The objective of this article is to review the quality requirements and recommended uses of the different types of face masks with a view to helping optimize their use and facilitating identification of nonconforming products.
MethodA literature search was conducted in PubMed, the Spanish Official State Gazette and Eudralex. The websites of the Ministry of Industry, Commerce and Tourism and of the Ministry of Health, as well as the relevant UNE standards were also reviewed.
ResultsThe different types of face masks available on the market meet different regulatory requirements. Community masks are not considered medical devices or personal protective equipment and do not require marketing authorization. They do not carry a CE mark and need not comply with the general regulations applicable to consumer products. Surgical masks, for their part, must meet the quality criteria defined in UNE-EN standard 14683: 2019. According to Regulation (EU) 745/2017 they are class I devices, subject to an EU declaration of conformity, and must bear a CE mark. Filtering masks are considered category III personal protective equipment, regulated by Regulation (EU) 2016/425, and must also bear a CE mark. In spite the abundant regulations in place, market control instruments have detected counterfeit face masks, which means that public authorities and users should ask manufacturers or suppliers for additional information in case of doubt.
ConclusionsThe legal and quality requirements of the masks are sufficient for their safe use. It is necessary for the general public to know these requirements to avoid the fraudulent use of high consumption products.
Revisar los requisitos de calidad y usos recomendados de los diferentes tipos de mascarillas con objeto de optimizar su uso y facilitar la identificacion de los productos no conformes.
MétodoSe hizo una busqueda bibliografica en PubMed, en el Boletin Oficial del Estado y Eudralex; se revisaron las paginas web de los Ministerios de Industria, Comercio y Turismo y Sanidad, asi como las normas UNE.
ResultadosLos diferentes tipos de mascarillas que se pueden encontrar en el mercado se acogen a diferentes exigencias regulatorias. Las mascarillas higienicas no se consideran productos sanitarios ni equipo de proteccion individual y no necesitan autorizacion. No llevan marcado CE y deben cumplir con la normativa general de los productos de consumo. Para las mascarillas quirurgicas, los criterios de calidad estan definidos en la UNE-EN 14683:2019, son productos sanitarios de clase I segun el Reglamento (UE) 745/2017, se les requiere declaracion UE de conformidad y debe colocar el marcado CE en el producto. Las mascarillas filtrantes son equipos de proteccion individual de categoria III, estan reguladas por el Reglamento (UE) 2016/425 y deben llevar marcado CE conforme al mismo. Por otro lado, los instrumentos de control de mercado han detectado mascarillas fraudulentas, por ello, ante cualquier duda se debe solicitar informacion adicional al fabricante o proveedor.
ConclusionesLos requisitos legales y de calidad de las mascarillas son suficientes para su uso seguro. Es necesario que el publico general conozca estos requisitos para evitar el uso fraudulento de estos productos de alto consumo.
The outbreak of COVID-19, caused by a new coronavirus named SARS‑CoV-2, was first identified in Wuhan, China, in December 20191,2. The new virus, which started out by killing thousands in China2, then went on to spread throughout the globe3. SARS-CoV-2 may be transmitted by Flügge droplets sprayed into the air when an infected individual talks, coughs or sneezes onto a susceptible person's mucosal or conjunctival membrane, or by direct contact with a surface that has been contaminated by such droplets. Measures adopted to prevent and control the spread of COVID-19 include the use of face masks, combined with other hygienic precautions, and social distancing4–6.
Different international organizations, such as the World Health Organization (WHO)7, the European Centre for Disease Prevention and Control (ECDC)8, and the Centers for Disease Control and Prevention of the United States (CDC)9 advise that healthy individuals should wear face masks where social distancing of at least 2 meters cannot be maintained. They also warn that disposable face masks should not be reused and that use of reusable face masks must follow the manufacturers’ recommendations with regard to the way they should be washed and the maximum number of washing cycles they may undergo10.
The Spanish Center for Coordination of Health Alerts and Emergencies has issued a series of recommendations on the use of face masks in the context of the COVID-19 pandemic10, which urge the general population to wear so-called community face masks. Use of surgical face masks is reserved for symptomatic persons, those in close contact with a confirmed case of COVID-19, and those living in collective residential facilities and penitentiary centers.
Persons belonging to a COVID-19 risk group (the elderly, persons with chronic conditions, pregnant women) are advised to use community, surgical10 or dual protection face masks depending on whether they are in a well-ventilated space or in a poorly ventilated and/or overcrowded environment such as a public transport vehicle.
From the healthcare point of view, face masks are not recommended in children aged 3 or below, people with respiratory conditions, persons with disabilities or who depend on caregivers or people with behavioral disorders. Their use is also advised against in the case of people engaging in activities that may complicate or prevent the use of face marks, such as intense exercise10. On the other hand, Order SND/422/2020 regulates the conditions under which face masks must compulsorily be worn during the COVID-19 crisis. Article 2 of the said Order imposes the obligatory use of face masks on persons aged 6 or above11.
The present study was conducted to review and provide information on the quality requirements and recommended usage of the different types of face masks in order to prevent the potential risks inherent in their inappropriate use.
MethodsA search was carried out in the Spanish Official State Gazette (BOE) to review the existing regulation, and in Pubmed to identify scientific articles on the subject. The keywords used were “face masks” and “COVID,” records being filtered by date of publication. Additional sources of information included the websites of the Ministry of Industry, Trade and Tourism (specifically the “Guidelines for the manufacturing and selection of face masks, face and eye protections, gloves, and safety outfits”) and of the Ministry of Health, as well as the UNE reference standards. The retrieved records were classified according to the different types of face masks discussed [community, surgical, or personal protection equipment (PPE)-grade].
ResultsThe following records were retrieved from the literature review: the “Guidelines for the manufacturing and selection of face masks, face and eye protections, gloves, and safety outfits”, the “Recommendations on the use of face masks in the community during the Covid-19 pandemic”, a document titled “What should you take into consideration when buying a face mask?”, as well as the reference documents of the Ministry of Industry, Trade and Tourism. UNE standards reviewed included: UNE 0064–1, UNE 0064–2, and UNE 0065 for community face masks; Directive 93/42 on medical devices (MDs), Regulation 2017/745 on MDs and UNE standard 14683:2019 for surgical face masks; and Regulation 2016/425 on PPE for face masks regarded as personal protection equipment.
Community face masksCommunity face masks are designed to protect the wearer and those around them as they reduce the spread and inhalation of respiratory droplets. They cover the mouth, the nose, and the chin and comprise one or more layers of textile fabric. They may be reused and are marketed in specific adult and child models10.
Regulatory aspectsCommunity face masks are not considered MDs or PPE and do not require marketing authorization. Market surveillance of these masks is the responsibility of the consumer affairs authorities of the different regional administrations. They cannot bear a CE mark12 and must comply with the general rules applicable to consumer products.
Types of community face masksa) Single-use face masks
These face marks must meet the specifications of UNE standard 0064 Non-reusable community masks. Materials, design, production, CE marking and use standards13 (Table 1). UNE standard 0064–1 defines use specifications for adults and UNE standard 0064–2 defines use specifications for children.
As regards the materials, community masks are manufactured with non-woven fabric and must under no circumstances contain any elastic components. A “non-woven fabric” is a predominantly planar fibrous assembly that has been given a designed level of structural integrity by physical and/or mechanical means, excluding weaving, knitting or paper making14.
b) Reusable community face masks
These face masks must meet the specifications of UNE standard 0065 Reusable community face masks for adults and children. Materials, design, production, CE marking and use standards15 (Table 1). These face masks must be able to endure at least five washing and drying cycles without compromise to their performance and ensuring complete virus eradication. The methods recommended by the Ministry of Health include16:
1. Washing and disinfection with ordinary detergent and water at 60–90 °C.
2. Soaking the face mask in a 1:50 diluted bleach/warm water solution for 30 minutes. Afterwards, the face mask should be washed with abundant soap to remove any bleach and allowed to dry.
Given the urgent need of virucidal products the Ministry of Health established that any of the virucidal products authorized for disinfecting public and private spaces (PT2)17 and compliant with standard 14476 (Chemical antiseptics and disinfectants. Quantitative virucidal suspension trial of chemical antiseptics and disinfectants used in medicine) and authorized for use by the general public could be used for this purpose.
Surgical face masksThese face masks are considered MDs. They constitute a barrier against direct transmission of infectious agents between surgical staff and patients18.
Regulatory aspectsTheir consideration as MDs stems from Directive 93/42/EEC on MDs, transposed to Spanish domestic legislation by Royal Decree 1591/2009.
Regulation (UE) 2017/745 of the European Parliament and of the Council on MDs came into force in 2017, superseding Directive 93/42/EEC. It was initially provided that the Regulation would start to apply from 26 May 2020, but following the outbreak of COVID-19, the EU adopted Regulation (EU) 2020/561 which postponed its entry into force by one year to guarantee unrestricted availability of MDs in the EU market in the context of the pandemic and the resulting public health crisis.
These face masks must comply with the essential requirements that may be applicable to them according to their intended use19.
Surgical face masks are classified as class I MDs on account of their lower risk. Manufacturers or their authorized representatives are only required to provide an EU declaration of conformity stating that the face masks in question meet the requirements of the Directive. These masks must bear a CE mark.
In terms of quality, these masks must comply with UNE EN standard 14683:2019+AC:2019 (Surgical masks. Requirements and testing methods20. The main purpose of these masks is to protect the surgical field. The standard specifies the procedures that must be followed in the manufacturing and design of these masks as well as all relevant performance requirements and testing methods.
They are classified into two types: type I and type II, depending on their bacterial filtration efficiency (Table 2). Type II surgical masks may be further classified as type IIR if they are splash resistant20. Table 2 specifies the performance requirements for these face masks.
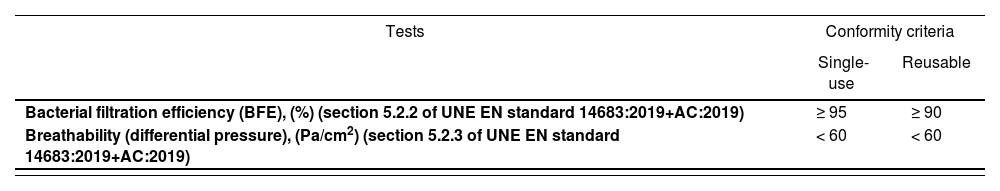
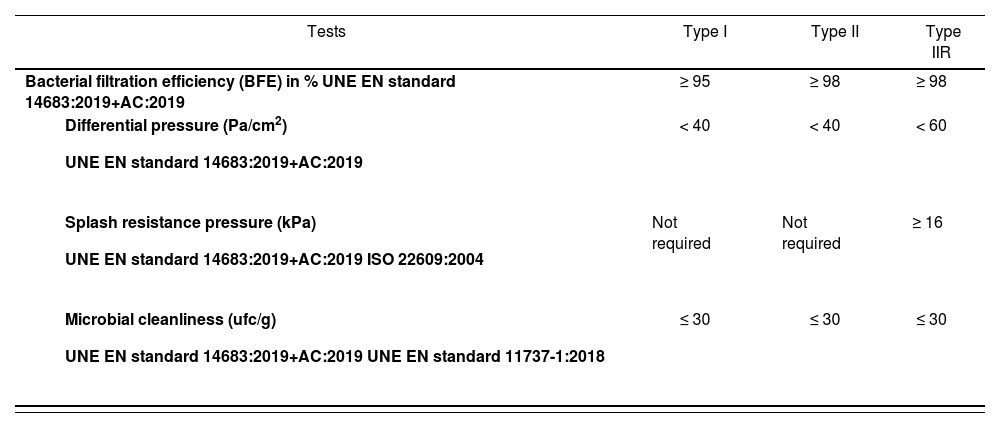
Performance requirements for surgical face masks20
| Tests | Type I | Type II | Type IIR |
|---|---|---|---|
| Bacterial filtration efficiency (BFE) in % UNE EN standard 14683:2019+AC:2019 | ≥ 95 | ≥ 98 | ≥ 98 |
| < 40 | < 40 | < 60 |
| Not required | Not required | ≥ 16 |
| ≤ 30 | ≤ 30 | ≤ 30 |
UNE EN standard 14683:2019 AC requires that these face masks be subjected to the following tests: breathability, splash resistance, biological load, and biocompatibility.
Type I surgical face masks should only be worn by patients and should be avoided by health personnel working in operating rooms or similar environments.
Personal protection equipment-grade face masksThese face masks are aimed at protecting wearers from inhalation of hazardous substances21. Table 3 describes the different kinds of PPE-grade face masks available:
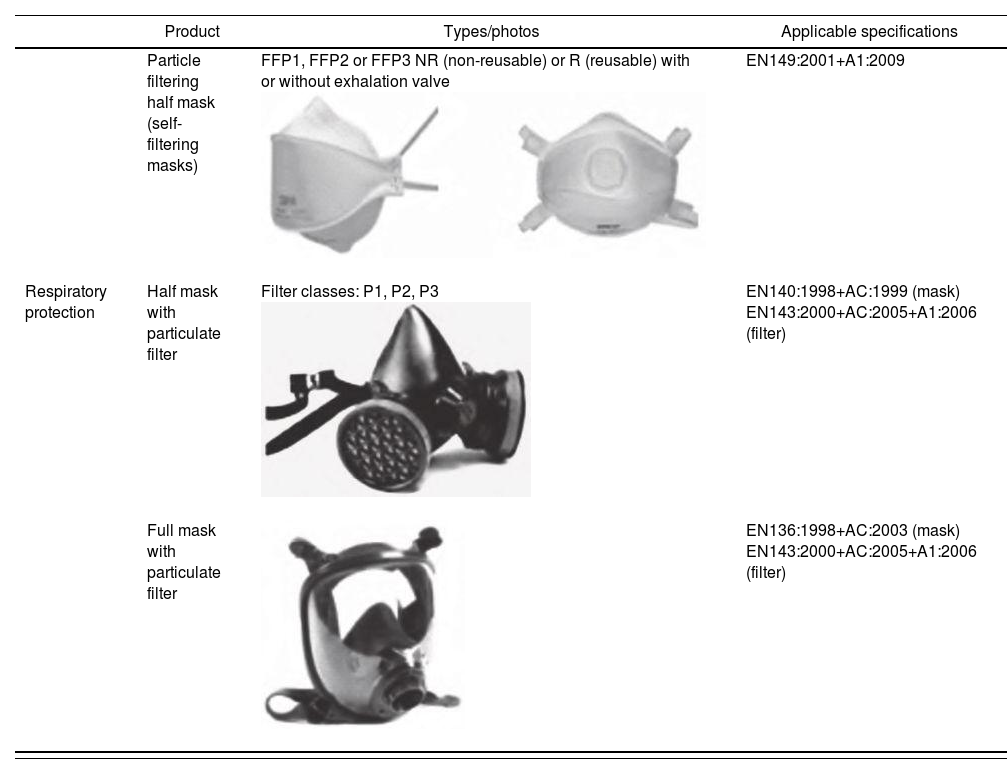
Types of PPE-grade masks. Adapted from Equipos de protección individual (EPI), productos sanitarios (PS) y otros12
| Product | Types/photos | Applicable specifications | |
|---|---|---|---|
| Particle filtering half mask (self-filtering masks) | FFP1, FFP2 or FFP3 NR (non-reusable) or R (reusable) with or without exhalation valve | EN149:2001+A1:2009 | |
| Respiratory protection | Half mask with particulate filter | Filter classes: P1, P2, P3 | EN140:1998+AC:1999 (mask) EN143:2000+AC:2005+A1:2006 (filter) |
| Full mask with particulate filter | EN136:1998+AC:2003 (mask) EN143:2000+AC:2005+A1:2006 (filter) |
A particle filtering half mask covers the nose, the mouth, and the chin, and may incorporate inhalation and/or exhalation valves. It is made almost entirely of filtering materials22. It should provide an airtight fit regardless of whether the wearer's face may be wet, or their head may be moving22.
Regulatory aspectsPPE is regulated by Regulation (EU) 2016/425 of the European Parliament and of the Council on PPE. PPE-grade face masks must bear a CE mark.
Use of particle filtering masks is currently regulated by harmonized EN standard 149:2001+A1:2009 Respiratory protective devices. Filtering half masks to protect against particles. Requirements, testing, marking22. Compliance with this standard provides presumption of conformity with Regulation (EU) 2016/425.
These face masks are divided into three categories (Table 3):
- –
FFP1: These face masks have a minimum filtration efficiency of 78% and a maximum inward leakage rate of 22%. They are normally used against inert particulate material.
- –
FFP2: These face masks have a minimum filtration efficiency of 92% and a maximum inward leakage rate of 8%. They are used against slightly or moderately toxic aerosols.
- –
FFP3: These face masks have a minimum filtration rate of 98% and a maximum inward leakage rate of 2%. They are used against highly toxic aerosols.
All of these masks may incorporate inhalation and/or exhalation valves. They may be single-use or reusable and they are all considered class III PPE23. Classification is carried out on the basis on conformity tests: inward leakage rate, skin compatibility, flammability, CO2 content, obstruction, and proper fit.
These face masks may incorporate an exhalation valve to reduce moisture and heat buildup inside the mask, providing the wearer with extra comfort and decreasing resistance to airflow18. They should not be used by persons infected with COVID-19. Their use should be reserved to healthcare staff24.
Dual protection face masks: PPE and MDThese face masks must comply with the standards and requirements applicable to both PPE and MDs.
In the face of the COVID-19 public health emergency, the Ministry of Industry, Trade and Tourism established that it would exceptionally and for a limited period of time allow in certain specific cases25 the distribution of PPE which does not bear the required CE mark but which complies with other harmonized standards such as NIOSH-42CFR84 or GB2626–200612.
DiscussionThe information provided in the present study has been drawn from the standards regulating the quality requirements to be met by face masks distributed in Spain. The reference standards for community masks are UNE 0064 and UNE 0065, the former for single-use and the latter for reusable masks. Quality criteria are based on bacterial filtration efficiency (≥ 95% for single-use vs. ≥ 90% for reusable masks) and differential pressure (< 60% for both). These two conditions ensure that these face masks confer both inbound and outbound protection which, together with their breathability factor, provide for adequate protection of the general population26. As regards surgical face masks, which are considered MDs, their quality requirements —partially overlapping those of community face masks— are described in UNE-EN standard 14683:2019. Apart from strict monitoring of filtration efficiency and differential pressure, these face masks require surveillance of microbial cleanliness and, in the case of class IIR masks, splash resistance pressure. Manufacturers must also have some kind of quality assurance system in place27. At the start of the pandemic, given the scarcity of these products, the Spanish Medicines Agency (AEMPS) exceptionally authorized face masks certified under other standards, such as those of the US or China, to be distributed in Spain28. Self-filtering masks are regulated by UNE EN standard UNE EN 149:2001. Close fitting to the wearer's face is essential for these masks to properly perform their particle filtering function24. If they are equipped with an exhalation valve, the exhaled air enters the environment without filtration, which makes these masks inappropriate for suspected, potential or confirmed COVID-19 patients as they do not prevent the spread of the virus28. As in the case of surgical face masks, at the beginning of the pandemic the authorities allowed the distribution of PPE-grade masks without the required CE mark if they were compliant with other harmonized standards28.
As recommended by Márquez Peiró et al.28, when in doubt as to whether a certain face mask could be counterfeit, additional information, such as the EU conformity declaration, the marketing authorization issued by a competent authority or an accredited laboratory report of the performance tests performed, should be requested from the manufacturer or supplier.
ConclusionsThe outbreak of COVID-19 and the recommendation of wearing face masks have made these products indispensable, which they will continue to be for the duration of the pandemic. Different types of face masks are available in the market. An understanding by consumers, healthcare practitioners and importers of the applicable quality standards will ensure that only regulation-compliant products are used and that counterfeit face masks are avoided.
FundingThis paper was not received any financial support.
Conflict of interestThe authors declare no conflict of interest.