïve vs. biologic non-naïve; male vs. female; and interruption vs. no interruption). Finally, we estimated the difference (expressed as a percentage) between the cost of the drug within the RSA framework and outside of it.
Statistical analyses were performed using the statistical program STATA version 14.2 (StataCorp, Texas, USA).
ResultsCharacteristics of the patientsA total of 66 patients were included in the study (women: 81.8%; n = 54) with a mean age of 37 years (SD: 12.3) (Table 1).
Characteristics of the study sample.
| Variable | Study sample, n (%) |
|---|---|
| Age in years, mean (SD) (n = 66) | 37 (12.3) |
| Sex (n = 66) | |
| Men, n (%) | 12 (18.2) |
| Women, n (%) | 54 (81.8) |
| Fertile age | n (57.4) |
| Wish to have children | 15 (27.8) |
| Breastfeeding | 2 (3.7) |
| None of the above | 6 (11.1) |
| BMI, mean (SD) (n = 66)BMI≥25, n (%) | 25.5 (5.3)32 (48.5) |
| Time since diagnosis in years, mean (SD) (n = 66) | 12.7 (8.4) |
| Tobacco use (n = 66) | |
| Smoker, n (%) | 15 (22.7) |
| Non-smoker, n (%) | 51 (77.3) |
| Family history of psoriasis (n = 66) | 29 (43.9) |
| Personal history of risk for the disease (diseases associated with msPs) (n = 62) | 12 (18.2) |
| Presence of comorbidities (non-articular) (n = 64) | 13 (20.3) |
| Presence of articular disorders (n = 66)Peripheral arthritis, n (%) | 13 (19.7)8 (12.0) |
| Previous systemic (non-biologic) treatment (n = 66) | 41 (62.1) |
SD, Standard deviation.
Regarding treatment at the baseline visit, 74.2% (n = 49) of patients were biologic naïve, 77.3% (n = 51) were receiving concomitant topical treatment for msPs, 7.6% (n = 5) were receiving other systemic (non-biologic) treatment; and only 3.1% (n = 2) were receiving phototherapy.
At the start of CZP treatment, patients had a mean absolute PASI score of 9.6 (SD: 5.8) (Table 2) and a mean BSA score of 13.1 (SD: 10.9).
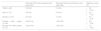
Evolution of the absolute PASI scores at baseline and follow-up visits.
| Absolute PASI score at baseline visit, mean (SD) | Absolute PASI score at follow-up visit, mean (SD) | Difference, mean (%) | |
|---|---|---|---|
| Total (n = 66) | 9.6 (5.8) | 1.6 (2.4) | −8.0 (−78.4) |
| Men (n = 12) | 9.0 (3.6) | 2.7 (3.5) | −6.3 (−72.5) |
| Women (n = 54) | 9.7 (6.2) | 1.3 (2.0) | −8.4 (−79.7) |
| Biologic naïve patient (n = 49) | 9.64 (5.16) | 1.55 (2.03) | −8.0 (−79.9) |
| Biologic non-naïve patient (n = 17) | 9.42 (7.49) | 1.55 (3.27) | −7.8 (−74.3) |
SD, Standard deviation.
Table 3 shows the absolute scores of the scales used to assess the severity and degree of disease involvement at baseline (PGA: mean 2.8, SD: 0.9; NAPSI: mean 4.1, SD: 8.0; and DLQI: mean 11.7, SD: 7.2).
Evolution of BSA, PGA, NAPSI, DLQI scores from baseline visit to follow-up visit.
| Scale | Baseline visit | Follow-up visit | Variation | |||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (%) | |
| BSA | 66 | 13.1 (10.9) | 66 | 1.7 (2.9) | 66 | −11.3 (−80.6) |
| PGA | 54 | 2.8 (0.9) | 53 | 0.9 (0.9) | 49 | −1.9 (−65.5) |
| NAPSI | 34 | 4.1 (8.0) | 33 | 1.0 (3.7) | 25 | −3.3 (−30.7) |
| DLQI | 53 | 11.7 (7.2) | 54 | 2.1 (2.6) | 47 | −9.0 (−66.4) |
SD, Standard deviation.
The maintenance dose of the drug was 200 mg (65.2%; n = 43) or 400 mg (34.8%; n = 23). At the follow-up visit, 46.6% (n = 26) of the patients received topical treatment, 4 patients continued with other systemic (non-biologic) treatments, and no patient continued with phototherapy.
RSA results and effectivenessAccording to the PASI scores, 90.9% (n = 60) of patients achieved the RSA target clinical response at the follow-up visit. However, 6 patients did not achieve this target and thus the cost of the drug for these patients was covered by the laboratory. The implementation of the RSA entailed cumulative savings of 9.1% at 16 weeks. Regarding subgroups, the target response was reached by biologic naïve patients (89.8%; n = 44) and non-naïve patients (94.1%; n = 16). In relation to sex, the target clinical response was reached by both men (91.7%; n = 11) and by women (90.7%; n = 49).
Response to treatmentOver the study period, there was an 8-point (−78.4%) decrease in the mean absolute PASI score to a mean of 1.6 (SD: 2.4) at the follow-up visit. The results also show that there were decreases in all the other scales at the follow-up visit (Table 3). The absolute scores of the BSA, PGA, NAPSIA, and DLQI decreased by 11.3 (−80.6%), 1.9 (−65.5%), 3.3 (−30.7%), and 9.0 points (−66.4%), respectively. A significant positive correlation was found between the decrease in PASI scores and DLQI scores (0.5405; P>.01).
Regarding subgroups, the absolute PASI scores decreased by 8.0 and 7.8 points in biologic naïve and non-naïve patients, respectively. In relation to sex, the absolute PASI score decreased by 6.3 points in men and 8.4 points in women.
Although 6 patients did not reach the target response, only 2 of them discontinued treatment. In the remaining 4 patients, the mean PASI score decreased by 58.9%, and there was an improvement on at least one of the other scales (Supplementary Table 2).
DiscussionThe implementation of an RSA for the treatment of msPs with CZP could mitigate the uncertainties associated with drug effectiveness, safety, and costs in real-world clinical practice.24 The results show that about 91% of patients met the RSA clinical target, in alignment with the current recommendations of the Psoriasis Group of the Spanish Academy of Dermatology and Venereology.9 The pharmaceutical laboratory reimbursed the hospitals for patients who did not reach the RSA clinical target, thus reducing the cost of treatment.
In Spain, manufacturers and hospitals commonly agree RSAs—especially those based on economic negotiations—and regions such as Catalonia have implemented outcomes-based RSAs25; nevertheless, there is little published evidence on such agreements in Spain.
To our knowledge, this is the first study on an outcomes-based RSA targeting psoriasis. Previous studies have explored the impact of this type of agreement in other diseases. In 2011, an RSA on the use of gefitinib for treating of non-small-cell lung cancer was implemented in the Spanish region of Catalonia. Although its efficacy has not been proven in clinical trials in the Spanish population, the results showed an association between the RSA and adequate effectiveness in real clinical practice, leading to direct and indirect benefits. In 2012, a risk-sharing program was implemented in a referral hospital for enzyme replacement therapy for lysosomal storage diseases.27 There were no cost savings because complete effectiveness was achieved in all patients, suggesting that the use of outcome-based RSAs should not only be based on economic aspects, but also on mitigating uncertainty regarding treatment effectiveness, especially in cases of rare diseases. In 2013, an RSA on the treatment of rheumatoid arthritis with CZP24 was established in Catalonia, and subsequently in other Autonomous Communities.28 The results showed cost savings for the pharmacy service and a reduction in the uncertainty associated with the actual efficacy of the drug. The results of our study are in line with these results, demonstrating that this type of RSA is an effective tool for improving efficiency within the healthcare system.
Regarding the clinical outcomes achieved in our study, there was an 8-point reduction in the absolute PASI score at follow-up, which was similar in the subgroups studied: men (6.3) vs. women (8.4) and biologic naïve patients (8.0) vs. non-naïve patients (7.4). Although the size of our sample was not comparable to that of the CZP clinical trials, our results are in line with those of the CIMPASI-1 and CIMPASI-218 clinical trials, confirming the efficacy and safety of the drug in routine clinical practice. Furthermore, the results of our study are also in line with those obtained in previous real-world studies.20,29–32 Carubbi et al. analysed 12 patients treated with CZP for psoriasis and psoriatic arthritis, finding a decrease in the absolute PASI score from 7.93 at the baseline visit to 0.29 at the end of follow-up.32 Vender et al. studied a total of 62 patients, observing a decrease in absolute PASI score from 13.0 points at baseline to 2.3 at follow-up.31 The results of our study were similar to those of Turkmen et al.,30 Vender et al.,31 and Carubbi et al.32 These studies found similar effectiveness between biologic naïve and non-naïve patients, suggesting that CZP is efficacious in patients with psoriasis regardless of their previous exposure to the biologic; however, these studies did not differentiate between men and women regarding its effectiveness. In women of childbearing age, CZP is an optimal drug of choice as well as the recommended drug because it is the only anti-TNF agent that does not cross the placenta during pregnancy, nor is it transferred through breast milk in lactation.33 Our study shows an improvement in effectiveness on all the scales used. In addition, the results obtained for men were also relevant, showing effectiveness in this group of patients.
Although the PASI is considered the gold-standard to evaluate effectiveness in psoriasis, other scales, such as the DLQI, BSA, and PGA, are relevant in therapeutic decision-making when treating patients with msPs with biologics, as they can be used to assess the impact of the disease on the patients' quality of life.13
Our study shows improvements on all the other scales used in relation to their baseline scores. In particular, we highlight the reduction in DLQI scores, given the impact of psoriasis on patients' quality of life. Of the studies cited, only those by Carubbi et al. and the CIMPASI-1 and CIMPASI-2 clinical trials used the DLQI; all these reported improvements of around 10 points, close to the 9-point improvement found in our sample. A DLQI score of 0/1 indicates no deterioration in health-related quality of life (HRQoL),34 whereas a decrease of ≥4 points is considered clinically significant35; thus, CZP had a beneficial impact on patients' HRQoL. We found a significant positive correlation between the DLQI and the PASI, which highlights the relevance of measuring HRQoL during treatment evaluation.
It is noteworthy that only 2 of the 6 patients who did not reach the RSA target discontinued treatment. The remaining 4 patients continued their treatment, possibly because they had shown a favourable response to the treatment they were receiving. In most of these patients, the results show that scores on the other scales were stable or improved during follow-up, indicating a clinically adequate response to treatment.
This study has several limitations. It is a retrospective study and relied on RSA data, with the PASI score as the primary outcome variable; thus, complete data were not obtained for the rest of the scales for the total sample. However, they were analysed in a large number of patients, thus complementing the treatment effectiveness data. The study is also limited by its sample size. The optimal sample size was not reached, likely due to the availability of multiple therapeutic alternatives for these patients and that in clinical practice they are mainly used in women of childbearing age. Furthermore, the sample size was insufficient to yield statistically significant results. Thus, larger sample sizes are needed validate the outcomes observed in real-world practice.
In conclusion, the female and male patients, and biologic naïve and non-naïve patients, who received CZP in real-world clinical practice experienced improvements in their PASI and DLQI scores of 80.6% and 66.4%, respectively, compared to their baseline scores. In addition, 90.9% of the sample achieved the target clinical response established in the RSA. The results show that the implementation of this type of agreement can generate direct or indirect benefits for all the agents involved in the process, as well as providing valuable information for decision-making.
Contribution to the scientific literatureIn line with the results of clinical trials, the study shows that CZP is effective in the treatment of moderate–severe psoriasis.
The real-world results of this study show that the uncertainties associated with the use of CZP can be mitigated via the implementation of a risk-sharing agreement.
Author statementAll the authors contributed substantially to study conception and design, data analysis and interpretation, and writing the article. All authors read and approved this version of the manuscript. Specifically, Andrés Navarro Ruiz and Fernando Toledo Alberola contributed to study design, data interpretation, and approval of the final version; Susana Aceituno contributed to data analysis and drafting the article; and the Arcoderm Group contributed to data collection and interpretation of the results.
FundingNone declared.
Ethical responsibilitiesThe study was approved by the Clinical Research Ethics Committee of the Hospital General Universitario de Elche, Spain (approved: 7 October 2021; code 12/2021).
CongressesPartial or preliminary results have been reported in the following congresses:
- XL + 1 Jornadas de Economía de la Salud (Zaragoza, Spain, 15–17 June 2022).
- 8th Psoriasis Congress (Madrid, Spain, 20–21 January 2023).
The ARCODERM Group: Sergio Santos Alarcón and Rafael Ubeda Bonete (Hospital Virgen de los Lirios, Alcoy); Francisco Javier Mataix Díaz and M. Ángeles Cía Barrio (Hospital Marina Baixa, Villajoyosa); Eva Vilarrasa Rull and Montserrat Masip Torné (Hospital de la Santa Creu i Sant Pau, Barcelona); Luca Schneller Pavelescu-Apetrei and Francisco Rodríguez Lucena (Hospital Vega Baja, Orihuela);Jaime E. Poquet Jornet and Juan Monte Serrano (Hospital de Denia, Denia); Amparo Talens Bolos and Ines Poveda Montoyo (Hospital de Elda, Elda); Rebeca Alcalá García and Joaquín Borrás Blasco (Hospital de Sagunto, Sagunto); Salvador Arias Santiago and Alberto Jiménez Morales (Hospital Virgen de las Nieves, Granada); and David Moreno Ramírez and Vicente Merino Bohórquez (Hospital Virgen Macarena, Seville).