Dimethyl fumarate is a medication approved for the treatment of relapsing-remitting multiple sclerosis. The purpose of the study was to evaluate the safety and persistence of dimethyl fumarate in clinical practice and analyze the occurrence of lymphopenia is patients treated with dimethyl fumarate over a period of at least 6 months.
MethodThis is a retrospective longitudinal observational study carried out between August 2015 and March 2019. The study cohort was made up of patients who had been treated with dimethyl fumarate for at least 6 months. Lymphocyte counts were recorded at different points of time (pre-treatment, at 3, 6, 12 months, and at the end of the study period). The evolution of lymphopenia was evaluated by means of a logistic regression statistical model. An analysis was performed of the relationship between a decreased lymphocyte count over the first 6 months of treatment and the development, by the end of the study, of grade II-III lymphopenia necessitating discontinuation of dimethyl fumarate. Other safety indicators were also evaluated including adverse events and interruptions or discontinuations of treatment. Persistence was determined by measuring the time to discontinuation of treatment.
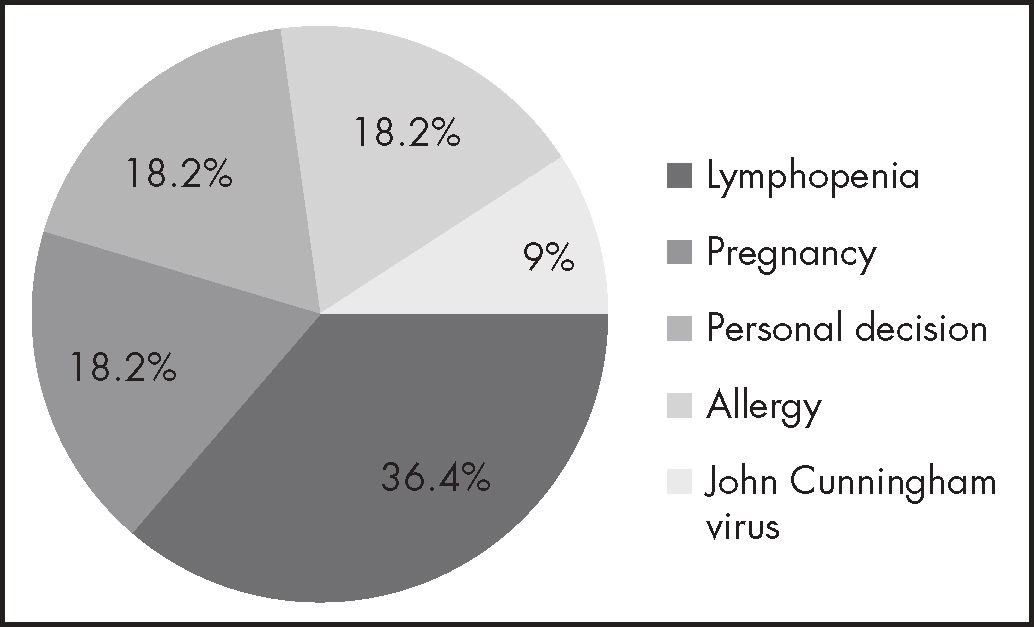
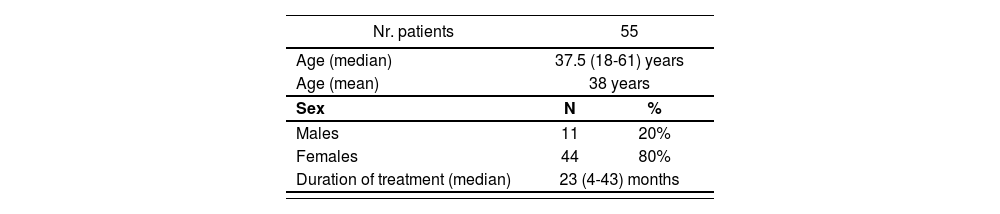
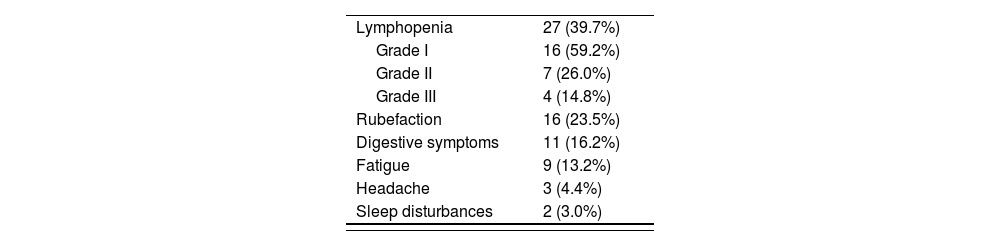
ResultsThe study included a total of 55 patients, of whom 80% were female. The most common adverse events were lymphopenia (27), rubefaction (16), digestive symptoms (11), fatigue (9), headache (3) and sleep disturbances (2). Eleven subjects interrupted/discontinued their treatment during the study period; reasons were as follows: pregnancy (2), personal decision (2), John Cunningham virus infection (1), allergy to the drug (2), and lymphopenia (4). Median duration of treatment was 23 months (4-43 months). A statistically significant association was found between a lower lymphocyte count over the first 6 months of treatment and the development of severe lymphopenia by the end of the study [OR = 1.34 (1.27-11.41); 95% CI (p = 0.001)].
ConclusionsThe adverse events observed in the present study are in line with those reported in previous analyses. Lymphopenia was the most common adverse event. The persistence of the medication was similar to that found in pivotal trials. The significant association found between a decreased lymphocyte count over the first 6 months of treatment and the development of severe lymphopenia by the end of the study suggests a connection between both variables, which could be instrumental in being able to predict and even prevent the occurrence of such lymphopenias.
Dimetilfumarato es un fármaco autorizado en el tratamiento de la esclerosis múltiple recurrente-remitente. El objetivo es evaluar la seguridad y persistencia del dimetilfumarato en la práctica clínica, y analizar la evolución de las linfopenias en pacientes en tratamiento con dimetilfumarato un mínimo de 6 meses.
MétodoEstudio observacional, longitudinal, retrospectivo entre agosto de 2015 y marzo de 2019. Se incluyeron todos los pacientes en tratamiento durante un periodo mínimo de 6 meses. Se recogieron los datos de recuento linfocitario a diferentes tiempos: pretratamiento, a los 3, 6, 12 meses y al final del periodo de estudio. Como modelo estadístico se utilizó la regresión logística para analizar la evolución de las linfopenias. Se estudió la relación entre el descenso del recuento linfocitario los primeros 6 meses de tratamiento y el desarrollo a tiempo final del estudio de linfopenias grado II/III que podrían ser motivo de suspensión. Además, se evaluaron otros indicadores de seguridad: reacciones adversas, suspensiones y abandonos de tratamiento. Para el análisis de la persistencia se contabilizaron los meses transcurridos desde el inicio hasta la suspensión del tratamiento.
ResultadosSe incluyeron 55 pacientes. El 80% fueron mujeres. Las reacciones adversas más frecuentes fueron: linfopenia (27), rubefacción (16), molestias digestivas (11), fatiga (9), cefalea (3) y alteraciones del sueño (2). Durante el periodo considerado hubo 11 abandonos/suspensiones de tratamiento, las razones fueron: embarazo (2), decisión propia (2), infección por virus John Cunningham (1), alergia al fármaco (2) y linfopenia (4). La mediana de duración de tratamiento fue de 23 meses (4-43 meses). Se encontraron diferencias estadísticamente significativas en el análisis de la relación entre el descenso de linfocitos los primeros 6 meses de tratamiento y el desarrollo de linfopenias graves a tiempo final del estudio, con una odds ratio de 1,34, un intervalo de confianza del 95% de 1,27-11,41 y un valor de p de 0,001.
ConclusionesLas reacciones adversas observadas siguen la línea de ensayos y estudios previos. La linfopenia fue la reacción adversa más frecuente. Los resultados muestran una persistencia del tratamiento similar a la de los ensayos pivotales. Las diferencias significativas observadas entre la reducción de linfocitos los primeros 6 meses de tratamiento y el desarrollo de linfopenias graves al final del estudio, sugieren una relación entre ambas variables y la posibilidad de predecir y evitar la aparición de dichas linfopenias.
Multiple sclerosis (MS) is a chronic inflammatory neurological disease that causes multifocal demyelination of the central nervous system (CNS). Although the condition's etiology has not been fully ascertained, it is believed to be an autoimmune disease triggered by an unknown stimulus in genetically predisposed individuals, involving cellular and humeral immunological abnormalities1.
The disease is thought to consist of two well-defined mechanisms: an autoimmune inflammatory mechanism that manifests itself in the first few years following the onset of the disease and is characterized by flare-ups and demyelinating lesions affecting both the white and the grey matter; and a degenerative mechanism triggered by irreversible axonal and neuronal damage which, although present from the initial stages of the disease, becomes more significant at later phases2.
MS a condition associated with a high social and healthcare burden due to its high incidence, its tendency to result in significant disability in young adults, its occupational repercussions, the high level of care required by patients and the high cost of treatment2.
MS has traditionally been categorized into four types3:
- a)
Relapsing-remitting multiple sclerosis (RRMS).
- b)
Secondary-progressive multiple sclerosis (SPMS).
- c)
Primary-progressive multiple sclerosis (PPMS).
- d)
Recurring progressive multiple sclerosis (RPMS).
Nowadays, however, a simpler, therapeutically-oriented classification has gained considerable acceptance, which classifies MS into forms with and without exacerbation potential1.
There is currently no curative treatment for the disease. Different medications have been approved in the last few years to be administered throughout the evolution of the disease which are aimed at decreasing the incidence of flare-ups and improving patients’ quality of life. One such drug is dimethyl fumarate (DMF), the use of which was approved in Spain in 2015 for first-line treatment of RRMS1.
DMF is a fumaric acid ester whose not-yet-fully-understood mechanism of action is known to activate the transcription pathway of nuclear factor NRF2. This pathway is presumed to be an important cellular defense mechanism against potentially toxic stimuli, including oxidative and inflammatory stress, both of them potentially implicated in MS pathogenesis1. DMF's adverse event (EA) profile could make it necessary for its administration to be interrupted or discontinued. The most common adverse effects recorded to date include rubefaction, gastrointestinal disturbances, and impaired hepatic function with high transaminase levels and lymphopenia4.
The aim of this study is to determine the safety and persistence of DMF therapy in our hospital's clinical practice, with special regard to the development of lymphopenia in patients with RRMS treated for at least 6 months.
MethodsA retrospective longitudinal observational study was undertaken to evaluate therapeutic safety and duration of symptoms in patients from our hospital treated with DMF for at least 6 months.
Data were obtained from the patients’ electronic medical record (EMR) and the hospital's pharmacy department's outpatient medication dispensing application. The data were anonymized in accordance with the procedure stipulated by Law 41/2002, of 14 November 2002, which regulates patient autonomy and clinical information and documentation-related rights and obligations5.
The variables analyzed included sex, age, date of treatment initiation and discontinuation, reason for discontinuation, identification of DMF-related AEs, and lymphocyte count.
Lymphocyte levels were measured at different points of time (T): prior to DMF treatment (T0), at 3 months (T3), at 6 months (T6), at 12 months (T12) and at completion of the study (TC).
Lymphocyte count at TC was considered a dichotomous variable (grade 0-I lymphopenia/grade II-III lymphopenia), the latter kind being the one leading to treatment discontinuation. An additional variable was used, which reflected the lymphocyte count difference between months T0 and T6 (T0-T6). This variable was recoded on three stratified levels.
An Independence test was performed between both variables using the chi-square value. In order to predict the evolution of lymphopenia toward more severe forms that could result in treatment discontinuation, an analysis was also made of the correlation between a decrease in lymphocyte count over the first 6 months of treatment (T0-T6) and the development of lymphopenia by the end of the study (TC). A logistic regression model was constructed to determine that correlation. Statistical significance was 95%. Statistical data were processed using the R Commander software package (version Rx64 3.6.1).
Safety was evaluated as a function of the presence (or absence) of AEs and interruptions/discontinuatio0ns of treatment. Lymphopenia was measured using the Common Terminology Criteria for Adverse Events (CTCAE) (Table 1)6. Persistence was determined by measuring the number of months elapsed from initiation to discontinuation of treatment for any cause.
CTCAE scale: Lymphocyte count decreased6
| Grade I | Grade II | Grade III | Grade IV | |
|---|---|---|---|---|
| Limphocyte | < LLN-800/mm3; | 800-500/mm3; | 500-200/mm3; | < 200/mm3; |
| count decreased | < LLN-0.8 × 109/L | 0.8-0.5 × 109/L | 0.5-0.2 × 109/L | < 0.2 × 109/L |
LLN: lower limit of normal.
Definition: A finding based on laboratory test results that indicate a decrease in number of lymphocytes in a blood specimen.
A total of 55 patients were included in the study, all of them on treatment with DMF. All patients received the dose recommended on the drug's label, i.e. 120 mg twice daily during the first week, followed by 240 mg every 12 hours1. The baseline characteristics of patients included in the study are presented in table 2.
The most common AEs were lymphopenia (27 patients), rubefaction (16), digestive symptoms (11), fatigue (9), headache (3) and sleep disturbances (2). Of the total of patients who developed lymphopenia, 16 presented with grade I lymphopenia, 7 with grade II lymphopenia and 4 patients with grade III lymphopenia6. Table 3 includes all AEs identified in the study.
AEs resulted in discontinuation of treatment in four patients: three of them developed grade III lymphopenia, which persisted for at least 6 months; the other developed a sudden grade II lymphopenia as well as a urinary infection.
Treatment discontinuations were due to the following reasons: two patients discontinued at 21 and 35 months, respectively, because they because they became pregnant. Both were clinically stable, with no signs of progression or any AEs at the time of interruption; two abandoned their treatment of their own accord, at 4 and 28 months, respectively, and are at present still reluctant to undergo any kind of therapy; one patient developed a John Cunningham virus (JCV) infection at 22 months and presented with no signs of progression or AEs at the time of discontinuation; and one patient developed an allergy to the drug (see Figure 1).
The binary logistic regression analysis conducted between the detection of lower lymphocyte levels during the first 6 months of treatment (T0-T6) and at the onset of lymphopenia by the end of the study (TC) showed statistically significant results (p = 0.001), with an odds ratio of 1.34 (1.27-11.41) (95% CI). According to this analysis, a decrease in the lymphocyte count over the first 6 months of treatment could be indicative of the development of lymphopenia in the course of treatment with DMF.
A chi-square test of independence performed between the two variables showed them to be independent, with chi-square 3.84, p = 0.14.
DiscussionMS is a chronic disease of the CNS characterized by inflammation, demyelination, glial scar formation and neuroaxonal damage leading to variable degrees of persistent neurological impairment2.
DMF is an oral agent approved in 2015 for treatment of the various clinical forms of RRMS. The drug is administered as a 120 mg capsule every 12 hours at the beginning of treatment, followed by a maintenance dose of 240 mg twice daily1.
The patient cohort included in this study had a mean age of 38 years [median: 37.5 (18-61)] and a mean duration of treatment of 23 months, which is in line with the DEFINE and CONFIRM pivotal trials, which included patients aged between 18 and 55 years (mean ages were 39 and 37 years, respectively) and a follow-up period of 24 months.
DMF's safety profile was established on the basis of the findings of the main clinical trials performed on the drug (C-1900, 109MS301, 109MS302 and 109MS303). The most common AEs identified by these trials were rubefaction and gastrointestinal symptoms. Less frequent AEs included leukopenia, elevated hepatic enzymes, itching and proteinuria1. In contrast, the most frequent AE observed in our study (in 49.1% of patients) was lymphopenia in its varying degrees of severity, followed by rubefaction and gastrointestinal symptoms, found in 29.1% and 20% of patients, respectively.
Lymphopenia is an AE that must be followed up over the long term due to the risk of persistence. Zecca C et al. reported the case of a patient where DMF had to be discontinued at 4 months of treatment following 3 months of persistent grade III lymphopenia. At 6 months from suspension, lymphopenia persisted at grade II7. For this reason, pre-marketing studies are essential to determine medium- and long-term safety.
Different studies have analyzed the AE profile of DMF with similar results to those of pivotal studies. Similarly to the pivotal trials, a randomized double-blind placebo-controlled phase III trial by Ralf Gold et al. involving 1,237 patients followed up for 96 weeks found the following AEs: flushing, gastrointestinal symptoms, proteinuria, itching, lymphopenia and elevated hepatic aminotransferase8. Lymphocyte levels decreased during the first year of treatment, with 4% of patients exhibiting a fall in lymphocyte levels to below 0,5 × 109/L, which corresponds to grade III lymphopenia. Subsequently, however, the lymphocyte count stabilized and remained within the normal range thereafter. No cases required discontinuation of treatment.
A 2015 Cochrane review of parallel-group randomized clinical trials evaluating the use of DMF as monotherapy, or in combination with other drugs versus placebo or other DMDs, concluded that the most common AEs included hot flushes and gastrointestinal disturbances. Lymphopenia and leukopenia were reported to be less common AEs, which differs from the findings of our study where lymphopenia was the most common AE. Moreover, a comparison of 240 mg of DMF every 12 hours vs. placebo revealed an increased risk of developing grade III and grade I lymphopenia in patients treated with DMF at two years (RR 5,69; 95% CI: [2.40-13.46]; p < 0.0001 and RR 6.53; 95% CI: [3.13-13.64]; p < 0.00001, respectively)9.
In a study of 1,089 patients published in 2018, Mirabella M. et al. observed that 16.5% of their subjects developed lymphopenia. A total of 12% developed grade I-II lymphopenia and 4.5% developed grade III lymphopenia. Mean time to onset of lymphopenia was 9.8 ± 6.8 months. DMF had to be suspended in 2.5% of patients due to persistent grade III lymphopenia10. In our study, up to 49.1% of patients developed lymphopenia of some severity (41.8% grade I-II and 7.3% grade III). Persistent grade III lymphopenia was responsible for discontinuation in 5.4% (3) of patients.
Miclea A. et al. carried out a retrospective analytical study of 644 patients and showed that 5.3% had to discontinue DMF due to lymphopenia. Mean time to discontinuation of DMF was 0.52 years11. A total of 7.27% of patients had to discontinue DMF due to lymphopenia of different degrees of severity.
In 2017, Raed Alroughani et al. published a 6-months prospective study of 119 patients diagnosed with RRMS and treated with DMF. Their aim was to evaluate the drug's effectiveness, tolerability, and safety, in addition to its effect on the occurrence of lymphopenia. Similarly to our study, they used the CTCAE scale to grade the severity of lymphopenia. They found that 8.4% of patients developed grade I-II, mostly transient, lymphopenia, and 2.5% of patients developed persistent grade III lymphopenia that resulted in discontinuation of DMF treatment12. As mentioned above, the percentage of patients in our study where treatment had to be suspended due to the development of persistent grade III lymphopenia was 5.4%.
A comparison of the results published in the literature with those obtained in the present study shows a higher incidence of lymphopenia of any grade among the patients in our study. Indeed, lymphopenia was the most common AE in our series. The comparison also reveals a higher incidence of grade III lymphopenia in our patients as well as a higher proportion of treatment discontinuations resulting from lymphopenia.
The statistically significant differences observed between T0-T6 and TC suggest a correlation between a lower lymphocyte count during the first 6 months of treatment and the development of grade II-III lymphopenia by the end of the study. Should this hypothesis be borne out, it would be mean that the development of lymphopenia toward more severe forms could be predicted (and prevented) from the sixth (T6) month of treatment.
The limitations of this study include the absence of data from the patients’ EMRs. This resulted in the lack of homogeneity observed in the lymphocyte counts measured following the first year of treatment. Patients with lower lymphocyte counts tend to be subjected to stricter analytical controls than those where the lymphocyte count is within the normal range. Future studies should take this into consideration and subject all patients treated with DMF to annual lymphocyte tests.
In a nutshell, the results of this study, which included patients of similar characteristics to those participating in pivotal studies, show a similar AE profile to the one reported by previous studies. Lymphopenia was the most common AE and the most likely cause for treatment discontinuation, with a higher incidence of the less severe forms. Moreover, the correlation identified between a decrease in the lymphocyte count over the first 6 months of treatment and the development of grade II-III lymphopenia by the end of the study suggests that it could be possible to introduce protocols capable of predicting the evolution of lymphopenia and preventing its progression toward the more severe forms that tend to result in discontinuation of treatment.
The persistence of DMF therapy found in our study was similar to that observed in the pivotal trials.
FundingNo funding.
Conflict of interestNo conflict of interests.
Contribution to the scientific literature
Multiple sclerosis is a chronic neurologic condition that causes demyelination of the central nervous system. There is currently no curative treatment for the disease; available treatments are aimed at reducing the frequency of flare-ups and improve patients’ overall quality of life. Dimethyl fumarate was approved in Spain in 2015 for the treatment of relapsing-remitting multiple sclerosis. The drug's adverse events include lymphopenia, which often requires discontinuation of treatment.
This study provides real-life data, evaluating the adverse events observed in patients with the disease in terms of their distribution, frequency, evolution, and their role in treatment discontinuation. Special consideration is given to lymphopenia and, particularly, to its evolution during the study period, its severity, and its role in the discontinuation of treatment with dimethyl fumarate. The results obtained from this study could constitute the basis for future efforts aimed at predicting the development of severe lymphopenia and preventing its occurrence in patients with multiple sclerosis treated with dimethyl fumarate.