Immunosuppressive drugs are necessary to avoid or reduce the risk of rejection of transplanted organs. The immunosuppression generated may result in these patients needing antibiotics and antivirals to be prescribed to them in conjunction with their immunosuppressants to avoid the risk of infection. This has generated an increase in neutropenia in patients treated with mycophenolate mofetil in combination with valganciclovir. The purpose of this study is to estimate the risk of neutropenia attributable to combination treatment of mycophenolate mofetil with valganciclovir in patients with a transplanted liver.
MethodThis is a retrospective cohort study. It included patients who received a liver transplant between 2012 and 2017 and who were treated with mycophenolate mofetil or with a combination of mycophenolate mofetil and valganciclovir. Minimum follow-up was 100 days post-transplantation. Children under 16 years of age and patients who died during follow-up were excluded. Binary logistic regression analysis was used to determine the association of neutropenia with sex, age, diabetes, creatinine at baseline and at discharge, and concomitant treatment of mycophenolate mofetil with valganciclovir. Relative risk and 95% CI were calculated using logistic regression coefficients.
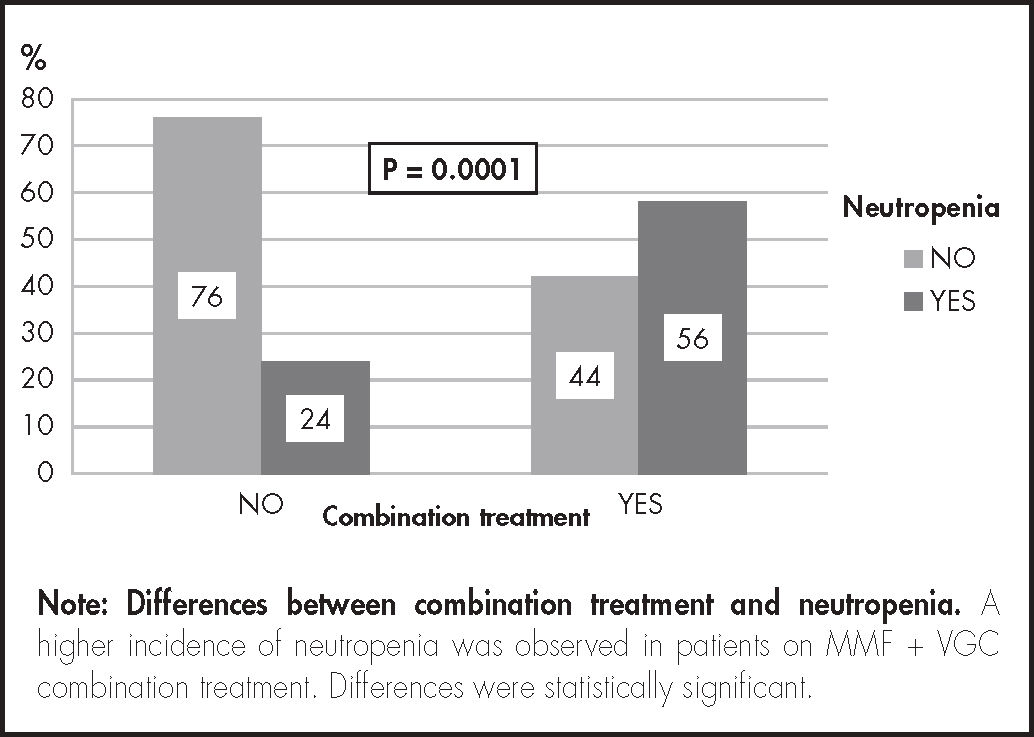
Results144 patients were analyzed, 87 were treated with mycophenolate mofetil and 57 received mycophenolate mofetil and valganciclovir together. An overall risk of neutropenia of 37% [95% CI (29-45)] was observed. The risk was significantly higher in patients who received the combination of mycophenolate mofetil and valganciclovir (56%) than in those treated with mycophenolate mofetil alone (24%), p = 0.001. Binary-logistic-regression analysis revealed that concomitant use of mycophenolate mofetil with valganciclovir was associated with an increased risk of neutropenia: Relative risk = 4.97, 95% CI [2.25-11.00].
ConclusionsOur study shows that concomitant use of mycophenolate mofetil and valganciclovir increases the risk of neutropenia in patients with a transplanted liver.
Los fármacos inmunosupresores son necesarios para evitar o reducir el riesgo de rechazo de órganos trasplantados. La inmunosupresión generada puede dar lugar a que estos pacientes necesiten recibir antibióticos y antivíricos con los inmunosupresores para evitar el riesgo de infecciones. Esto ha generado un incremento de neutropenia en pacientes tratados conjuntamente con micofenolato de mofetilo y valganciclovir. El objetivo de este estudio es estimar el riesgo de neutropenia atribuible al tratamiento concomitante de micofenolato de mofetilo y valganciclovir en pacientes trasplantados hepáticos.
MétodoEstudio de cohorte retrospectiva. Se incluyeron pacientes receptores de hígado entre 2012 y 2017 tratados con micofenolato de mofetilo o con la combinación de micofenolato de mofetilo y valganciclovir, con al menos 100 días de seguimiento postrasplante. Se excluyeron menores de 16 años y pacientes fallecidos durante el seguimiento. El análisis de regresión logística binaria se utilizó para determinar la asociación del riesgo de neutropenia con el sexo, edad, diabetes, creatinina basal y al alta, y tratamiento concomitante de micofenolato de mofetilo y valganciclovir. El riesgo relativo y los IC 95% se calcularon mediante los coeficientes de regresión logística.
ResultadosUn total de 144 pacientes fueron analizados, 87 se trataron con micofenolato de mofetilo y 57 recibieron conjuntamente micofenolato de mofetilo y valganciclovir, observándose un riesgo de neutropenia del 37%, IC 95% [29-45]. Este riesgo fue significativamente mayor en pacientes que recibieron la combinación de micofenolato de mofetilo y valganciclovir (56%) respecto a los tratados solo con micofenolato de mofetilo (24%), p = 0,001. El análisis de regresión logística binaria reveló que el uso concomitante de micofenolato de mofetilo y valganciclovir se asociaba a un mayor riesgo de neutropenia: riesgo relativo = 4,97, IC 95% [2,25-11,00].
ConclusionesNuestro estudio demuestra que el uso concomitante de micofenolato de mofetilo y valganciclovir aumenta el riesgo de neutropenia en pacientes trasplantados hepáticos.
Liver transplantation has allowed patients with progressive and irreversible liver conditions to extend their life expectancy with a satisfactory quality of life. There are multiple factors that may result in an irreversible dysfunction of the liver, including alcoholic cirrhosis (30%), hepatitis C virus (HCV)-related cirrhosis (23%), and hepatocarcinoma (21%)1.
The beginnings of liver transplantation date back to 1963 when Dr. Starzl performed the first liver transplant in humans. In those years, survival of these patients was extremely short due to the complexities inherent in the procedure, the lack of experience of the surgeons who performed it, and the failure to indicate an appropriate immunosuppressive treatment capable of preventing rejection of the transplanted liver. In the 1980’s, however, thanks to the advent of cyclosporine, the long-term survival of these patients started to gradually increase2.
A few years later, the development of new immunosuppressors marked a true revolution in the realm of organ transplantation, these drugs becoming key to the success of these procedures. Moreover, the subsequent honing of surgical and diagnostic procedures together with a more rigorous selection of transplant candidates led to a significant increase in 1, 5 and 10-year survival rates, which currently stand at 85%, 72% and 61%, respectively3.
Immunosuppressive treatment in transplanted patients usually consists in a combination of three drugs: a corticosteroid, a calcineurin inhibitor (CNI), usually tacrolimus, and mycophenolate mofetil (MMF), an antiproliferative agent. However, as a result of the immunosuppression induced by these drugs, patients are at a higher risk of infection, which often leads to the (prophylactic or therapeutic) prescription of a wide range of antibiotics and antivirals together with the immunosuppressive medication. This often leads to the appearance of adverse events caused by the interactions between the different antibiotics and antivirals, which come on top of the adverse reactions brought about by the immunosuppressive drugs themselves4.
One of the most widely used antivirals in transplanted patients is valganciclovir (VGC). VGC is an orally administered prodrug of ganciclovir that is prophylactically or therapeutically used for cytomegalovirus infections. One of the potential adverse reactions of VGC is neutropenia, which has also been reported following administration of MMF5.
An increased incidence of neutropenia has been observed in the last few years in patients receiving VGC and MMF concomitantly5–9. The risk of neutropenia has been shown to be even higher if these patients have received a liver transplant. The purpose of this study is to estimate the risk of neutropenia attributable to concomitant treatment with MMF and VGC in patients with a transplanted liver.
MethodsThis was a retrospective observational cohort study performed at Nuestra Señora de La Candelaria University Hospital, a third-level center located in Tenerife, Spain, which has the largest transplantation program in the Canary Islands. Subjects were liver transplant recipients treated with MMF or a combination of MMF and VGC between 2012 and 2017, with at least 100 days’ post-transplantation follow-up.
To be included in the study, subjects had to be over 16 years of age and had to have been subjected to a liver transplant between December 2012 and June 2017. Patients under 16 years of age and those who passed away during follow-up were excluded from the study. Patients were followed up until the appearance of the event of interest or until the end of the study period.
The subjects’ clinical and analytical data were retrospectively collected from their electronic medical records. All of them were liver transplant recipients concomitantly treated with MMF and VGC during the study period, following routine clinical practice.
According to the hospital's protocol, the immunosuppressive treatment prescribed to liver transplant recipients at discharge usually comprises three kinds of drugs: a corticosteroid, a CNI, usually tacrolimus, and MMF, an antiproliferative agent. The standard dose of MMF is 2 g daily, distributed in 2 doses of 1 g each, although 3 g/day regimens are also common. In the event of hematologic toxicity (particularly leukopenia), or of any other kind of toxicity, a dose reduction may be required. Should the neutrophil count fall below 1,300/µL the treatment must be discontinued. In cases of gastrointestinal toxicity, fractionation of the dose (500 mg/6 h) may be attempted. If this is not enough, the dose must be reduced. Administration should be oral whenever possible.
The recommended dose of VGC to be used as prophylaxis against cytomegalovirus in patients transplanted with a solid organ such as a liver is 900 mg once a day, provided that their glomerular filtration rate from the tenth to the 100th day post-transplantation (or 200th day in high-risk patients) is >60 mL/min.
The main variables of the study were as follows:
- •
Combination treatment (exposure factor): this was a dichotomous variable that identified whether MMF was used alone or in combination with VGC.
- •
Neutropenia (response variable): this was a dichotomous variable that measured whether neutropenia occurred or not. Neutropenia was considered to have occurred when the neutrophil count fell below 1.5 x 103/µL10.
The co-variables of our studies included:
- •
Demographic parameters: age and sex.
- •
Creatinine levels at baseline (before transplantation) and at discharge.
- •
Presence of hypertension and diabetes.
- •
Clearance values at baseline (before transplantation) and at discharge: values were obtained using the Cockcroft-Gault formula.
Assuming an exposure prevalence of 40%, a 28% risk in the unexposed group and a 42% risk in the exposed group resulted is a sample size of 375 subjects (150 exposed and 225 unexposed) with a 95% confidence interval (95% CI), and a statistical power of 80%8,9.
An initial descriptive analysis was performed by calculating the mean and median standard deviation (SD) and the interquartile range (IQR) [P25-P75] for numerical variables, and the percentages (%) for qualitative variables.
The risk of neutropenia was estimated using a 95% CI All hypothesis tests were bilateral and considered statistically significant at p = 5%. Pearson's chi-squared test was used for nominal variables, Mann-Whitney's U test for ordinal variables, and Student's t test for normally distributed data. A binary logistic regression analysis was performed to determine the association of neutropenia with sex, age, diabetes, creatinine levels (at baseline and at discharge), and MMF and VGC concomitant treatment. Relative risk (RR) and 95% CIs were calculated by means of logistic regression coefficients.
The analyses were carried out using the EPIDAT software package v.3 (Regional Ministry of Health of Galicia and Pan American Health Organization, PAHO-WHO), and the SPSS/PC for Windows statistical package v.24 (SPSS, Inc, Chicago, IL).
The study meets the ethical requirements laid down in Order SAS 3470/2009 and the Helsinki Declaration of the World Medical Association on the ethical principles to be followed by medical research involving humans, and its subsequent amendments. It also complies with the regulations applicable to studies of its characteristics. For those reasons, the Ethics Committee for Research with Medicinal Products of the University Hospital Complex of the Canary Islands (Tenerife Province) gave its consent to the performance of the study.
ResultsOne hundred sixty-five liver transplants were performed between 2012 and 2017, of which 21 were excluded following the patients’ death. This means that the final sample of our study was made up of 144 subjects (Figure 1).
Reasons for transplantation included cirrhosis of the liver (113), biliary cirrhosis (10), autoimmune hepatitis (10), and others (11). The most usual etiology in the hepatic cirrhosis group was alcoholic (36%), followed by HCV (19%) and compound etiology (13%).
Of all patients, 99 (69%) had to discontinue treatment with MMF. Causes for discontinuation included neutropenia (54%), change to a dual immunosuppressive regimen on medical orders (15%), tumor (10%) and other causes (21%).
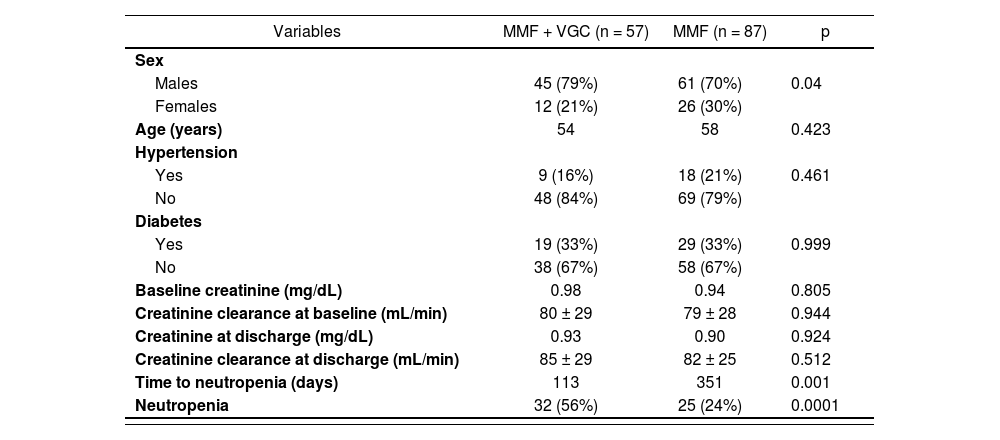
The characteristics of the studied population are summarized in Table 1.
Demographic and clinical characteristics of the sample
| Variables | MMF + VGC (n = 57) | MMF (n = 87) | p |
|---|---|---|---|
| Sex | |||
| Males | 45 (79%) | 61 (70%) | 0.04 |
| Females | 12 (21%) | 26 (30%) | |
| Age (years) | 54 | 58 | 0.423 |
| Hypertension | |||
| Yes | 9 (16%) | 18 (21%) | 0.461 |
| No | 48 (84%) | 69 (79%) | |
| Diabetes | |||
| Yes | 19 (33%) | 29 (33%) | 0.999 |
| No | 38 (67%) | 58 (67%) | |
| Baseline creatinine (mg/dL) | 0.98 | 0.94 | 0.805 |
| Creatinine clearance at baseline (mL/min) | 80 ± 29 | 79 ± 28 | 0.944 |
| Creatinine at discharge (mg/dL) | 0.93 | 0.90 | 0.924 |
| Creatinine clearance at discharge (mL/min) | 85 ± 29 | 82 ± 25 | 0.512 |
| Time to neutropenia (days) | 113 | 351 | 0.001 |
| Neutropenia | 32 (56%) | 25 (24%) | 0.0001 |
MMF: mycophenolate mofetil; VGC: valganciclovir.
Of the 144 patients, 87 did not receive VGC and 57 were put on concomitant MMF + VGC treatment. Time to neutropenia was shorter in patients on combination treatment, p = 0.001. Thirty-seven percent of all patients (95% CI = 29-45) developed neutropenia during follow-up. Of those on MMF + VGC combination treatment, 56% developed neutropenia as compared with 24% in patients who only took MMF (Figure 2).
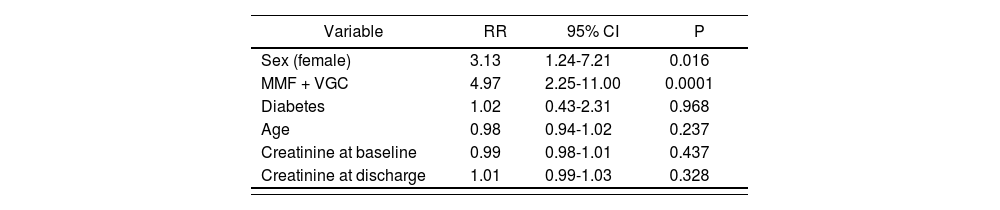
A multivariate analysis adjusted for sex, age, diabetes, creatinine levels (at baseline and at discharge) and concomitant MMF and VGC treatment was conducted to determine the association of these variables with the risk of neutropenia. The binary logistic regression analysis confirmed that the risk of neutropenia was associated with the patients’ sex and with concomitant use of MMF and VGC. Specifically, it was found that the risk of neutropenia involved in treating transplanted patients with MMF and VGC together was 4.97 times higher than that of treating them without that combination of drugs (RR: 4.97; IC95%: 2.25-11.00). As regards sex, it was found that females were 3.13 more likely to develop neutropenia than males (RR: 3.13; 95% CI: 1.24-7.21) (Table 2).
Variables of the study adjusted to a binary logistic regression model
| Variable | RR | 95% CI | P |
|---|---|---|---|
| Sex (female) | 3.13 | 1.24-7.21 | 0.016 |
| MMF + VGC | 4.97 | 2.25-11.00 | 0.0001 |
| Diabetes | 1.02 | 0.43-2.31 | 0.968 |
| Age | 0.98 | 0.94-1.02 | 0.237 |
| Creatinine at baseline | 0.99 | 0.98-1.01 | 0.437 |
| Creatinine at discharge | 1.01 | 0.99-1.03 | 0.328 |
RR: relative risk; CI: confidence interval; MMF: mycophenolate mofetil; VGC: valganciclovir.
The increased risk of neutropenia posed by concomitant use of MMF and VGC has been reported in previous studies5–9. Our study, however, unlike Molina et al., found statistically significant differences between concomitant use of these drugs and the appearance of neutropenia. This discrepancy could be due to the differences in patient ages, which in our study were higher (54 ± 10 years) than in Molina et al. (49.7 ± 12.7 years). Another factor that differs between the two studies was the VCG dose used as prophylaxis, which in Molina et al. was 1,440 mg/24 h on average, whereas in our study it was 900 mg/24 h.
Brum et al.6 and Zafrani et al.8 observed an increased incidence of neutropenia in patients with a renal transplant receiving concomitant treatment of MMF and VGC. It is to be expected that these same findings would apply to patients with a liver transplant like those included in our study.
Our study shows that concomitant use of MMF and VGC increases the risk of neutropenia in patients receiving a liver transplant, which may place them at a higher infection risk11. This is particularly significant in transplanted patients as the development of an infection could result in the loss of the transplanted organ12. An understanding of the interactions between MMF and VGC could prompt specialists to take steps to prevent the risks posed by their concomitant use. These could involve a reduction of the VGC dose6,13,14, the replacement of MMF by another immunosuppressor15, or the reduction/discontinuation of MMF with a concomitant readjustment of the dosing of the other immunosuppressors.
Some studies propose potential solutions to reduce neutropenia13–16. Savvidaki et al. suggest substituting MMF with everolimus, an immuno-suppressor with a different mechanism of action (selective mTOR inhibitor), which was shown to be associated to a lower risk of neutropenia. For their part, Imamura et al., Halim et al. and Kalil et al. concluded that low doses of VGC (450 mg/24 h) were as efficient as high doses (900 mg/24 h) of the drug at preventing a cytomegalovirus infection. Moreover, those high doses resulted in a lower incidence of neutropenia. However, it must be considered that none of these studies was specifically dedicated to patients with a liver transplant.
Limitations of this study have to do with the general limitations inherent in any retrospective cohort study, particularly selection bias. The reason for this is that these studies are typically commenced when the majority of patients have already experienced the event of interest, which is likely to increase the likelihood that such patients might participate in the study. Another limitation has to do with the fact that patients were receiving concomitant treatment with multiple drugs, which could have influenced the development of neutropenia. Moreover, variables such as etiology, BMI, and rejection episodes were not evaluated. Lastly, the size of the sample was not large enough to achieve the power estimated at the beginning of the study.
Performance of randomized studies will be necessary in the future to demonstrate our hypothesis. Future research should determine which therapeutic intervention can more safely and effectively decrease the risk of neutropenia, especially in patients receiving concomitant treatment with MMF and VGC: a reduction in the dose or duration of VGC, or a reduction in the dose of MMF with or without changing the rest of the immunosuppressive treatment. Whichever the decision, the alternative selected should not alter the effectiveness of the immunosuppressive treatment and of prophylaxis against cytomegalovirus.
It should also be underscored that this study found a higher incidence of neutropenia in females, which had not been observed by other authors. This finding should be corroborated in the future by larger well-designed studies.
In a nutshell, our study shows that the overall incidence of neutropenia in patients with a liver transplant is of 37% (95% CI = 29-45), with transplanted patients using MMF and VGC concomitantly showing the highest risk (56% vs 24% in patients receiving MMF alone; p = 0.001). This demonstrates that concomitant use of MMF and VGC increases the risk of neutropenia (RR: 4.97; IC95%: 2.25-11.00). Moreover, it was observed that female sex could play a role in the development of neutropenia (RR: 3.13; IC95%: 1.24-7.21).
FundingNo funding.
Conflict of interestsNo conflict of interests.
Presentation at congressesThis study was presented at the 63rd National Congress of the Spanish Society of Hospital Pharmacists.
Contribution to the scientific literature
This manuscript shows that concomitant use of mycophenolate mofetil with valganciclovir increases the risk of neutropenia in patients with a hepatic transplant. This increased risk of neutropenia has been studied in patients with a renal transplant but not in liver transplant recipients. Neutropenia is a characteristic reaction to the two drugs analyzed. The study demonstrates that a synergistic effect occurs when they are used concomitantly.
Both drugs analyzed are part of the therapeutic arsenal commonly used in patients who have undergone liver transplantation. This means that understanding the risk faced by patients of developing neutropenia could be used by healthcare providers to adjust their clinical practice.