The purpose of this article is to describe the PeOpLe study protocol, developed to assess patient-reported health outcomes in advanced or metastatic non-small-cell lung cancer in routine clinical practice using the methodology provided by the International Consortium for Health Outcomes Measurement tool.
MethodThe study envisaged will be multicenter, longitudinal, ambispective and observational. Two groups will be compared: a control group (followed up according to standard clinical practice) and an experimental group (followed up using the International Consortium for Health Outcomes Measurement methodology adapted to the Spanish setting for 6 months). The variables collected will be related to demography (age, sex, degree of family support), clinical factors (smoking, comorbidities, lung capacity), the neoplasm (histology, staging, mutations), pharmacotherapy (treatment schedule, modifications, and complications), health status (functional status, quality of life, satisfaction and overall survival) and resource consumption (emergency visits, hospital admissions and time spent by health providers). The PeOpLe study protocol has been approved by the Ethics Committee for Research into Medicinal Products of the Gregorio Marañón General University Hospital and will be conducted in compliance with prevailing ethical principles and standards.
ConclusionsThe PeOpLe study will explore how patient-reported outcomes collection can be developed and integrated with the clinical processes used in the management of patients with locally advanced or metastatic non-small cell lung cancer what patient-reported outcomes can be measured with systems that can conveniently be used both by patients and by healthcare providers. Systematic evaluation of patient-reported outcomes will help determine their impact in terms of effectiveness (survival), safety (complications of systemic therapy), and quality of life and patient satisfaction. The multidisciplinary and multicenter nature of the study will facilitate a comprehensive view of the subject analyzed and allow external reproducibility.
El objetivo es describir el protocolo del estudio PeOpLe, cuyo fin es evaluar los resultados en salud centrados en el paciente con cáncer de pulmón no microcítico avanzado o metastásico en la práctica clínica habitual mediante una metodología adaptada de la herramienta del International Consortium for Health Outcomes Measurement.
MétodoEstudio observacional, ambispectivo, longitudinal y multicéntrico. Se compararán dos grupos: grupo control (seguimiento según práctica clínica habitual) frente a un grupo intervención (seguimiento mediante la metodología del International Consortium for Health Outcomes Measurement adaptada al entorno español) durante un período de 6 meses. Las variables recogidas incluirán aspectos demográficos (edad, sexo, apoyo familiar), clínicos (hábito tabáquico, comorbilidades, capacidad pulmonar), del tumor (histología, estadiaje, mutaciones), farmacoterapéutico (esquema de tratamiento, modificaciones y complicaciones), grado de salud (estado funcional, calidad de vida, satisfacción y supervivencia global) y consumo de recursos (visitas a urgencias, ingresos hospitalarios y tiempo dedicado por los profesionales sanitarios). El protocolo del estudio PeOpLe ha sido aprobado por el Comité de Ética de la Investigación con medicamentos y se realizará respetando los principios y las normas éticas básicas.
ConclusionesEl estudio PeOpLe explorará cómo se pueden desarrollar e integrar los procesos de medición de resultados en salud centrados en los pacientes, especialmente los patient-reported outcomes, en pacientes con cáncer de pulmón no microcítico localmente avanzado o metastásico en la práctica clínica. La evaluación sistemática de estos patient-reported outcomes permitirá conocer su impacto en términos de efectividad (supervivencia), seguridad (complicaciones de la terapia sistémica) y calidad de vida y satisfacción. El carácter multidisciplinar y multicéntrico facilitará una visión integral y su reproducibilidad externa.
There is currently a generalized consensus between patients, healthcare providers and administrators around the need to move towards a healthcare system that is based on the creation of value. Our healthcare system is at present in the throes of a trend towards placing the patient at the center of all care processes, striving to ensure that healthcare goals are aligned with patients’ needs and expectations1. Patients must be the protagonists of the steps taken to follow-up their condition and of all the decisions made regarding their disease. Evaluating and integrating patient-reported outcomes (PROs) is a key factor in achieving this goal2,3. These PROs are basically related to the quality-of-life dimension, which encompasses the patients’ health status and/or symptoms as well as adherence to treatment, among other factors. Evaluation of PROs is normally performed by means of questionnaires that are rigorously developed and validated to ensure their clarity, reliability and reproducibility. Among other aspects, PROs have shown that the patients’ perception on the severity of their symptoms and on the latter's impact on their quality of life is different from that measured by healthcare providers4–6.
There are a series of conditions that make PROs particularly noteworthy such as those pertaining to chronic or end-stage conditions, diseases that result in disability or which are associated with a high social or occupational impact or treatment with limited effectiveness or a high incidence of adverse events. Generally speaking, the higher the variability of a given process and the greater the uncertainty regarding its results, the higher the impact of PROs. Despite the benefits of evaluating PROs in some of these situations, their use in clinical practice is anecdotal. As a first step to expand their application, significant work has been done in recent years to systematize their collection and analysis.
The International Consortium for Health Outcomes Measurement (ICHOM) was created with the aim of promoting the development of valuebased healthcare. With that purpose in mind, the ICHOM developed a series of standard sets of patient-centered outcome measurements7. These standard sets have to date been developed for 40 conditions, including five malignant neoplasms: colorectal cancer, breast cancer, lung cancer, localized prostate cancer and advanced prostate cancer. Cancer patients are prime candidates for benefiting from the ICHOM health outcomes assessment methodology. This is clearly exemplified by lung cancer patients. Approaches to metastatic lung cancer is becoming increasingly complex, mainly due to the appearance of novel high-impact medicines and the short life expectancy of patients. Against a background where treatments are usually not curative and may in addition hamper the patients’ quality of life with meagre increases in survival, the role of PROs becomes particularly significant8.
The evidence on the impact that these healthcare interventions have on the health status of patients with lung cancer in clinical practice is very limited. This is due to several reasons such as the fact that many healthcare providers are not aware of the existence of this methodology, the difficulty to integrate PROs with healthcare information systems, and above all, the lack of a systematic method for gathering and evaluating them9. In other to promote implementation of PROs in the Spanish health system, an adaptation of ICHOM's standard set of outcome measurements for lung cancer was made taking into considerations the characteristics of the Spanish health system10.
Systematic evaluation of the health outcomes from real-world individuals, not restricted by the stringent criteria of clinical trials, favors the participation of patients in decision-making, optimizing results and spearheading the sustainable management of resources11.
The purpose of this study is therefore to design a protocol to evaluate patient-centered health outcomes in individuals with locally advanced metastatic or non-small cell lung cancer (NSCLC) obtained following adaptation and implementation of ICHOM's standard set.
MethodsDesignA multicenter longitudinal ambispective observational study will be conducted aimed at evaluating the implementation of PRO measurements following the ICHOM methodology. A control group, comprising patients followed up through standard clinical practice, will be retrospectively compared with an experimental group, comprising patients prospectively followed up through the methodology proposed in the study. The recruitment period will last 12 months while the follow-up period in both groups will be of 6 months.
ScopePatients will be recruited from three university hospitals from the Madrid region (Gregorio Marañón General University Hospital, La Paz University Hospital, and Fuenlabrada University Hospital).
Studied populationAn sample size of 100 patients (50 in each arm) is envisaged.
Inclusion criteria:
- –
Control group: Adult patients with early-onset (unresectable stage IIIB or IV) locally advanced or metastatic NSCLC started on palliative antineoplastic treatment (chemotherapy, immunotherapy or targeted oral therapies) within 6 months prior to recruitment.
- –
Experimental group: Adult patients with early-onset (unresectable stage IIIB or IV) locally advanced or metastatic NSCLC started on palliative antineoplastic treatment (chemotherapy, immunotherapy or targeted oral therapy). Exclusion criteria:
- –
Patients presenting with language, cultural or cognitive barriers that prevent them from participating in the study interview or understanding the questionnaires that need to be filled out.
- –
Patients started on treatment at a facility different from those participating in the study.
- –
Patients started on treatment within the context of a clinical trial.
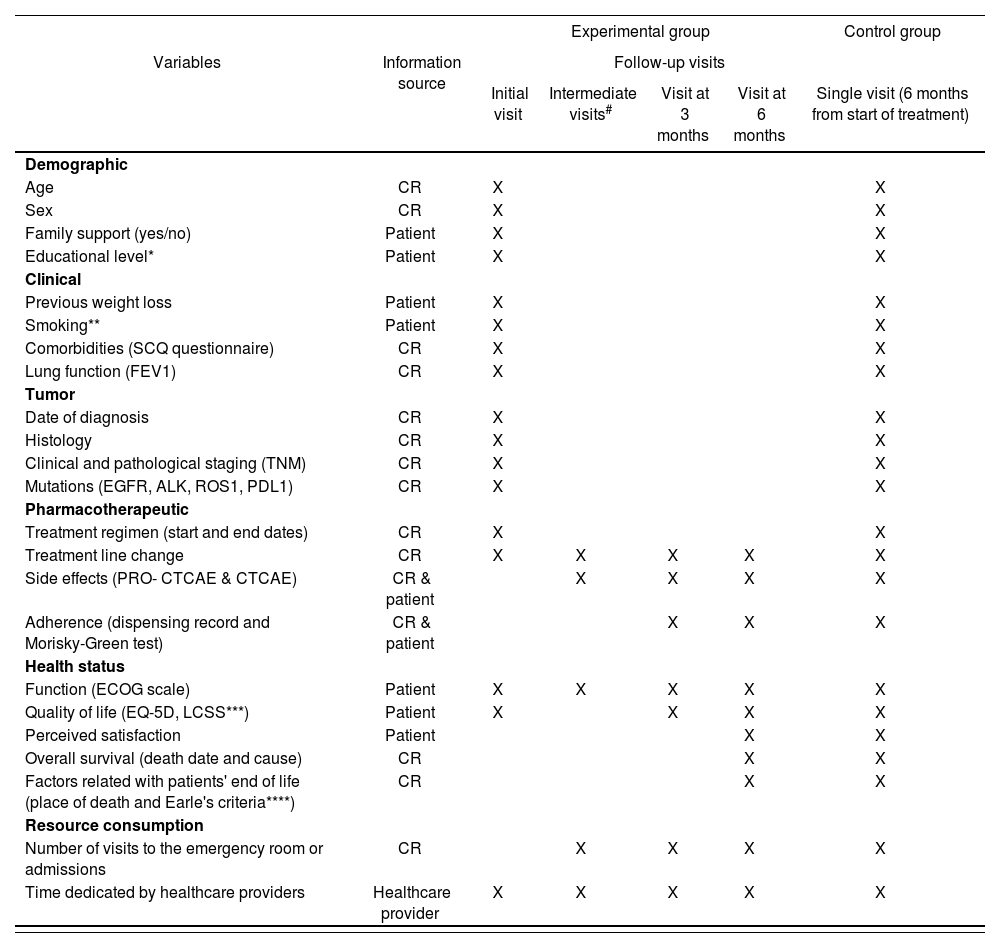
The variables collected as part of the PeOpLe study in the course of the interviews conducted with patients, as well as their frequency and sources of information are described in table 1. The main endpoint of the study will be health-related quality of life as evaluated using the EQ-5D questionnaire. An analysis will be made of the association between type of treatment (chemotherapy; immunotherapy, chemotherapy + immunotherapy or targeted oral therapy) and the result of the main endpoint.
Variables of the PeOpLe study: sources, collection frequency and measurement instruments
| Experimental group | Control group | |||||
|---|---|---|---|---|---|---|
| Variables | Information source | Follow-up visits | ||||
| Initial visit | Intermediate visits# | Visit at 3 months | Visit at 6 months | Single visit (6 months from start of treatment) | ||
| Demographic | ||||||
| Age | CR | X | X | |||
| Sex | CR | X | X | |||
| Family support (yes/no) | Patient | X | X | |||
| Educational level* | Patient | X | X | |||
| Clinical | ||||||
| Previous weight loss | Patient | X | X | |||
| Smoking** | Patient | X | X | |||
| Comorbidities (SCQ questionnaire) | CR | X | X | |||
| Lung function (FEV1) | CR | X | X | |||
| Tumor | ||||||
| Date of diagnosis | CR | X | X | |||
| Histology | CR | X | X | |||
| Clinical and pathological staging (TNM) | CR | X | X | |||
| Mutations (EGFR, ALK, ROS1, PDL1) | CR | X | X | |||
| Pharmacotherapeutic | ||||||
| Treatment regimen (start and end dates) | CR | X | X | |||
| Treatment line change | CR | X | X | X | X | X |
| Side effects (PRO- CTCAE & CTCAE) | CR & patient | X | X | X | X | |
| Adherence (dispensing record and Morisky-Green test) | CR & patient | X | X | X | ||
| Health status | ||||||
| Function (ECOG scale) | Patient | X | X | X | X | X |
| Quality of life (EQ-5D, LCSS***) | Patient | X | X | X | X | |
| Perceived satisfaction | Patient | X | X | |||
| Overall survival (death date and cause) | CR | X | X | |||
| Factors related with patients' end of life (place of death and Earle's criteria****) | CR | X | X | |||
| Resource consumption | ||||||
| Number of visits to the emergency room or admissions | CR | X | X | X | X | |
| Time dedicated by healthcare providers | Healthcare provider | X | X | X | X | X |
ALK: anaplastic lymphoma kinase; CR: clinical record; CTCAE: Common Terminology Criteria for Adverse Events; ECOG: Eastern Cooperative Oncology Group; EGFR: epidermal growth factor receptor; EQ-5D: European Quality of Life-5 Dimensions; FEV1: Forced exhaled volume in 1 second; LCSS: Lung Cancer Symptom Scale; PDL1: Programmed death ligand 1; PRO- CTCAE: patient-reported outcome (PRO) measurement system; ROS1: receptor tyrosine kinase encoded by ROS1 gene; SCQ: Self-administered Comorbidity Questionnaire. #Intermediate visits: visits prior to consultations with the oncologists that do not coincide with the 3 or 6-month consultations.
Pack years + smoking classification: never smoker (< 100 cigarettes per lifetime), ex-smoker (quit the habit > 1 year prior to diagnosis), smoker.
The symptoms defined in ICHOM's standard set, fatigue, pain, cough and dyspnea, are collected through the LCSS questionnaire.
Earle's criteria: (1) patient receives chemotherapy or some other antineoplastic therapy during the last 14 days of life; (2) the patient starts a new antineoplastic treatment in the last month of life; (3) the patient visits the emergency room more than once during the last month of life or is admitted to the intensive care unit; (4) the patient dies in an acute patient unit; (5) the patient does not receive palliative care before death; (6) the patient was started on palliative care within 72 hours before death.
The information will be obtained from the patients’ medical records and from the questionnaires they filled in during the clinical interviews. Quality-of-life questionnaires will be prepared by pharmacists. Adverse events will be recorded jointly by pharmacists and oncologists, on the basis the CTCAE v5.0. classification. Moreover, other PROs not contemplated by the ICHOM tool will be measured such as the PRO-CTCAE items, as well as other outcomes related to the health system itself such as the consumption of resources. The identity of participants will be pseudonymized in an encrypted file only the members of the research team will be able to access (by introducing a password). The data will be recorded using the REDCap system, a secure web application used for creating databases for research and clinical trials (Project-redcap.org).
Data collection- 1.
Recruitment and initial interview.
Control group: Patients who meet the inclusion criteria and who have been on active treatment for at least 6 months will be scheduled for a visit to the pharmacy department during which they will sign their informed consent form and participate in an interview where the data will be collected. These patients will only be scheduled for one visit at 6 months from the beginning of treatment.
Experimental group: At their first appointment with the oncologist after diagnosis, patients who meet the inclusion criteria will be given the relevant details about the study. Patients who agree to participate will be scheduled for a visit to the pharmacy department during which they will sign their informed consent form and participate in their initial (data-gathering) interview.
- 2.
Follow-up visits.
Patients in the experimental group will be followed up until the end of the study, death or loss to follow up. All the defined variables will be measured, according to the frequencies described in table 1. Follow-up visits will always take place before the different appointments with the oncology department and may be held onsite or remotely depending on whether the patient has an onsite or remote appointment with their oncologist.
The final follow-up visit will coincide with the patients’ 6th-month appointment with the oncologist.
- 3.
Evaluation of the healthcare providers’ perception.
A record will be made of the length of time and the resources needed to develop, integrate and implement the PRO measurement protocol. At the end of the study, semi-structured interviews will be held with the members of the healthcare team to identify potential measures that could be taken to increase the sustainability and acceptability of systematic measuring PROs in the long term. Healthcare providers will be asked semi-structured questions to explore their perceptions on the impact that measuring PROs may have on their workload, on effective decision-making and on improving the infrastructure processes and factors needed to compile and use PROs in an efficient and clinically relevant way.
The results of continuous variables will be presented as means and standard deviation. For categorical variables, results will be presented as frequencies and percentages. Numerical variables with non- normal distribution will be presented as medians and interquartile ranges (25th-75th percentile). The normality analysis will be conducted through the Kolmogorov-Smirnov test. Numerical variables will be compared using Student's t test or the Mann-Whitney test, depending on the normality of data distribution and on the total number of patients in each group. The association between qualitative variables will be analyzed using the Pearson's chi-squared test or Fisher's Exact Test. The corresponding measures of risk and association will be calculated along with their confidence intervals. Overall survival will be calculated by means of Kaplan-Meier curves. The statistical analysis will be carried out sing the SPSS v. 21.0. software package Results will be considered statistically significant if the p value < 0.05.
LimitationsThe main limitations associated with systematizing the evaluation of PROs in clinical practice are related with the need to implement technological tools capable of facilitating the process, the integration of PROs in the established workflows, and the necessary engagement of healthcare providers and patients. The development of pilot studies and the publication of work methodologies and fresh scientific evidence, such as the one arising from the PeOpLe study, are likely to contribute to the gradual breakdown of those barriers.
Ethical considerationsThe protocol for the PeOpLe study was approved by the Ethics Committee for Research into Medicinal Products of the Gregorio Marañón General University Hospital (study code: VEV-PUL-2017–01). The study will be undertaken abiding by the basic ethical principles and norms included in the current version (adopted in Fortaleza in 2013) of the Declaration of Helsinki, adopted by the World Medical Association and the Oviedo Convention, and by the regulatory requirements contained in Royal Decree 957/2020 of 3 November, which regulates the way in which observational studies on medicinal products for human use should be conducted.
The study will be carried out in accordance with what has been stated in this protocol. The performance of the study will under no circumstances interfere with physicians’ prescribing habits. Suspected adverse reactions will be recorded and reported in accordance with the current legislation and best pharmacovigilance practices.
To participate in the study, patients will be required to sign an informed consent form.
Data will be collected in a data logbook (Redcap®). The collection, processing and analysis of data will be carried out in accordance with the General Data Protection Regulation [Regulation (EU) 2016/679 of 27 April], Organic Law 03/2018 of 5 December on the protection of personal data and digital right guarantees, and Law 41/2002, of 14 November, which regulated patient autonomy and the rights and obligations in the field of clinical information and documentation.
DiscussionThe PeOpLe study was developed with the aim of adapting and implementing ICHOM's standard sets tool in clinical practice in Spain, identifying the barriers that may prevent its adoption, and propose solutions that allow incorporation of this innovative way of measuring health outcomes in the Spanish healthcare system.
The PeOpLe study will allow an analysis of the impact of systematically evaluating PROs in patients with locally advanced or metastatic NSCMC in terms of effectiveness (survival), safety (complications from systemic therapy), quality of life and satisfaction, identifying the healthcare interventions capable of contributing the highest value. It will also allow an evaluation and a comparison of the PROs obtained from different pharmacotherapeutic alternatives (chemotherapy, immunotherapy and targeted oral therapies) in order to provide evidence that may guide healthcare providers in their decision-making processes, particularly when high-impact medicines are involved.
Several publications exist that report on different experiences of the implementation of ICHOM standard sets in clinical practice for conditions such as hip and knee osteoarthritis12, harelip and cleft palate13, coronary artery disease14, and Parkinson's disease15. According to those reports, the standard sets have had a positive impact at all the different stages of the process. As regards cancer care, there is nowadays a growing debate on the value added by the use of PROs in daily clinical follow-up. Several studies on cancer patients have shown that systematic measurement of PROs is associated with more effective physician-patient communication16, higher patient satisfaction levels17, and improved symptoms control18. Basch et al. observed that managing the symptoms reported by patients on chemotherapy improved their quality of life, decreased the frequency of their visits to the emergency room, enhanced their tolerance of chemotherapy, and improved survival19. Despite these benefits, implementation of PRO measurement in clinical practice is still scarce. Classical recording of PROs is associated with a series of drawbacks such as the requirement of additional resources, transcription and the difficulties inherent in keeping a real-time and continuous record. The PeOpLe study will explore how PRO compilation processes can be developed and integrated, what PROs can be measured with systems that can conveniently be used both by patients and by healthcare providers20. The study will also look into the consumption of resources required by the development and integration of a systematic and longitudinal assessment of PROs at hospital level. On the one hand, the multidisciplinary approach of the PeOpLe study, which will involve hospital pharmacists and oncologists, will produce results based on a comprehensive perspective of the care administered to patients with lung cancer. The project will strengthen the relationship and coordination between pharmacy and oncology professionals and improve the care provided to patients. However, the circuit should be adapted as efficiently as possible to the needs of each hospital. In this regard, the role of nurses may be very significant, particularly in those facilities with advanced nurse practitioners on staff or those that have no pharmacist in their daycare centers. On the other hand, the multicenter nature of the study will make it possible to extrapolate the methodology used to implement the tool to other facilities, contributing to the reproducibility of the study and to the measurement of patient-reported outcomes in clinical practice.
FundingThis study has been funded by a grant under the 2016–2017 Oncology Research and Innovation Program sponsored by the Spanish Society of Hospital Pharmacists (SEFH).
Conflict of interestsNo conflict of interests.
PeOpLe study researchers team:
Rosa Álvarez Álvarez. Oncology Department. Hospital General Universitario Gregorio Marañón. Madrid.
Antonio Calles Blanco. Oncology Department. Hospital General Universitario Gregorio Marañón. Madrid.
Julia Calzas Rodríguez. Oncology Department. Hospital Universitario de Fuenlabrada. Fuenlabrada (Madrid).
Beatriz Candel García. Pharmacy Department. Hospital Universitario de Fuenlabrada. Fuenlabrada (Madrid).
Gema Casado Abad. Pharmacy Department. Hospital Universitario La Paz. Madrid.
Roberto Collado Borrell. Pharmacy Department. Hospital General Universitario Gregorio Marañón. Madrid.
Javier de Castro Carpeño. Oncology Department. Hospital Universitario La Paz. Madrid.
Vicente Escudero Vilaplana. Pharmacy Department. Hospital General Universitario Gregorio Marañón. Madrid.
Ana Beatriz Fernández Román. Pharmacy Department. Hospital Universitario de Fuenlabrada. Fuenlabrada (Madrid).
Mar Galera López. Oncology Department. Oncology Department. Hospital General Universitario Gregorio Marañón. Madrid.
Eva González-Haba Peña. Pharmacy Department. Hospital General Universitario Gregorio Marañón. Madrid.
Javier Letellez Fernández. Pharmacy Department. Hospital Universitario de Fuenlabrada. Fuenlabrada (Madrid).
Belén Marzal Alfaro. Pharmacy Department. Hospital General Universitario Gregorio Marañón. Madrid.
Francisco Moreno Ramos. Pharmacy Department. Hospital Universitario La Paz. Madrid.
José Luis Revuelta Herrero. Pharmacy Department. Hospital General Universitario Gregorio Marañón. Madrid.
Ana Sierra Muñoz. Pharmacy Department. Hospital Universitario La Paz. Madrid.
Cristina Villanueva Bueno. Pharmacy Department. Hospital General Universitario Gregorio Marañón. Madrid.
Early Access date (05/05/2022).