Chronic lymphocytic leukaemia places a considerable economic burden on the Spanish National Health System. This study estimated the direct costs of chronic lymphocytic leukaemia oral targeted therapies from 2011 to 2025, inclusive, in a scenario with fixed treatment oral targeted therapies and in a scenario without them.
MethodThe clinical course of adult chronic lymphocytic leukaemia patients was represented by a Markov model with four health states: watchful waiting, first-line treatment, relapse, and death. The treatment pattern was defined according to patient type by disease status or situation, age, presence or absence of deletion in the short arm of chromosome 17, immunoglobulin heavy chain mutation status, and year of treatment. The treatment algorithm was simulated from 2011 to 2025, and included therapies funded by the Spanish National Health System and their use in routine clinical practice, validated by leading experts. A single treatment option was assumed for each type of patient and time period (the most widely option used at each time point). Direct costs were included: pharmacological, administration, tests performed, routine visits, hospitalizations, and adverse events.
ResultsFrom 2011 to 2025, there would be a mean annual chronic lymphocytic leukaemia prevalence of 16,436 patients in the scenario without fixed treatment oral targeted therapies and 16,413 in the scenario with fixed treatment oral targeted therapies. In the same period, the total costs in the scenario without fixed treatment oral targeted therapies would be €4,676.7 million and in the scenario with fixed treatment oral targeted therapies they would be €4,111.8 million. Thus, the introduction of fixed treatment oral targeted therapies would entail a saving of €564.9 million (12.1% of the total cost of care of chronic lymphocytic leukaemia patients during the period assessed). In this period, the total cost per patient would decrease from €266,019 in the scenario without fixed treatment oral targeted therapies to €236,852 in the scenario with fixed treatment oral targeted therapies, representing a saving of €29,167 per patient.
ConclusionsThis study estimates that, between 2011 and 2025, the introduction of fixed treatment oral targeted therapies for the treatment of chronic lymphocytic leukaemia would entail €564.9 million cost savings for the Spanish National Health System (12.1% of the total cost of care of chronic lymphocytic leukaemia patients during the period assessed).
La leucemia linfocítica crónica supone una carga económica considerable para el Sistema Nacional de Salud español. Este estudio estimó los costes directos de las terapias orales dirigidas para leucemia linfocítica crónica desde 2011 a 2025, inclusive, en un escenario con terapias orales de duración fija y en un escenario sin ellas.
MétodoSe representó el curso clínico de pacientes adultos con leucemia linfocítica crónica mediante un modelo de Markov con cuatro estados de salud: vigilancia activa, tratamiento de primera línea, recaída y muerte. Patrón de tratamiento definido por tipo de paciente: estado o situación de la enfermedad, edad, presencia o no de deleción en el brazo corto del cromosoma 17, estado mutacional de la cadena pesada de inmunoglobu- linas y año de tratamiento. Algoritmo de tratamiento simulado desde 2011 a 2025, incluyendo terapias financiadas por el Sistema Nacional de Salud español y su uso en práctica clínica habitual, validado por expertos de referencia. Se asumió una opción de tratamiento por tipo de paciente y periodo de tiempo (la más ampliamente utilizada en cada momento). Se incluyeron costes directos: farmacológicos, administración, pruebas realizadas, visitas rutinarias, hospitalizaciones y acontecimientos adversos.
ResultadosSe estimó una prevalencia media anual de leucemia linfocítica crónica desde 2011 a 2025 de 16.436 pacientes en el escenario sin terapias orales de duración fija y 16.413 en el escenario con terapias orales de duración fija. Los costes totales desde 2011 a 2025 en el escenario sin terapias orales de duración fija ascendieron a 4.676,7 millones de € y a 4.111,8 millones de € en el escenario con terapias orales de duración fija. Así, la introducción de las terapias orales de duración fija supondría un ahorro de 564,9 millones de € (12,1% del total del coste de atención de los pacientes con leucemia linfocítica crónica durante el periodo evaluado). El coste total por paciente en este periodo de tiempo pasaba de 266.019 € en el escenario sin terapias orales de duración fija a 236.852 € en el escenario con terapias orales de duración fija, suponiendo un ahorro de 29.167 € por paciente.
ConclusionesEste estudio estima que la introducción de las terapias orales de duración fija para el tratamiento de la leucemia linfocítica crónica entre 2011 y 2025 supone un ahorro para el Sistema Nacional de Salud español de 564,9 millones de € (12,1% del total del coste de atención de los pacientes con leucemia linfocítica crónica durante el periodo evaluado).
Chronic lymphocytic leukaemia (CLL) is the most common type of leukaemia in adults, accounting for 30% of adult leukaemia cases in Western countries1. The mean age at diagnosis is 71.7 years2.
In Spain, the incidence rate in 2010 was an estimated 13.6/100,0003. According to the Institut National de la Santé et de la Recherche Médicale (INSERM), in 2016, the prevalence of CLL in Europe was an estimated 27/100,0001.
Chronic lymphocytic leukaemia has a considerable impact on the survival and health-related quality of life (HRQoL) of CLL patients. In addition to the impact on patients’ health, CLL places a considerable economic burden on the Spanish National Health System (NHS)1.
In recent years, great progress has been made in understanding the biology of CLL, leading to significant advances in the treatment of this disease. In particular, oral targeted therapies have demonstrated remarkable results in CLL patients, improving both progression-free survival (PFS) after treatment and overall survival (OS). As stated in the latest clinical practice guideline of the European Society for Medical Oncology (ESMO), the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have recently approved various combinations of oral drugs for the treatment of CLL in both first-line and relapse settings4. Other therapies are expected to become available in the near future1,5,6.
New treatments pose a major challenge, mainly due to their economic burden1. A specific concern is their long treatment duration, an aspect that is expected to improve with the development of CLL therapies with a defined fixed treatment duration. The aim of this study was to perform a cost minimisation analysis of CLL for the Spanish NHS under two scenarios: the first, without oral targeted therapies; and the second, with the introduction of fixed time duration (FTD) oral therapies. The specific FTDs included in the model were the combinations venetoclax and obinutuzumab and venetoclax and rituximab.
MethodsWe conducted the CLL cost minimisation analysis from the perspective of the Spanish NHS by adapting a model previously used in the United States5 and Canada7,8. This model was developed to analyse the economic burden of CLL before and after the introduction of FTDs and was subsequently adapted to the Spanish healthcare setting. Thus, on 19 November 2019, we conducted a literature search on the OVID platform, combining terms relating to the pathology of interest with terms relating to the inputs needed to adapt the model and terms to identify references in the Spanish population. The search was limited to references published in the last 5 years in English or Spanish. It was completed with a review of the grey literature. Subsequently, an expert group (the authors of this article) validated the scenarios, assumptions, and inputs by consensus and completed the information needed to perform the analysis.
Study design and populationThe population included in the analysis comprised adult patients (at least 18 years) diagnosed with CLL from 2011 to 2025 in Spain. These patients were divided according to deletions on the short arm of chromosome 17 (del(17p)), fit or unfit status, age less than 65 or at least 65 years, and immunoglobulin heavy chain mutation (IGHV) status as key criteria to guide treatment. The initial population for 2011 was calculated by including new incident cases from 2000 to 2010 in Spain1,3 in the model, stratified according to clinical practice from this time period. Following this procedure, the model generated a population representing 2011 epidemiological data (prevalence). Data on PFS and OS were obtained from the clinical trials of the treatments considered in the model. Finally, data on overall mortality of the Spanish population was also used9.
The clinical course of CLL patients was represented in a Markov model including four health states: watchful waiting, first-line treatment, relapse, and death5. We assumed that most of the patients with a new diagnosis of CLL were not treated with active therapies and they were therefore included in the model in the watchful waiting state10,11. Once patients required treatment, they were moved to the first-line treatment state. After first-line treatment failure, patients were moved to the relapsed state and received second-line treatment. If treatment duration was fixed, patients continued in the first-line or relapsed state without active treatment. Patients with second-line treatment failure discontinued the active treatment they were receiving. Patients could transition to the dying state from any of the other states described. We estimated the probabilities of transition between the different health states based on treatment time, PFS, and OS data from the clinical trials.
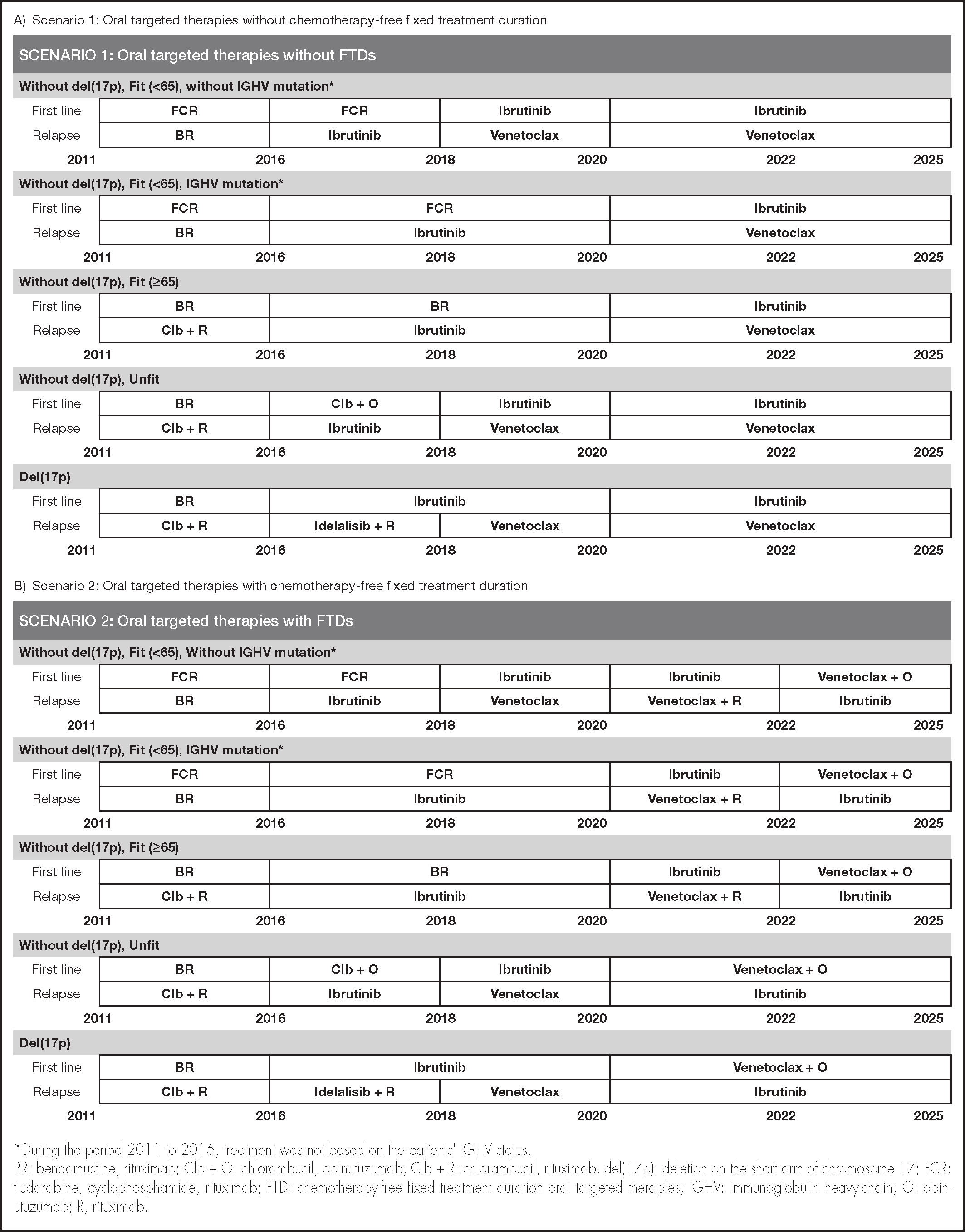
Treatment strategiesThe CLL treatment pattern was defined according to patient type by disease status, age, presence or absence of del(17p) determined by fluorescence in situ hybridisation (FISH), IGHV mutation status, and year of treatment. Table 1 shows the different parameters included in the model, as well as each of their values.
Parameters included in the model
| Parameter | Value | Source | |
|---|---|---|---|
| Probability of watchful waiting at the time of diagnosis | 85.0% | 5 | |
| Prevalence of del(17)p | 7.0% | 1 | |
| Probable age at diagnosis | < 65 years | 35.0% | |
| 65-70 years | 15.0% | 1 | |
| > 70 years | 50.0% | ||
| Probability of the patient being fit | < 65 years | 87.5% | |
| 65-70 years | 50.0% | Expert opinion | |
| > 70 years | 25.0% | ||
| IGHV mutation rate | 60.0% | Expert opinion | |
| IGHV nonmutation rate | 40.0% | Expert opinion | |
| Oral | 5.0% | ||
| Administration route | IV | 95.0% | Expert opinion |
| Probability of discontinuation of oral targeted therapy for each 4-week cycle in 1st-line treatment | 0.7% | 19 | |
| Probability of discontinuing oral targeted therapy for each 4-week cycle in relapsed patients | 1.4% | 19 |
del(17p): deletions in the short arm of chromosome 17; IGHV: immunoglobulin heavy-chain; IV: intravenous.
The treatment algorithm was simulated from 2011 to 2025, dividing this time interval into five periods (2011-2015, 2016-2017, 2018-2019, 2020-2021, and 2022-2025), representing the evolution of standard care, as well as changes in actual clinical practice due to the entry of oral targeted therapies, all of which was validated by the clinical expert group. The second model was of a parallel clinical scenario which included the introduction of the FTD regimen.
The proposed scenarios were defined taking into account the therapies available in Spain and funded by the Spanish NHS in each time period. To simplify the model, a single treatment option was assumed for each type of patient and time period, such that 100% of the patients were on treatment with the most widely used option at each time point. In this respect, both scenarios were identical in terms of treatments and CLL management in the period from 2011 to 2019, inclusive, because FTD treatments only started to be marketed in Spain at the end of 2019 in Spain. Thus, for the purposes of the analysis, it was assumed that FTDs were marketed at the beginning of 2020 (Figure 1).
Direct costsThe cost minimisation analysis was conducted from the perspective of the Spanish NHS. Thus, the only costs considered were the following direct health care costs: pharmacological, administration, tests performed, routine visits, hospitalisations, and the management of adverse events. All these costs were updated to 2019 euros based on the average annual consumer price index published for the year of the cost used and its correction to 201912 without including any discount rate.
To estimate the pharmacological costs, we used the Summary of Product Characteristics for each drug to obtain the dose, frequency in each of the cycles, and administration route of the treatment regimens included in the model13, all of which were validated by the expert group in case of doubt or nonspecific dosage. Regarding drugs with variable dosing according to patient weight or body surface area, we assumed a weight of 79.0 kg and a body surface area of 1.92 m2, which are averages for the CLL patient population14. For each drug, we used the laboratory selling price (LSP) excluding VAT as published in the Official College of Pharmacists database15 as well as the deductions established by Royal Decree-Law 8/2010, as amended by Royal Decree-Law 9/201116. In the case of different pharmaceutical forms of the same drug, we used the average cost price per milligram of all the forms (Table 2).
Dose, administration route, and cost of chronic lymphocytic leukaemia treatments included in the model13
| Active ingredient | Administration route | First treatment cycle | Subsequent treatment cycles (2 to 6 inclusive) | Average LSP/mg* |
|---|---|---|---|---|
| Bendamustine | IV | 90 mg/m2 on d 1 and d 2 (1st line) | € 2.01 | |
| 70 mg/m2 on d 1 and d 2 (2nd line) | ||||
| Cyclophosphamide | Oral | 200 mg/d | € 0.00 | |
| Cyclophosphamide | IV | 250 mg/m2/d from d 1 to d 3 | € 0.01 | |
| Chlorambucil | Oral | 0.5 mg/kg/d on d 1 and d 15 | € 0.01 | |
| Fludarabine | Oral | 40 mg/m2/d from d 1 to d 5 | € 2.21 | |
| Fludarabine | IV | 25 mg/m2/d, from d 1 to d 5 | € 1.00 | |
| Ibrutinib | Oral | 420 mg/d | € 0.52 | |
| Idelalisib | Oral | 150 mg/b.i.d | € 0.58 | |
| Obinutuzumab | IV | 1,000 mg on d 1, d 8, and d 15 | 1,000 mg on d 1 | € 3.97 |
| Rituximab | IV | 375 mg/m2 on d 1 | 500 mg/m2 on d 1 | € 2.08 |
| Venetoclax | Oral | Week 1: 20 mg/d; Week 2: 50 mg/d | ||
| Week 3: 100 mg/d; Week 4: 200 mg/d | 400 mg/d | € 0.60 | ||
| Week 5: 400 mg/d | ||||
b.i.d: twice a day; d: day; IV: intravenous; LSP: laboratory selling price.
The cost of administration was taken into account only for intravenous (IV) administration, which reached an estimated cost of €312.19 per session17. Fludarabine and cyclophosphamide are marketed for both oral and IV administration. Based on the criteria of experts in clinical practice, we assumed that 95% of these drugs were administered IV. The model assumes that the number of IV administrations for each treatment regimen was the maximum number of times per cycle that the patient attended for the IV administration of that treatment regimen, as indicated in each drug's SPC13 and that no treatment was administered during the maintenance period.
Monitoring costs included routine patient visits and tests as well as hospitalisations. The amount of each of these resources was quantified using expert judgement, while unit costs were obtained from the health care cost database17. The model included different monitoring costs depending on the treatment and the treatment cycle and/or period. Based on the monitoring validated with experts, the watchful waiting period entailed an estimated cost of €184.94, irrespective of treatment. In addition, the model estimated a cost of €5,928.19 for CLL patients who progressed to second-line treatment, regardless of first-line treatment.
The cost estimate for adverse events associated with each treatment regimen was limited to grade 3 or 4 adverse events: neutropenia, thrombocytopenia, anaemia, infections (non-specific viral/bacterial), atrial fibrillation, hypertension, and bleeding. The percentage of patients experiencing each adverse event was obtained from the literature review. The cost of each adverse event, irrespective of treatment, was estimated using the cost of the corresponding diagnosis-related groups (DRGs) weighted by the number of cases of each DRG in the same year18. The cost of infections was estimated as the mean of the DRGs for bacterial and viral infections of unspecified location.
ResultsIn the scenario without FTDs, the total number of people diagnosed with CLL was estimated to increase from 13,726 in 2011 to 19,357 (41.0% increase) in 2025. With the introduction of FTDs, the total number of people with CLL in 2025 would be 19,196 (39.9% increase) by 2025. The average annual prevalence of CLL from 2011 to 2025 was estimated to be 16,436 patients in the scenario without FTDs and 16,413 patients in the scenario with FTDs.
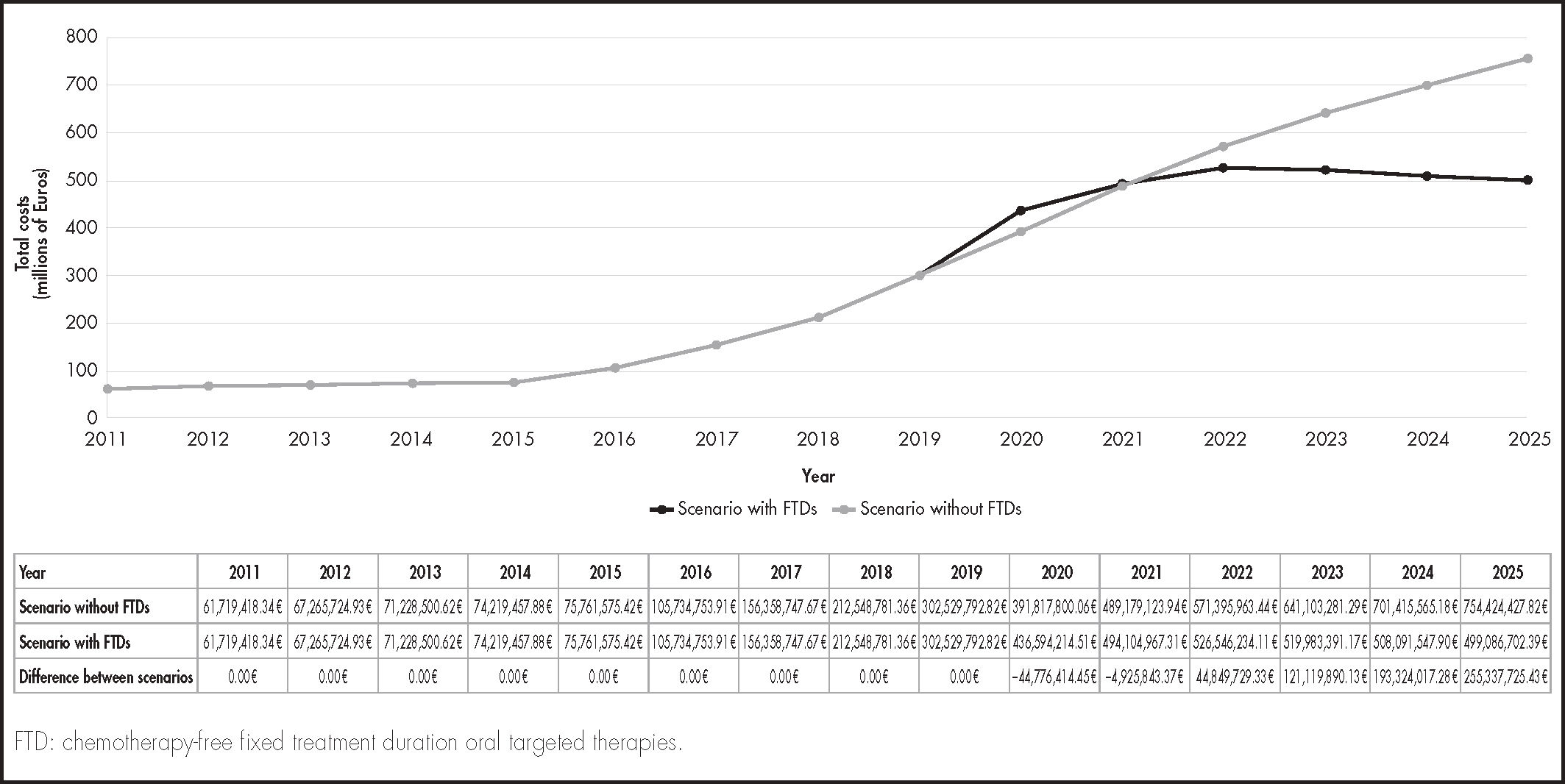
Table 3 shows the different costs for each of the treatments included in the model. From the perspective of the Spanish NHS, the total costs from 2011 to 2025 would be €4,676.7 million in the scenario without FTDs and €4,111.8 million in the scenario with FTDs (Figure 2): thus, the introduction of FTDs would result in savings of €564.9 million. These savings would imply a 12.1% reduction in the cost of caring for CLL patients over the period assessed. As shown in figure 2, the differences between the alternatives assessed begin to be seen from 2020 onward, which is the year in which FTDs become available for use in clinical practice in Spain. After a slight increase in total costs during 2020, from 2022 until the end of the study period, there would be a decrease in total costs of care for LLC patients, which is related to the fixed duration of some of the treatments used.
Costs associated with each of the chronic lymphocytic leukaemia treatments included in the model
| Treatment | Type of cost | Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | Cycle 6 | Further cycles | Maintenance | Treatment- and Progression-Free Period |
|---|---|---|---|---|---|---|---|---|---|---|
| Pharmacological | 1,751.57 € | 2,251.28 € | 2,251.28 € | 2,251.28 € | 2,251.28 € | 2,251.28 € | − | − | − | |
| FCR (IV) | Administration | 936.57 € | 936.57 € | 936.57 € | 936.57 € | 936.57 € | 936.57 € | − | − | − |
| Monitoring | 1,577.59 € | 341.72 € | 341.72 € | 341.72 € | 341.72 € | 341.72 € | − | − | 69.70 € | |
| Pharmacological | 2,241.42 € | 2,741.13 € | 2,741.13 € | 2,741.13 € | 2,741.13 € | 2,741.13 € | − | − | − | |
| FCR (Oral) | Administration | 312.19 € | 312.19 € | 312.19 € | 312.19 € | 312.19 € | 312.19 € | − | − | − |
| Monitoring | 1,577.59 € | 341.72 € | 341.72 € | 341.72 € | 341.72 € | 341.72 € | − | − | 69.70 € | |
| Pharmacological | 11,434.37 € | 3,811.97 € | 3,811.97 € | 3,811.97 € | 3,811.97 € | 3,811.97 € | − | − | − | |
| Clb + O | Administration | 1,248.76 € | 312.19 € | 312.19 € | 312.19 € | 312.19 € | 312.19 € | − | − | − |
| Monitoring | 1,450.49 € | 242.09 € | 242.09 € | 242.09 € | 242.09 € | 242.09 € | − | − | 69.70 € | |
| Pharmacological | 1,499.92 € | 1,999.64 € | 1,999.64 € | 1,999.64 € | 1,999.64 € | 1,999.64 € | − | − | − | |
| Clb + R | Administration | 312.19 € | 312.19 € | 312.19 € | 312.19 € | 312.19 € | 312.19 € | − | − | − |
| Monitoring | 1,450.49 € | 242.09 € | 242.09 € | 242.09 € | 242.09 € | 242.09 € | − | − | 69.70 € | |
| Pharmacological, 1st line | 2,193.10 € | 2,692.82 € | 2,692.82 € | 2,692.82 € | 2,692.82 € | 2,692.82 € | − | − | − | |
| BR | Pharmacological, 2nd line | 2,038.89 € | 2,538.61 € | 2,538.61 € | 2,538.61 € | 2,538.61 € | 2,538.61 € | − | − | − |
| Administration | 624.38 € | 624.38 € | 624.38 € | 624.38 € | 624.38 € | 624.38 € | − | − | − | |
| Monitoring | 1,651.10 € | 341.72 € | 341.72 € | 341.72 € | 341.72 € | 341.72 € | − | 69.70 € | ||
| Pharmacological | 5,898.48 € | 5,898.48 € | 5,898.48 € | 5,898.48 € | 5,898.48 € | 5,898.48 € | 5,898.48 € | − | − | |
| Ibrutinib | Administration | − | − | − | − | − | − | − | − | − |
| Monitoring | 1,822.57 € | 346.07 € | 346.07 € | 346.07 € | 346.07 € | 346.07 € | − | 473.58 € | − | |
| Pharmacological | 6,031.65 € | 6,531.36 € | 6,531.36 € | 6,531.36 € | 6,531.36 € | 6,531.36 € | 4,532.50 € | − | − | |
| Idelalisib + R | Administration | 312.19 € | 312.19 € | 312.19 € | 312.19 € | 312.19 € | 312.19 € | − | − | − |
| Monitoring | 1,822.57 € | 346.07 € | 346.07 € | 346.07 € | 346.07 € | 346.07 € | − | 473.58 € | − | |
| Pharmacological | 1,432.99 € | 6,196.70 € | 6,196.70 € | 6,196.70 € | 6,196.70 € | 6,196.70 € | 6,196.70 € | − | − | |
| Venetoclax | Administration | − | − | − | − | − | − | − | − | − |
| Monitoring | 2,105.55 € | 406.32 € | 406.32 € | 406.32 € | 406.32 € | 406.32 € | − | 636.81 € | − | |
| Pharmacological | 2,932.13 € | 8,195.56 € | 8,195.56 € | 8,195.56 € | 8,195.56 € | 8,195.56 € | 96,125.10 €* | − | − | |
| Venetoclax + R | Administration | 312.19 € | 312.19 € | 312.19 € | 312.19 € | 312.19 € | 312.19 € | − | − | − |
| Monitoring | 2,905.13 € | 406.32 € | 406.32 € | 406.32 € | 406.32 € | 406.32 € | − | 636.81 € | 13.79 € | |
| Venetoclax + O | Pharmacological | 12,866.59 € | 10,007.90 € | 10,007.90 € | 10,007.90 € | 10,007.90 € | 10,007.90 € | 36,014.51 €* | − | − |
| Administration | 1,248.76 € | 312.19 € | 312.19 € | 312.19 € | 312.19 € | 312.19 € | − | − | − | |
| Monitoring | 2,716.92 € | 408.06 € | 408.06 € | 408.06 € | 408.06 € | 408.06 € | − | 403.20 € | 13.17 € |
BR: bendamustine, rituximab; Clb + O: chlorambucil, obinutuzumab; Clb + R: chlorambucil, rituximab; FCR: fludarabine, cyclophosphamide, rituximab; IV: intravenous; O: obinutuzumab; R: rituximab.
The total savings would amount to €475.1 million (84.1%) in first-line treatments after watchful waiting and €90.8 million (15.9%) in treatments after relapse or the discontinuation of first-line treatment (see Figure 2).
The total cost per patient from 2011 to 2025 would rise from €266,019 in the scenario without FTDs to €236,852 in the scenario with FTDs, saving the Spanish NHS €29,167 per patient.
DiscussionThe introduction of oral targeted therapies for the treatment of CLL led to improved OS in CLL patients compared to OS under standard therapies. This improvement was a relevant advance for CLL patients19, although there was an increase in their cost of treatment. Thus, the introduction of FTDs represents a significant advance in CLL treatment, allowing health care systems to predict treatment duration and the cost of care of CLL patients. Our study projected an annual increase in the number of CLL patients and in the total direct costs of managing these patients. Specifically, the total costs from 2011 to 2025 would be €4,676.7 million in the scenario without FTDs, representing an increase of 1,222.35% over this period, and €4,111.8 million in the scenario with FTDs, representing an increase of 808.64% over the same period (Figure 2). Thus, the introduction of FTDs would generate considerable resource savings of €564.9 million (12.1% of the total cost of care for CLL patients) for the Spanish NHS over the entire period under assessment. If these results are confirmed in further studies, FTDs would be a treatment alternative offering good efficacy results for CLL patients, while optimising the use of health care resources in health systems.
An analysis of the total direct costs shows that, for all treatments, the highest costs are pharmacological ones. The highest pharmacological cost was that of ibrutinib as monotherapy (€41,289.36), which is one of the most recommended treatments in both first-line and relapse settings6.
The highest administration cost was that of IV fludarabine, cyclophosphamide, and rituximab in combination (€5,619.42) because it was the treatment included in the model with the most IV administra- tions13.
Monitoring costs ranged from €2,730.64 for the chlorambucil combinations to €5,58733 for the venetoclax and rituximab combination, In the latter case, more resources were used, mainly in the form of routine visits.
Few studies have investigated CLL disease burden or its cost minimisation and of these, none have been conducted in Spain. A study conducted in Germany in 2008 estimated a CLL prevalence of 4.9/10,000 inhabitants, with an estimated CLL disease burden of €4,946 per patient and an average total annual cost of €201 million within the setting of the German NHS20. The mean cost per patient in our study was much higher in the scenarios without and with FTDs (€266,019 and €236,852 per patient and a total cost of €311.8 million and €274.1 million, respectively). However, the studies are not comparable because the German study was conducted before the introduction oral targeted therapies which, as we have highlighted, brought about a radical change in the management of the disease. Therefore, the pharmacological cost was much lower in that study than in ours.
This study has some limitations, the first of which stems from the fact that the model represents a simplification of reality which, although based on the best possible evidence, can never be an accurate representation of real clinical practice. In this sense, one of the limitations is the assumption of a single treatment option for each type of patient and time period, which means that the costs associated with other treatments were not taken into account. This simplification of reality implies that the savings estimated in our analysis would be the maximum achievable, since all patients would follow the therapeutic strategies presented in the analysis. Although the choice of treatment was made on a case-bycase basis, the clinical experts agreed that the typical patient in each of the categories described in the model used the treatment alternatives most widely used in each of the time periods considered. On the other hand, the lack of data was completed and validated by the expert panel based on the literature review, thus reducing the model's uncertainty. Another limitation is that the analysis was conducted using the public prices of medicines: thus, the results could be affected by differences between these prices and the prices of the same drugs, which are reimbursed by the health care service. This aspect could impact the magnitude of the difference between the strategies assessed. However, any such difference is unlikely to affect the direction of the results obtained in favour of strategies that include FTDs, and thus, the conclusion of the study. Another potential limitation is that some of the patient subtypes included in the model did not differentiate between patients with or without the IGHV mutation status or did not include TP53 mutation status. After assessing this aspect, we assumed that the treatment and management differences in these patient subtypes between the two scenarios would be minimal and the model was simplified. Although these limitations could lead to underestimations or overestimations of the costs included in the model, the analysis is considered to be reflecting real clinical practice. The model included a population with a range of health states, treatments, and stratifications, for which we do not have data on specific prevalence. This implies that this prevalence had to be estimated from the incidence rates of previous years, and so the results of this study should be interpreted taking this nuance into account. On the other hand, the PFS and OS data included in the model were taken from clinical trials, which may lead to overestimations compared to these aspects in clinical practice (e.g. exclusion of comorbidities, better stratification, or age of patients). Finally, in the absence of a sensitivity analysis, it was not possible to assess the effect of uncertainty in variations in the model parameters on the results. Although this effect cannot be ignored, it was minimised by the expert panel, who provided their expertise and realworld knowledge of the disease, such that the model would be as close as possible to real clinical practice.
This economic analysis shows how the direct cost of CLL varies in the era of oral targeted therapies with the introduction of FTDs. From the perspective of the Spanish NHS, this introduction would result in savings of €564.9 million (12.1% of the total health costs for CLL patients during the period assessed). Although further studies are needed to assess the full effects and costs associated with CLL treatment, the present study may help to define future costsaving therapeutic strategies.
FundingThe study was designed, conducted, and funded by AbbVie.
AcknowledgmentsThe authors would like to acknowledge the support of María del Carmen Barrull Santamaría and Daniel Callejo Velasco de IQVIA in study design, data analysis, and medical writing. This support was funded by AbbVie.
Conflicts of interestDr. Montañés: Advisory board of AbbVie. Dr. Casado: Advisory board of AbbVie. Dr. Medina: Advisory board of AbbVie. Dr. Nieto: Consultancies for AbbVie and Boehringer, as well as training activities for Roche, Janssen, and Astellas. Dr. Ramírez: Advisory boards and presentations for Novartis, AbbVie, BMS, Gilead, Takeda, Janssen, Roche, Pfizer, Amgen, EUSA Pharma.
Presentation at congressesThe results of this work were previously presented at the LXII National Congress of the SEHH and XXXVI National Congress of the SETH.
Early Access date (01 /11 /2022).