Primary sclerosing cholangitis (PSC) is a rare, progressive, liver disease associated with high morbidity and mortality rates. A high percentage of patients develop concomitant inflammatory bowel disease (IBD), most frequently ulcerative colitis (UC). There is no effective treatment for PSC and patients are commonly refractory to different lines of treatment. Thus, the management of PSC remains a challenge for clinicians.1 The composition of the colonic microflora has been suggested to induce changes in immune response and signaling. Hence, the microbiome, along with other factors such as inherited predisposition or bile acid homeostasis, may play a major role in the development of PSC. Systemic absorption of oral vancomycin, an antibiotic traditionally used for pseudomembranous colitis, is often negligible. This agent modifies the intestinal microbiome and reduces bacterial populations, which are typically elevated in UC patients.2 However, there is limited evidence that oral vancomycin is effective in restoring abnormal liver enzymes.
We report the case of a pediatric patient diagnosed with CSP secondary to IBD who was refractory to multiple lines of treatment until oral vancomycin was introduced.
Case reportWe present the case of a 13-year-old patient diagnosed with ulcerative pancolitis and CSP. At 6 years, the patient presented with antral gastritis, lymphoid nodular hyperplasia, and hypertransaminasemia, which led to a diagnosis of IBD. One year later, different immunotherapies (5-aminosalicylic acid, azathioprine, and mercaptopurine) had not been effective, and biological therapy with adalimumab (a tumor necrosis factor α inhibitor) was initiated as first-line treatment. Due to secondary failure, treatment was switched to infliximab. At 3 years, the treatment was discontinued since it was no longer effective. Vedolizumab (α4β7 anti-integrin) therapy was then prescribed off-label. In 2021, the hospital pharmacy was requested to prepare the master formula of tacrolimus suppositories (2 mg), due to a flare of the disease that was refractory to previous treatments. The same year, ustekinumab (anti-IL12/23) was prescribed off-label.
Diagnosis of CSP was established in 2019, although the etiology was unclear. Autoimmunity tests revealed that the patient was HLA B8-positive, which confers a higher risk for the disease. Additionally, the patient was heterozygous for DR3 and DR2, which is associated with a poorer prognosis.1 Ursodeoxycholic acid was administered at high doses of 30 mg/kg/day, as they have been demonstrated to improve inflammatory processes and laboratory parameters, and delay fibrosis progression.3
In a routine checkup carried out in February 2022, blood test was normal, except for calprotectin >2000, and the patient had severe, persistent diarrhea. In March, the patient was admitted to ER with fever consistent with urinary tract infection (UTI). The same month, the patient was readmitted to the hospital due to a poor clinical course, with abnormal levels of gamma-glutamyl transferase (GGT), calprotectin, and C-reactive protein (CRP).
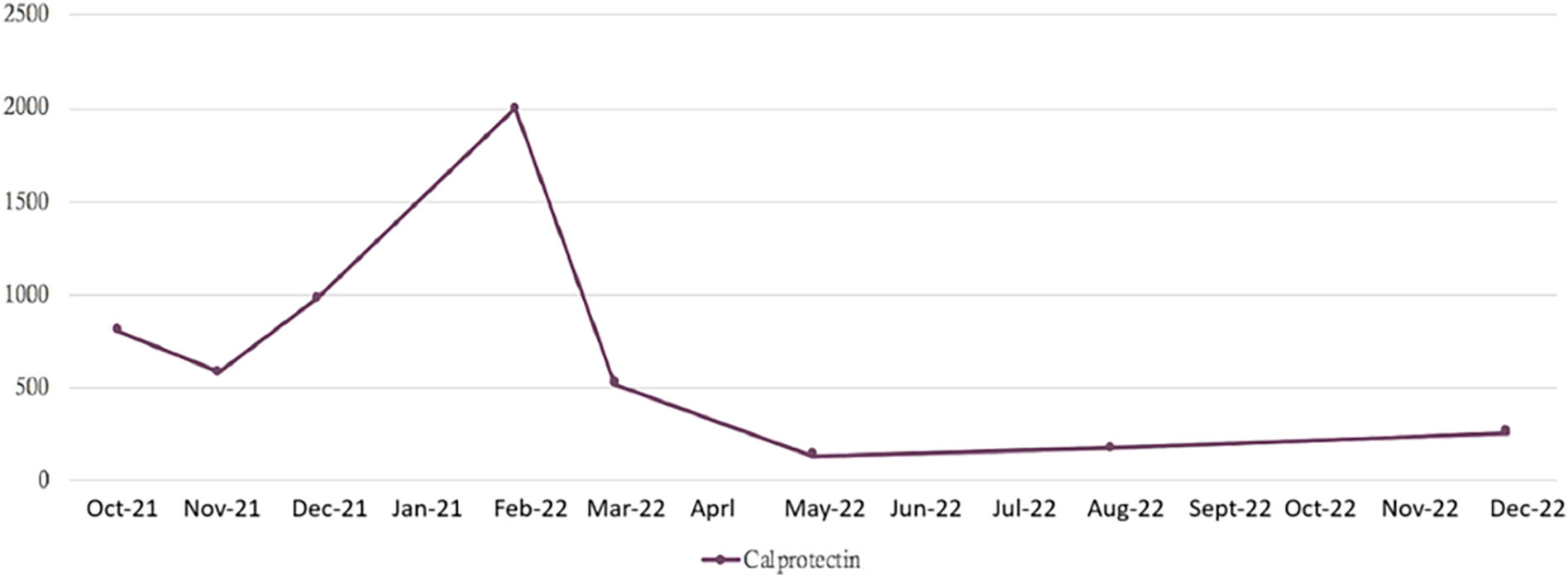
Treatment with oral vancomycin was initiated at doses of 50 mg/kg/day. Capsules of 125 mg were dispensed by the Hospital Pharmacy under a regimen of 375 mg/6 h. With the combination of ustekinumab and vancomycin, he evolved from 8 bloody liquid stools per day to 2 formed stool depositions per day, after 18 days of treatment. Since then, the patient has maintained between 1-2 formed stool depositions per day, without blood in stool or abdominal pain, and clinical improvement has been maintained. After 18 days receiving a combination therapy with ustekinumab plus vancomycin, bowel movements decreased from 8 bloody loose movements per day to 2 solid bowel movements per day. Since then, the patient has 1–2 solid bowel movements daily, without blood or abdominal pain, and calprotectin has improved (Fig. 1), with the patient having remained clinically stable. The remaining laboratory parameters returned to normal with the introduction of vancomycin in March of 2022 (Table 1).
Evolution of laboratory parameters.
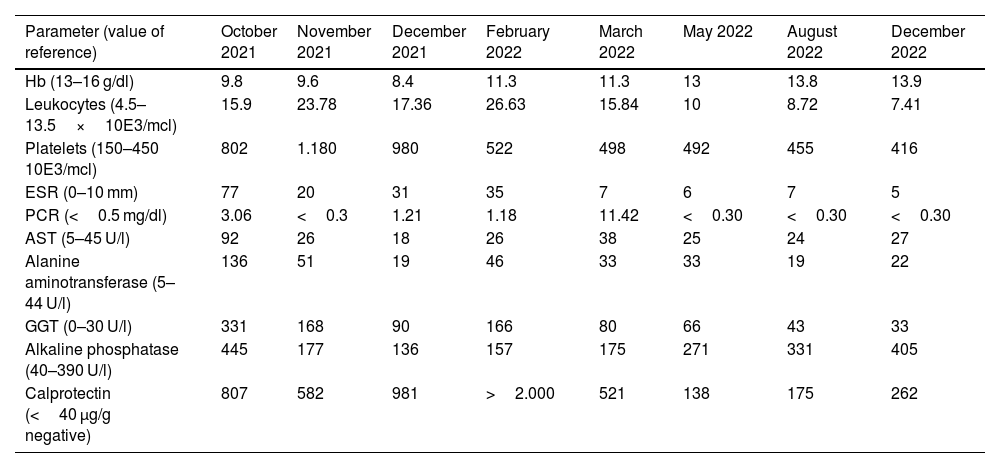
| Parameter (value of reference) | October 2021 | November 2021 | December 2021 | February 2022 | March 2022 | May 2022 | August 2022 | December 2022 |
|---|---|---|---|---|---|---|---|---|
| Hb (13–16 g/dl) | 9.8 | 9.6 | 8.4 | 11.3 | 11.3 | 13 | 13.8 | 13.9 |
| Leukocytes (4.5–13.5×10E3/mcl) | 15.9 | 23.78 | 17.36 | 26.63 | 15.84 | 10 | 8.72 | 7.41 |
| Platelets (150–450 10E3/mcl) | 802 | 1.180 | 980 | 522 | 498 | 492 | 455 | 416 |
| ESR (0–10 mm) | 77 | 20 | 31 | 35 | 7 | 6 | 7 | 5 |
| PCR (<0.5 mg/dl) | 3.06 | <0.3 | 1.21 | 1.18 | 11.42 | <0.30 | <0.30 | <0.30 |
| AST (5–45 U/l) | 92 | 26 | 18 | 26 | 38 | 25 | 24 | 27 |
| Alanine aminotransferase (5–44 U/l) | 136 | 51 | 19 | 46 | 33 | 33 | 19 | 22 |
| GGT (0–30 U/l) | 331 | 168 | 90 | 166 | 80 | 66 | 43 | 33 |
| Alkaline phosphatase (40–390 U/l) | 445 | 177 | 136 | 157 | 175 | 271 | 331 | 405 |
| Calprotectin (<40 μg/g negative) | 807 | 582 | 981 | >2.000 | 521 | 138 | 175 | 262 |
AST: aspartate aminotransferase; GGT: gamma-glutamyl transferase; Hb: Hemoglobin; PCR: C-reactive protein; ESR: Erythrocyte sedimentation rate.
There is limited experience with the use of oral vancomycin in children with CSP. The first study, published in 1998, revealed that oral vancomycin improved gastrointestinal symptoms and levels of liver enzymes in 3 children. However, when treatment was discontinued, symptoms and abnormal laboratory findings recurred.4
A more recent study published in 2021 assessed the efficacy of vancomycin in 21 children who were unresponsive to standard therapy. Results revealed that vancomycin had reduced levels of aspartate aminotransferase (AST) and GGT in 81% of patients. Six patients who achieved complete response were classified as low/medium risk according to the SCOPE (Sclerosing Cholangitis Outcomes in Pediatrics) index. The fact that none of these patients were classified as high risk suggests that patients with lower disease activity are more likely to achieve biochemical response.5 Similar results were obtained in another study that revealed that a low SCOPE index at initiation of treatment was an independent predictor of response.6
Davies et al. reported that transaminase levels and erythrocyte sedimentation rate (ESR) returned to normal in 14 pediatric patients with PSC-IBD. When treatment with vancomycin was discontinued, symptoms recurred and liver enzymes increased. When treatment was re-initiated, liver function was restored. Seven patients received long-term treatment, ranging from 4 to 56 months. Their liver enzymes remained within normal limits, and patients remained clinically asymptomatic.6 No side effects or infectious complications were observed in the long-term in any these studies.5,7
Oral vancomycin is poorly absorbed in the gastrointestinal tract, and the mechanism by which this agent induces an improvement of biochemical parameters is unclear. Vancomycin has been documented to exert immunomodulatory effects on T lymphocytes.8–10 Some authors have posited that these effects may be mediated by the antimicrobial activity of vancomycin on unknown gastrointestinal pathogens or on the normal microbiome, which cause abnormal immune reactions subsequent to their migration to the liver.7
In conclusion, in agreement with previous studies, this case report shows the effectiveness of orally administered vancomycin in children with CSP and IBD. However, further studies are necessary to confirm that this agent can be used safely and routinely in the long-term. In our case, the combination of vancomycin plus ustekinumab for CSP-IBD was effective in restoring liver function and reducing inflammation and disease activity.
FundingNone.
AuthorshipCristo Yared Pérez Martin and Jenifer González Chávez contributed to the study conception and design, data collection, and to manuscript drafting and edition.
José Ramón Alberto Alonso contributed to study conception and design, literature search, clinical trials and data collection.
María Pilar Díaz Ruiza, Marta Suárez González and Javier Merino Alonso reviewed the manuscript.
All authors approved the final version of the manuscript.
Liability and assignment of rightsAll authors accept responsibility as defined by the International Committee of Medical Journal Editors (available at http://www.icmje.org/).
In the event of publication, the authors assign exclusively the right of reproduction, distribution, translation and public presentation (by any sound, audiovisual or electronic means) of our manuscript to Farmacia Hospitalaria and, by extension, to the SEFH. To such purpose, a right assignment letter will be signed upon submission of the paper via the online paper management system.