The health crisis resulting from the rapid spread of SARS-CoV-2 worlwi-de, added to the low evidence of currently used treatments has led to the development of a large number of clinical trials (CT) and observational studies. Likewise, important measures have been adopted in healthcare and research centers aimed at halting the pandemic as soon as possible.
The objective of this study is to gather the main aspects of the clinical research studies undertaken by the Pharmacy Department (PD) of Spain during the COVID-19 crisis.
The decision of the Spanish Society of Hospital Pharmacy (SEFH) to sponsor CTs made it possible that 13% of Pharmacy Department (PD) had been led at least one CT. The Spanish Agency for Medicines and Medical Devices (AEMPS), in coordination with Institutional Review Boards, has adopted a fast-track review procedure to accelerate authorizations for CTs related to the treatment or prevention of COVID-19. There have also been numerous public and private calls for financing research projects aimed at contributing to the fight against this virus.
Despite the pandemic, actions have been taken to continue ongoing CTs and studies while the safety and well-being of patients are guaranteed. More specifically, the AEMPS and the European Medicines Agency (EMA) have issued guidelines that incorporate changes to CT protocols that will have to be applied until the pandemic is over.
In this health emergency, the scientific community has found itself in a race against time to generate evidence. It is at this moment that hospital pharmacists emerge as key players in clinical research and are contributing to a rational, effective and safe healthcare decision-making.
La presente crisis sanitaria derivada de la rápida expansión del virus SARS-CoV-2 a nivel mundial, así como la falta de evidencia de los tratamientos empleados actualmente, ha provocado la aparición de un gran número de ensayos clínicos y estudios observacionales. Del mismo modo, ha ocasionado la puesta en marcha de importantes medidas en el entorno sanitario e investigador con el fin de conseguir detener la evolución de la pandemia lo antes posible.
El objetivo del actual trabajo es recopilar aspectos fundamentales relacionados con la investigación clínica desarrollada por los servicios de farmacia hospitalaria durante la crisis provocada por la COVID-19.
La iniciativa de la Sociedad Española de Farmacia Hospitalaria de actuar como promotor de ensayos clínicos ha posibilitado que el 13% de estos servicios de farmacia hospitalaria haya podido liderar uno. En este sentido, la Agencia Española de Medicamentos y Productos Sanitarios, junto con los Comités de Ética de Investigación, ha acelerado los procedimientos de autorización de nuevos ensayos clínicos destinados a tratar o prevenir la COVID-19. Asimismo, han sido numerosas las convocatorias públicas y privadas destinadas a la financiación de proyectos de diversa índole con el fin de contribuir a la lucha contra este virus.
A pesar de la irrupción de la pandemia, también han surgido acciones destinadas a mantener las actividades de los ensayos clínicos y estudios puestos previamente en marcha, garantizando la seguridad y bienestar del paciente. Concretamente, la Agencia Española de Medicamentos y Productos Sanitarios y la Agencia Europea de Medicamentos han publicado guías que incluyen cambios en los protocolos de los ensayos clínicos que deben mantenerse mientras dure la pandemia.
La emergencia sanitaria actual ha obligado a la comunidad científica a la generación de evidencia a contrarreloj. Por ello, en este momento en el que se requiere del mayor rigor posible, el farmacéutico de hospital debe alzarse como una figura clave en la investigación en salud, contribuyendo a que las decisiones sanitarias sean racionales, eficientes y seguras.
The SARS-CoV-2 outbreak has posed an unprecedented challenge to health systems worldwide. The transmission rate and the threat of the collapse of the health system, coupled with the economic impact of the pandemic, have forced researchers of all fields to a race against time to seek solutions in the shortest possible time1. The absence of preventive and therapeutic therapies against this virus has caused over 240,000 deaths related to COVID-19 worldwide in two months, which has resulted in a outburst of clinical trials2. Moreover, a series of strategies have been carried out to facilitate the development of and access to therapies based on the creation of therapeutic accelerators as those supported by Bill & Melinda Gates, Wellcome and Mastercard. Hospital pharmacy has jumped on the bandwagon of research, as there is much to explore and no time to lose3.
The scarcity of evidence on the treatments currently used and the urgent need for new effective drugs have resulted in the adoption of novel measures to facilitate and accelerate the clinical trial authorization process and classification of observational studies. Although this system may be beneficial, it may also affect the quality of studies and evidence generated4. The objective of this article is to provide an insight into the fundamentals of clinical research in Pharmacy departments (PD) during the COVID-19 pandemic.
Strategy developed: design, cycle and stages, implementationHospital pharmacy in the COVID-19 pandemicUp to date, 1,324 studies on COVID-19 have been registered in ClinicalTrials, 783 of which are interventional CTs (86 phase I, 310 phase II, 209 phase III, 48 phase IV and 130 unclassified), 524 observational and 17 expanded-access studies. Of all, 4 have been terminated, 519 have not yet started patient recruitment and only 49 have been completed5. As to CTs in Europe, 176 CTs have been recorded in the EU Clinical Trials Register6, with Spain ranking first in Europe in number of CTs (68), followed by France (42), United Kingdom (28), Denmark (19), Germany (17) and the Netherlands (12).
Of the 68 CTs where Spain is involved, 66 (1 phase I, 28 phase II, 23 phase III and 14 phase IV) have been recorded in the Spanish Registry of Clinical Trials7. Of all, 27 have not yet started and 38 are recruiting patients. Only a CT has been completed and none has published results. Additionally, 155 observational studies with drugs for COVID-19 have been registered in Spain8.
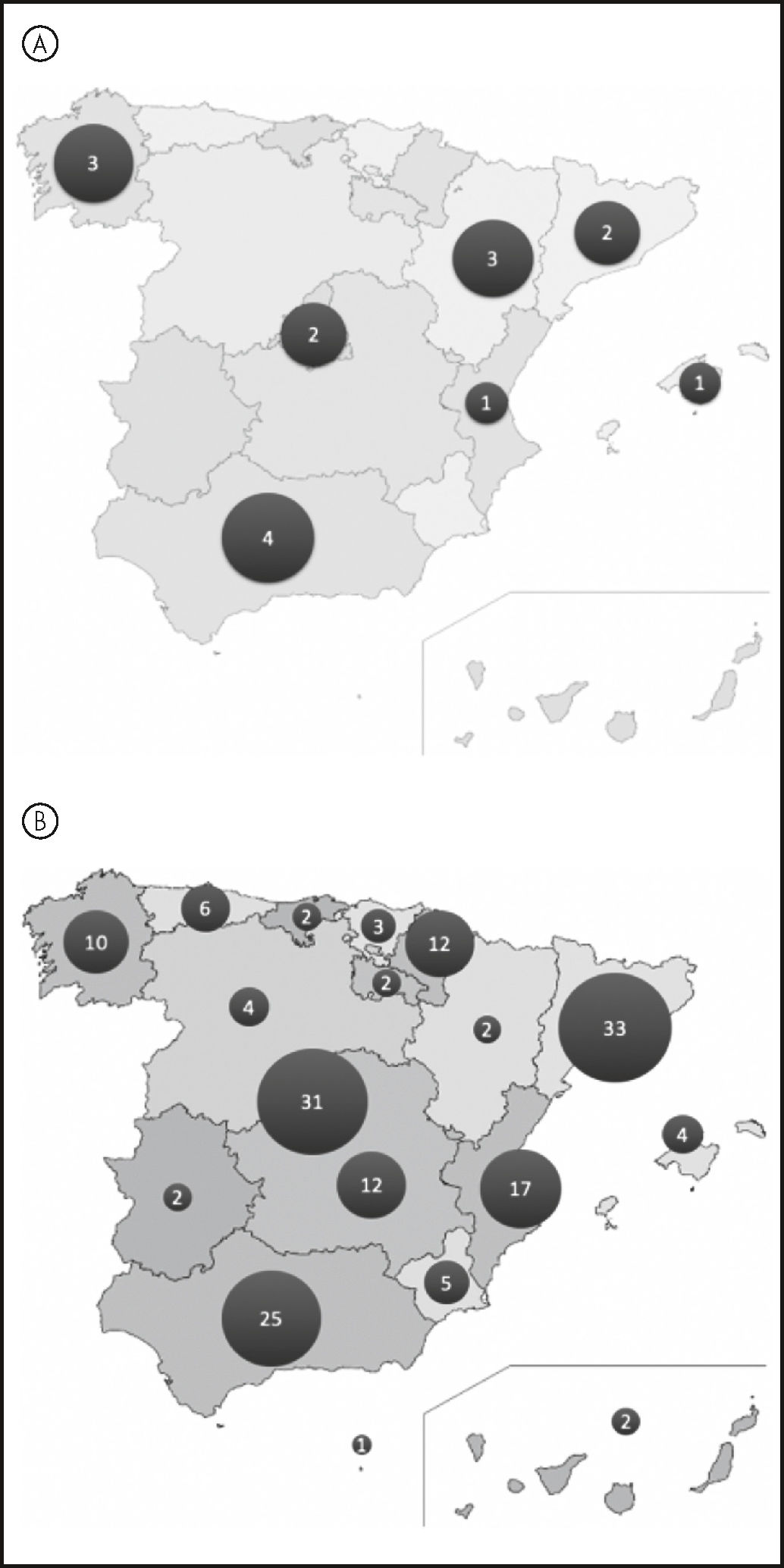
To understand the role of hospital pharmacy in research on COVID-19, we conducted a survey and obtained response from 133 centers. Of note, the initiative of the SEFH to sponsor CTs has boosted research on the disease. This has allowed that 13% of PD have led a CT in their center. The QUINAVID-19 deserves special mention. It is a multicentric CT intended to assess the effectiveness and safety of chemoprophylaxis with hydroxychloroquine in healthcare professionals (Figure 1A). Another CT sponsored by SEFH is the phase III intervention trial (ALCOVID-19, EUDRACT No. 2020-001760-29), currently under evaluation by the AEMPS. This trial will assess the effectiveness and safety of a new therapy with inhaled anti-infectives.
Of special note is the high level of participation of PD in CTs as collaborators, with 34% of participation in CTs sponsored by other entities, and 22% in CTs carried out in their center. You can find a list of PD that have taken part in CTs promoted by entities other than SEFH at: Mapa InvestigaSEFH COVID-19, https://www.google.com/maps/d/viewer?mid=1QOUibqh6RIm62UEvnUdYyDP0FFdgX2zV≪=39.626362653230885%2C-2.8221781500000143&z=6.
With regard to observational studies, 60% of PD have taken part in the “RERFAR-COVID-19: Spanish Registry of Pharmacotherapy Results against COVID-19” study promoted by SEFH, with 173 participating centers (Figure 1B). An excellent response has been obtained from researchers, who have recruited more than 7,740 patients. In addition, parallel projects have been proposed to assess the effectiveness of a prognostic analysis based on the characteristics of patients. In addition, authorization was recently granted for the international collaboration requested by the Universidad Austral de Chile for the use of the database. Finally, 14% of PD are taking part in studies with other sponsors, 13% in local studies, and 5% lead their own observational studies (Mapa InvestigaSEFH COVID-19): https://www.google.com/maps/d/viewer?mid=1QOUibqh6RIm62UEvnUdYyDP0FFdgX2zV≪=39.626362653230885%2C-2.8221781500000143&z=6.
Specific actions for the authorization and support of research on COVID-19With regard to the authorization of new CTs, the AEMPS, along with Institutional Review Boards, prioritized the evaluation of CTs related to the prevention or treatment of the disease caused by coronavirus, with a 15 days deadline for obtaining an official response. The authorization process to start a post-authorization observational, prospective, follow-up studies promoted or sponsored by healthcare authorities (EPA-AS) have also been simplified. Recently, after the high number of applications received, the AEMPS encourages researchers to consider joining ongoing CTs, consulting the websites enabled for this.7–9.
Funding is necesary to conduct clinical research. Thus, since the beginning of the health crisis derived from the worldwide expansion of SARS-CoV-2, there have been numerous public and private initiatives for financing several projects in order to contribute to the fight against this virus. In our country, the Instituto de Salud Carlos III (ISCIII) has created the COVID-19 Fund, with a budget of 24 million euros aimed at promoting projects and programs that generate knowledge of the virus and seek short-term solutions to improve the quality of life of patients and facilitate the work of healthcare professionals and researchers10. Similarly, ISCIII has extended the contracts of clinical researchers (Río Hortega, Juan Rodés, to name a few) as long as the state of alarm is in force. ISCIII has also facilitated the hiring of clinicians by regional health systems to face the peak of the pandemic11.
Other similar initiatives have been launched, such as “Caixaimpulse COVID-19” of La Caixa Foundation in Spain and Portugal12, with a budget of 1.5 million euros aimed at supporting clinical and translational research projects based on the use of cutting-edge technologies for the prevention, treatment, follow-up and diagnosis of COVID-19. Likewise, the Mutua Madrileña Foundation has adopted new actions to fight the social and clinical effects of the COVID-19 pandemic. Thus, Mutua Madrileña has announced two extraordinary social and medical research aid schemes with a budget of 500,000 euros each13. The Autonomous Communities with a strong tradition of scientific research have funded numerous and ambitious projects. Such is the case of the Regional Government of Catalonia, which has allocated 4 million euros to 19 research projects on COVID-1914. In Europe, the Innovative Medicines Initiative (IMI) deserves special mention, with the European Commission having provided 45 million euros to support research projects for the rapid development of antivirals and other type of therapies.
This fund is also aimed at the development of diagnostic techniques for coronavirus, whereas preventive vaccines are specifically excluded. The pharmacy industry is expected to contribute 90 million euros to the European program15. In addition, the European Union and its partners have organized a Donor Conference with the purpose of raising funds that guarantee the collaborative development and global implementation of diagnostic, therapeutic and preventive tools against coronavirus. As of May 10th, 7,400 million euros have been gathered, with Spain having contributed 1,250 million euros16.
Effects of the pandemic on research in pharmacy services on issues unrelated to COVID-19During the pandemic, some actions have been taken to guarantee the safety and well-being of patients who were participating in clinical trials prior to the outbreak. The AEMPS and the EMA have issued guidelines that incorporate changes to CT protocols, which will be maintained as long as the emergency continue17,18. These protocols include recommendations for participants about the quarantine, the prevention of transmission and reallocation of clinicians, among other actions. The purpose of these recommendations is to provide consistent information for Member States to implement it in accordance with their laws and regulations18,19.
According to these guidelines, new CTs unrelated to coronavirus are postponed, whereas ongoing CTs have suspended patient recruitment, as changes are expected to be done to protocols during the COVID-19 pandemic20,21. The continuity of patients taking part in ongoing CTs must be maintained, while visits are restricted as much as possible, with priority given to teleconsultation whenever possible. The same occurs with the monitoring of participating centers, which is now conducted telematically. Data verification has also been put off to avoid overloading the hospital staff with extra work, as their tasks and duties within their service may have changed.
Another measure adopted where PD are directly involved is the dispensing of medicines being tested in a CT to authorized persons or participants at home. The dispensing of experimental drugs is performed through dispensing cycles established by PD, which were mostly created during the public health emergency (SEFH provides this information on its website22). Occasionally, experimental therapies are administered by CT sponsors under the direction of the pharmacy service23.
As other services, the capacity of PD has been strained by the high demand caused by the massive spread of the SARS-CoV-2 virus across the country. This critical situation has impacted research activity, as all human and material resources devoted to research have been repurposed to fight the global emergency, in detriment of other ongoing investigational activities.
Finally, PD with strong connections with universities and biomedical research entities have been affected by the closure of these institutions, as they were declared as “non-essential” at the start of the state of alarm.
Lessons learned. Future applicability in pharmacy services
In the current public health emergency, the scientific community has found itself in a race against time24,25. Therefore, it is not striking that we are overwhelmed by an avalanche of information, poor-quality articles withdrawn by their own authors, or inconsistent evidence26–28. The urgency to make progress makes us fall back. There are no shortcuts in science. Results are obtained after many years of research with the adequate resources29.
In a time where high-quality evidence is needed, the hospital pharmacist emerges as an essential figure in biomedical research. Hospital pharmacists contribute with their critical evaluation skills and lead research for the generation of evidence, thereby facilitating rational, effective and safe clinical decision-making30.
Research is crucial to address the challenges that arise in clinical practice. The role that hospital pharmacists have played in research during the pandemic is demonstrated by their participation in CTs supported by local and external sponsors. Another example is the high level of participation of hospital pharmacists in the observational study “RERFAR-COVID-19”. PD must keep the momentum going and boost active participation in research projects, which will help bring the pandemic under control in the shortest possible time. Hospital pharmacists emerge as a key to the discovery of more cost-effective therapies against SARS-CoV-2.
Likewise, the Spanish Society of Hospital Pharmacy has supported research through the creation of its own Research Committee and the active promotion of clinical trials. One of the projects with the greatest impact was performed under the auspices of the consortium established with REDCap, an international collaborative network widely used by the scientific community. Being a member of the consortium has enabled SEFH to offer its members free access to a platform for the management, design, and coordination of research projects. This has facilitated the building of a large, invaluable database for biomedical research31.
Apart from access to the platform for future studies, SEFH offers advisory services to PD provided by a contract research organization (CRO) (Delos Clinical, S. L.). This CRO provides guidance on the design and conduct of biomedical research projects, which has been crucial to set studies in motion in a short time.
Consultancy services range from the design and authorization of observational studies and clinical trials to the preparation of essential documents, through monitoring the start-up and safety of the study, and performing data collection and statistical analysis. It is an integral service that adapts to the needs of all biomedical researchers.
In a recent editorial, the Editor-in-Chief of the journal Farmacia Hospitalaria raised the question of where research is and where it is going. The Editor emphasized the research activity of hospital pharmacists and the need for high-quality research studies32. This scenario is a good example of the situation of our professionals. We must have a critical spirit, lead biomedical research, and generate reliable evidence. There is no time better than this to step forward.
In critical situations such as the one caused by the COVID-19 pandemic, the need arises for an effective public network of science and research. Clinicians are grateful for the daily applause, but what we really need is that enough resources are allocated to be able to do our work, which includes a budget for R&D. In a country where science is not valued enough, this is the right time for policy-makers to lay the foundation for a long-term plan of research, which has been repeatedly claimed by the scientific community.
To the Spanish Society of Hospital Pharmacy for its commitment to research and its role as a sponsor of clinical trials and studies led by hospital pharmacies. To the Carlos III Health Institute for the JR18/00014 and CM18/00090 grants.