Characterize the health-related quality of life among patients undergoing kidney replacement therapy and to explore associated factors.
MethodA descriptive observational study was conducted using the Kidney Disease Quality of Life Short Form questionnaire to assess health-related quality of life. The Dader Method was employed to evaluate negative outcomes associated with medications. Face-to-face interviews and clinical records were utilized to collect sociodemographic and clinical data from patients undergoing kidney replacement therapy at the Nephrology Department of Virgen de las Nieves University Hospital (Granada, Spain). We explored the association between independent variables (clinical and demographic factors) and dependent variables (Mental Component Score and Physical Component Score) using the linear regression method.
ResultsNinety-one participants were included, 47 (48.35%) were females. The mean age was 62 years, 52 patients (57.14%) were on hemodialysis, 13 patients (14.29%) on peritoneal dialysis, and 26 patients (28.57%) on other forms of kidney replacement therapy. The study revealed a mean Physical Component Score of 40.89 and a Mental Component Score of 47.19. Additionally, 98.90% of the patients experienced negative outcomes associated with medications. Influential factors include age, comorbid conditions, the number of medications, and clinical parameters such as vitamin D and calcium levels.
ConclusionsThis study underscores significant findings in patients undergoing kidney replacement therapy, indicating low Mental Component Score and Physical Component Score, accompanied by negative outcomes associated with medications.
Caracterizar la calidad de vida relacionada con la salud en tratamiento renal sustitutivo y explorar los factores asociados.
MétodoSe llevó a cabo un estudio observacional descriptivo utilizando el cuestionario Kidney Disease Quality of Life Short Form para evaluar la calidad de vida relacionada con la salud. Se empleó el Método Dáder para evaluar los resultados negativos asociados con la medicación. Se realizaron entrevistas y se utilizaron historias clínicas para recopilar datos sociodemográficos y clínicos de pacientes sometidos a terapia de reemplazo renal en el servicio de nefrología del Hospital Universitario Virgen de las Nieves (Granada, España). Se exploró la asociación entre las variables independientes (factores clínicos y demográficos) y las variables dependientes (Puntuación del Componente Mental y Puntuación del Componente Físico) utilizando el método de regresión lineal.
ResultadosSe incluyeron 91 participantes, 47 (48,35%) eran mujeres. La edad media fue de 62 años, 52 pacientes (57,14%) estaban en hemodiálisis, 13 pacientes (14,29%) en diálisis peritoneal, y 26 pacientes (28,57%) en otras formas de tratamiento renal sustitutivo. El estudio reveló una puntuación media del componente físico de 40,89 y una puntuación del componente mental de 47,19. Además, el 98,90% de los pacientes presentaba resultados negativos asociados a la medicación. Entre los factores asociados se incluyen: la edad, el número de comorbilidades, el número de medicamentos prescritos y parámetros clínicos como los niveles de vitamina D y calcio.
ConclusionesEste estudio subraya hallazgos significativos en pacientes con tratamiento renal sustitutivo, indicando bajas puntuaciones de los componentes mental y físico medidas por el cuestionario, acompañadas de resultados negativos asociados con la medicación.
The concept of health-related quality of life (HRQoL) is complex and focuses on how patients' well-being is affected by their health. For individuals with end-stage renal disease (ESRD), a low HRQoL is associated with higher risks of mortality and hospitalization.1–8 Assessing HRQoL is crucial for evaluating treatment outcomes in chronic kidney disease (CKD) patients undergoing kidney replacement therapies (KRT). Various tools are being tested to improve patient outcomes by measuring HRQoL, which is significantly impacted by lifestyle changes, emotional disturbances, and physical and psychosocial symptoms.8–12
Kidney transplantation (KT) greatly improves HRQoL,9,13 with patients often achieving scores similar to those of healthy individuals.9 However, dialysis patients typically experience a compromised HRQoL, facing challenges similar to those with other chronic conditions like cancer and heart failure.2 While medications have improved health and HRQoL,14 their extensive use has also led to a rise in adverse side effects, known as negative outcomes associated with medications (NOM).15,16
Several factors influence HRQoL in ESRD patients, including country, ethnicity, and demographic characteristics. Studies on HRQoL have included patients undergoing hemodialysis (HD)3–5,12,17 or peritoneal dialysis (PD)3,6 across various countries.3,11 Understanding these factors can lead to better dietary, lifestyle, and educational recommendations, integrating HRQoL assessment into patient-centered care to enhance symptom relief, patient care, and rehabilitation.10,11
There is limited literature on HRQoL and NOM in KRT patients, with most studies focusing on the relationship between KRT modality and HRQoL.3,5,6,8–11 The present study aims to characterize HRQoL among KRT patients and explore the associated factors to provide a comprehensive understanding that can inform healthcare practices and patient management.
MethodAn observational study was conducted at the Nephrology Department of Virgen de las Nieves University Hospital in Granada, Spain. The research period extended from February 2, 2021, to July 31, 2023, spanning 29 months, and focused on patients with ESRD undergoing KRT.
All outpatient individuals who met the following criteria were included in the study: being over 18 years of age, undergoing KRT such as HD, PD, or KT at the nephrology department during the study period, and expressing a willingness to participate. Patients with cognitive impairments were excluded.
Eligible patients consulting the Nephrology Department at Virgen de las Nieves University Hospital were invited to participate. Informed consent was obtained from all participants, and variables were collected from medical records and through personal interviews. These interviews were used to gather information on HRQoL and symptoms not available in medical records. Patients meeting the inclusion criteria were recruited by nephrology experts.
HRQoL was assessed using the Kidney Disease Quality of Life Short Form (KDQOL-SF) Questionnaire, a 36-item tool specifically designed to evaluate quality of life in patients with chronic renal disease, including those undergoing dialysis. Although originally designed for dialysis patients, it has also been validated for use in transplant patients. The questionnaire includes domains such as the Physical Component Score (PCS), Mental Component Score (MCS), Symptoms and Problems of Kidney Disease (SPKD), Burden of Kidney Disease (BKD), and Effect of Kidney Disease (EKD). Scores were standardized on a scale from 0 to 100, with higher scores indicating better quality of life.18 Medication adherence was assessed using the Simplified Medication Adherence Questionnaire (SMAQ), a brief and reliable tool for evaluating adherence to medication regimens.19 Both questionnaires have been validated in the Spanish population.19,20
Data were collected using a combination of methods, including electronic medical record reviews, semi-structured interviews, and questionnaires administered through face-to-face interviews. The Dáder Method, developed by the Pharmaceutical Care Research Group at the University of Granada, was used to identify and classify DRP/ NOM according to the Granada Consensus.15 Pharmacists facilitated discussions with nephrology experts to ensure comprehensive data collection. The pharmacist conducted thorough reviews of electronic medical records and interviews to assess prescribed medications, baseline demographics, comorbidities, clinical laboratory data, allergies, and the number of NOM or DRP.
The stage of kidney disease was determined using the estimated glomerular filtration rate, calculated from serum creatinine levels using the Chronic Kidney Disease Epidemiology Collaboration equation, as documented in electronic medical records. Laboratory tests were recorded as part of routine clinical practice.
NOM were defined as health outcomes affecting the patients that are, or may be, associated with medication use. A DRP was defined as an event or circumstance involving drug therapy that interferes, or potentially interferes, with desired health outcomes.15
Statistical analyses were conducted at a 5% significance level using R software version 4.3.2 (©2023 The R Foundation for Statistical Computing, R Studio 2023.09.1 © 2009–2023 Posit Software, PBC). Microsoft Excel was used for data entry and preliminary editing before analysis. Descriptive statistics, including frequency distributions, percentages, means, standard deviations (SD), and medians, were calculated for continuous data.
The association between independent variables (clinical and demographic factors) and dependent variables (PCS or MCS) was explored using linear regression. Additionally, relationships between independent variables (clinical, demographic, PCS, or MCS) and the dependent variable (medication adherence) were assessed using univariate and multivariate logistic regression methods.
Institutional Review Board approval was obtained from the Andalusian Biomedical Research Ethics Committee (FIS-IRB-2020-01) on July 28, 2020. Written informed consent was obtained from all study participants, ensuring adherence to ethical standards and respect for participant autonomy.
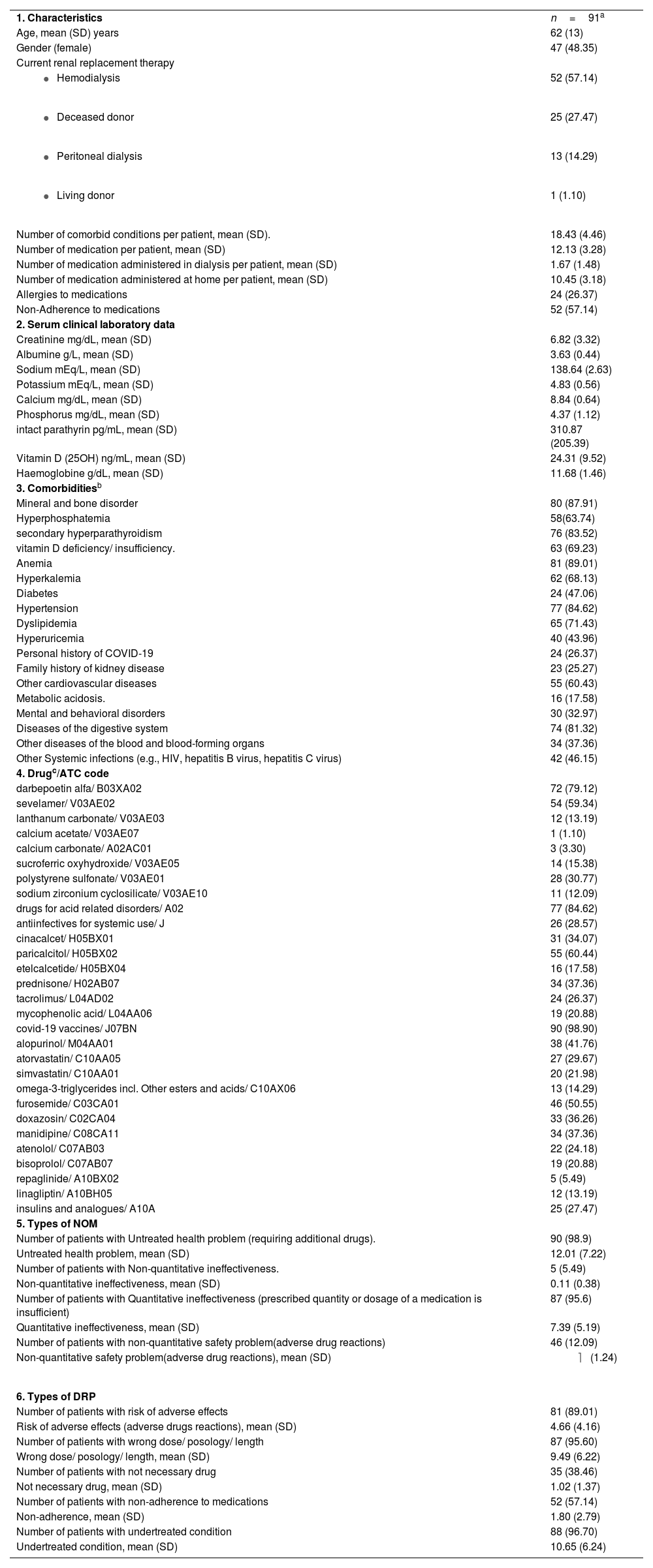
ResultsA cohort of 91 out of 117 patients undergoing KRT was included in the study. Throughout the study period, five patients from the cohort passed away due to causes related to kidney disease or complications of their health conditions. The sociodemographic and clinical characteristics of the study population are shown in Table 1.
Sociodemographic and clinical characteristics of patients.
| 1. Characteristics | n=91a |
| Age, mean (SD) years | 62 (13) |
| Gender (female) | 47 (48.35) |
| Current renal replacement therapy | |
| 52 (57.14) |
| 25 (27.47) |
| 13 (14.29) |
| 1 (1.10) |
| Number of comorbid conditions per patient, mean (SD). | 18.43 (4.46) |
| Number of medication per patient, mean (SD) | 12.13 (3.28) |
| Number of medication administered in dialysis per patient, mean (SD) | 1.67 (1.48) |
| Number of medication administered at home per patient, mean (SD) | 10.45 (3.18) |
| Allergies to medications | 24 (26.37) |
| Non-Adherence to medications | 52 (57.14) |
| 2. Serum clinical laboratory data | |
| Creatinine mg/dL, mean (SD) | 6.82 (3.32) |
| Albumine g/L, mean (SD) | 3.63 (0.44) |
| Sodium mEq/L, mean (SD) | 138.64 (2.63) |
| Potassium mEq/L, mean (SD) | 4.83 (0.56) |
| Calcium mg/dL, mean (SD) | 8.84 (0.64) |
| Phosphorus mg/dL, mean (SD) | 4.37 (1.12) |
| intact parathyrin pg/mL, mean (SD) | 310.87 (205.39) |
| Vitamin D (25OH) ng/mL, mean (SD) | 24.31 (9.52) |
| Haemoglobine g/dL, mean (SD) | 11.68 (1.46) |
| 3. Comorbiditiesb | |
| Mineral and bone disorder | 80 (87.91) |
| Hyperphosphatemia | 58(63.74) |
| secondary hyperparathyroidism | 76 (83.52) |
| vitamin D deficiency/ insufficiency. | 63 (69.23) |
| Anemia | 81 (89.01) |
| Hyperkalemia | 62 (68.13) |
| Diabetes | 24 (47.06) |
| Hypertension | 77 (84.62) |
| Dyslipidemia | 65 (71.43) |
| Hyperuricemia | 40 (43.96) |
| Personal history of COVID-19 | 24 (26.37) |
| Family history of kidney disease | 23 (25.27) |
| Other cardiovascular diseases | 55 (60.43) |
| Metabolic acidosis. | 16 (17.58) |
| Mental and behavioral disorders | 30 (32.97) |
| Diseases of the digestive system | 74 (81.32) |
| Other diseases of the blood and blood-forming organs | 34 (37.36) |
| Other Systemic infections (e.g., HIV, hepatitis B virus, hepatitis C virus) | 42 (46.15) |
| 4. Drugc/ATC code | |
| darbepoetin alfa/ B03XA02 | 72 (79.12) |
| sevelamer/ V03AE02 | 54 (59.34) |
| lanthanum carbonate/ V03AE03 | 12 (13.19) |
| calcium acetate/ V03AE07 | 1 (1.10) |
| calcium carbonate/ A02AC01 | 3 (3.30) |
| sucroferric oxyhydroxide/ V03AE05 | 14 (15.38) |
| polystyrene sulfonate/ V03AE01 | 28 (30.77) |
| sodium zirconium cyclosilicate/ V03AE10 | 11 (12.09) |
| drugs for acid related disorders/ A02 | 77 (84.62) |
| antiinfectives for systemic use/ J | 26 (28.57) |
| cinacalcet/ H05BX01 | 31 (34.07) |
| paricalcitol/ H05BX02 | 55 (60.44) |
| etelcalcetide/ H05BX04 | 16 (17.58) |
| prednisone/ H02AB07 | 34 (37.36) |
| tacrolimus/ L04AD02 | 24 (26.37) |
| mycophenolic acid/ L04AA06 | 19 (20.88) |
| covid-19 vaccines/ J07BN | 90 (98.90) |
| alopurinol/ M04AA01 | 38 (41.76) |
| atorvastatin/ C10AA05 | 27 (29.67) |
| simvastatin/ C10AA01 | 20 (21.98) |
| omega-3-triglycerides incl. Other esters and acids/ C10AX06 | 13 (14.29) |
| furosemide/ C03CA01 | 46 (50.55) |
| doxazosin/ C02CA04 | 33 (36.26) |
| manidipine/ C08CA11 | 34 (37.36) |
| atenolol/ C07AB03 | 22 (24.18) |
| bisoprolol/ C07AB07 | 19 (20.88) |
| repaglinide/ A10BX02 | 5 (5.49) |
| linagliptin/ A10BH05 | 12 (13.19) |
| insulins and analogues/ A10A | 25 (27.47) |
| 5. Types of NOM | |
| Number of patients with Untreated health problem (requiring additional drugs). | 90 (98.9) |
| Untreated health problem, mean (SD) | 12.01 (7.22) |
| Number of patients with Non-quantitative ineffectiveness. | 5 (5.49) |
| Non-quantitative ineffectiveness, mean (SD) | 0.11 (0.38) |
| Number of patients with Quantitative ineffectiveness (prescribed quantity or dosage of a medication is insufficient) | 87 (95.6) |
| Quantitative ineffectiveness, mean (SD) | 7.39 (5.19) |
| Number of patients with non-quantitative safety problem(adverse drug reactions) | 46 (12.09) |
| Non-quantitative safety problem(adverse drug reactions), mean (SD) |
|
| 6. Types of DRP | |
| Number of patients with risk of adverse effects | 81 (89.01) |
| Risk of adverse effects (adverse drugs reactions), mean (SD) | 4.66 (4.16) |
| Number of patients with wrong dose/ posology/ length | 87 (95.60) |
| Wrong dose/ posology/ length, mean (SD) | 9.49 (6.22) |
| Number of patients with not necessary drug | 35 (38.46) |
| Not necessary drug, mean (SD) | 1.02 (1.37) |
| Number of patients with non-adherence to medications | 52 (57.14) |
| Non-adherence, mean (SD) | 1.80 (2.79) |
| Number of patients with undertreated condition | 88 (96.70) |
| Undertreated condition, mean (SD) | 10.65 (6.24) |
Abbreviations: kidney replacement therapies (KRT); negative outcomes associated with medications (NOM), drug-related problems (DRP).
The main causes of ESRD were as follows: glomerulonephritis (26 cases; 28.57%), unknown etiology (20 cases; 21.98%), polycystic kidney disease (12 cases; 13.19%), and diabetic nephropathy (11 cases; 12.09%).
The principal comorbidities among the group of patients undergoing KRT were mineral and bone disorder (80 cases; 87.91%), anemia (81 cases; 89.01%), and hypertension (77 cases; 84.62%).
Overall, the group of patients used 1093 medications: 951 medications at home and 152 at the dialysis unit. Table 1 displays the principal types of medications used in the treatment of patients.
Overall, we identified 1875 NOM and 2578 DRP during the study period, with a rate of 20.6 NOM per patient and 28.3 DRP per patient. The principal types of NOM and DRP are presented in Table 1.
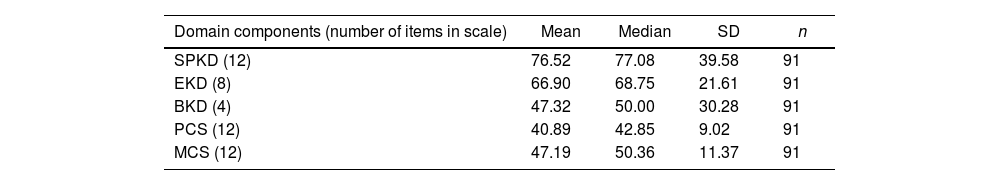
Table 2 shows the distribution of scores for the five components of KDQOL-SF.
Comprehensive Assessment of Kidney Disease Quality of Life short form (KDQOL-SF): Breakdown of Domain Components and Results.
| Domain components (number of items in scale) | Mean | Median | SD | n |
|---|---|---|---|---|
| SPKD (12) | 76.52 | 77.08 | 39.58 | 91 |
| EKD (8) | 66.90 | 68.75 | 21.61 | 91 |
| BKD (4) | 47.32 | 50.00 | 30.28 | 91 |
| PCS (12) | 40.89 | 42.85 | 9.02 | 91 |
| MCS (12) | 47.19 | 50.36 | 11.37 | 91 |
Abbreviations: Kidney Disease Quality of Life short form questionnaire (KDQOL-SF), Physical Component Score (PCS), Mental Component Score (MCS), Symptoms and Problems of Kidney Disease (SPKD), Burden of Kidney Disease (BKD), and Effect of Kidney Disease (EKD).
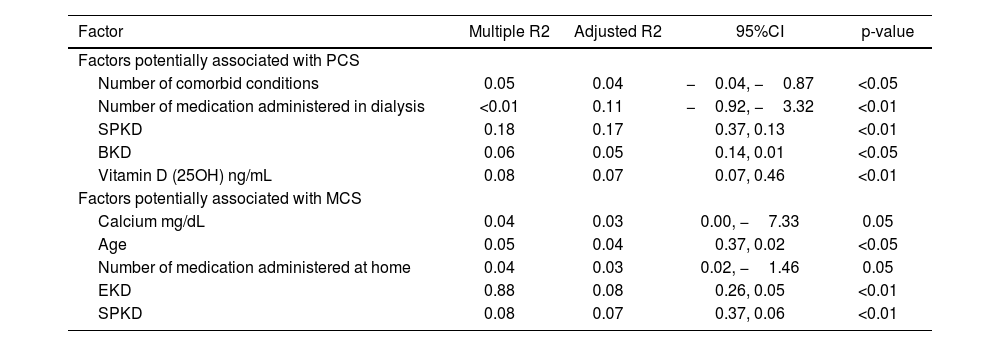
Moreover, we examined the relationship between independent variables and dependent variable (PCS or MCS) through the application of linear regression method.
The linear regression analysis (Table 3) showed that factors such as number of comorbid conditions, number of medication administered in dialysis, SPKD, BKD, or Vitamin D (25OH) are associated with PCS.
Linear regression analysis of factors potentially associated with PCS and MCS.
| Factor | Multiple R2 | Adjusted R2 | 95%CI | p-value |
|---|---|---|---|---|
| Factors potentially associated with PCS | ||||
| Number of comorbid conditions | 0.05 | 0.04 | −0.04, −0.87 | <0.05 |
| Number of medication administered in dialysis | <0.01 | 0.11 | −0.92, −3.32 | <0.01 |
| SPKD | 0.18 | 0.17 | 0.37, 0.13 | <0.01 |
| BKD | 0.06 | 0.05 | 0.14, 0.01 | <0.05 |
| Vitamin D (25OH) ng/mL | 0.08 | 0.07 | 0.07, 0.46 | <0.01 |
| Factors potentially associated with MCS | ||||
| Calcium mg/dL | 0.04 | 0.03 | 0.00, −7.33 | 0.05 |
| Age | 0.05 | 0.04 | 0.37, 0.02 | <0.05 |
| Number of medication administered at home | 0.04 | 0.03 | 0.02, −1.46 | 0.05 |
| EKD | 0.88 | 0.08 | 0.26, 0.05 | <0.01 |
| SPKD | 0.08 | 0.07 | 0.37, 0.06 | <0.01 |
Abbreviations: Physical Component Score (PCS), Symptoms and Problems of Kidney Disease (SPKD), Burden of Kidney Disease (BKD), Mental Component Score (MCS), Effect of Kidney Disease (EKD).
Table 3 shows that factors such as calcium, age, number of medication administered at home, EKD, or SPKD are associated with MCS.
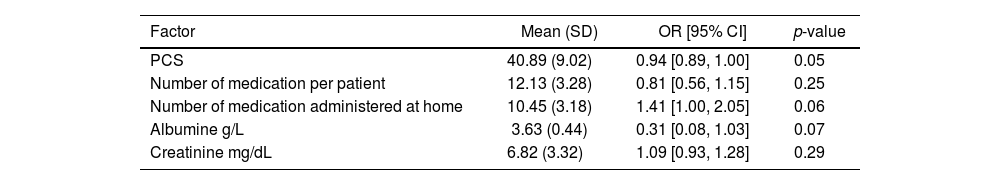
We examined the relationship between independent variables and the dependent variable (adherence to medications) using univariate and multivariate logistic regression methods.
The univariate logistic regression analysis showed that the PCS, number of medications, number of medications administered at home, albumin, and creatinine were associated with adherence to medications.
Variables with a p-value <0.05 in bivariate analysis were subsequently included in the multiple logistic regression. Table 4 shows the results of the multivariate analysis.
Influential Factors Affecting Medication Adherence.
| Factor | Mean (SD) | OR [95% CI] | p-value |
|---|---|---|---|
| PCS | 40.89 (9.02) | 0.94 [0.89, 1.00] | 0.05 |
| Number of medication per patient | 12.13 (3.28) | 0.81 [0.56, 1.15] | 0.25 |
| Number of medication administered at home | 10.45 (3.18) | 1.41 [1.00, 2.05] | 0.06 |
| Albumine g/L | 3.63 (0.44) | 0.31 [0.08, 1.03] | 0.07 |
| Creatinine mg/dL | 6.82 (3.32) | 1.09 [0.93, 1.28] | 0.29 |
Abbreviation: kidney replacement therapies (KRT); Physical Component Score (PCS).
Furthermore, we examined the relationship between the type of KRT (KT or dialysis) and BKD or EKD (univariate logistic regression analysis). The BKD (OR = 1.024; 95%CI = 1.003–1.051; p < 0.05) and EKD (OR = 1.038; 95%CI = 1.004–1.088; p < 0.05) were found to be associated with the type of KRT.
DiscussionThe present study explores the HRQoL among individuals undergoing KRT and its associated factors, including NOM. HRQoL has emerged as a recommended clinical tool for evaluating patients on KRT, serving as a primary endpoint in various studies aimed at elucidating the effectiveness of comprehensive disease management.20
In the present study, low HRQoL was found within the sample, as evidenced by a mean PCS of 40.89 (SD: 9.02) and MCS of 47.19 (SD: 11.37). Our findings align with other studies that have reported low HRQoL among individuals with ESRD undergoing KRT.3,9,11,12
We found that the PCS and MCS exhibited the lowest scores, aligning with findings from a prior study11 that used the KDQOL-SF questionnaire. Specifically, the previously identified most affected dimensions, namely the PCS and the BKD, has also demonstrated significant impacts in our study. Notably, the dimension of SPKD consistently yielded the lowest scores, indicating a substantial degree of impairment in this specific facet of health-related QoL.11
Several factors were identified as contributors to these lower HRQoL scores. The PCS was associated with variables such as the number of comorbid conditions, the total number of medications administered during dialysis, the presence of SPKD, the BKD, and Vitamin D (25OH) levels.
Similarly, the MCS exhibited associations with factors including calcium levels, age, the number of medications administered at home, the EKD, and the manifestation of SPKD. These insights underscore the multifaceted nature of HRQoL in individuals undergoing KRT for ESRD and emphasize the importance of considering various health-related factors in assessing their well-being.
Previous research has indicated that factors such as depression, sleep quality, and lower calcium levels are associated with a negative impact on mental well-being.20
Furthermore, within this cohort of patients, we identified NOM, notably characterized by the prevalence of untreated health problems (98.9%) and quantitative ineffectiveness (95.6%), where the prescribed quantity or dosage of a medication was deemed insufficient. These findings are consistent with the existing literature, which consistently reports low HRQoL in patients with NOM.16
Patients undergoing KRT often experience high rates of undertreated conditions due to the complexity of their comorbidities, the primary focus on managing their kidney disease, and the overlap of symptoms which can lead to misattribution. Frequent healthcare visits, medication management challenges, and specialized provider focus contribute to gaps in holistic care. Additionally, logistical barriers, limited patient education, and psychosocial factors like depression further complicate comprehensive treatment. Improving care for these patients requires a multidisciplinary approach, better coordination among healthcare providers, and enhanced patient education to address both their renal and non-renal health needs effectively.11–13
Identifying specific patterns of NOM sheds light on critical aspects of healthcare delivery, emphasizing the imperative to address gaps in treatment and optimize medication regimens to enhance overall patient well-being.
Understanding the risk factors associated with poor HRQoL is crucial for identifying vulnerable ESRD patients and developing targeted interventions to support them. Determinants of HRQoL in ESRD extend beyond clinical factors to include mental factors. Importantly, these mental factors are potentially modifiable, presenting an opportunity for interventions addressing the physical and mental aspects of well-being in this patient population. This comprehensive approach holds promise for improving the overall QoL for individuals with ESRD.3
Our study revealed a significant association between vitamin D levels and the PCS, and between calcium levels and the MCS. These findings align with prior research investigating the relationship between laboratory values—such as hematocrit, potassium, phosphorus, and calcium—and patient attributes in the context of HRQoL.17,21
Some limitations of the present study include the relatively small sample size, which may hinder our ability to detect significant differences in the type of KRT and PCS or MCS. Additionally, the study design only allows us to establish associations between variables and prevents the identification of causal relationships.
In conclusion, our study reveals that patients undergoing KRT exhibit a low percentage of MCS and PCS scores as measured by the KDQOL-SF questionnaire and demonstrate instances of NOM.
Various factors emerge as significant contributors to HRQoL, including age, the prevalence of comorbid conditions, the quantity of medications administered during dialysis or at home, the manifestation of kidney disease symptoms, the overall burden of kidney disease, and clinical parameters such as vitamin D (25OH) and calcium levels.
Identifying these influencing factors underscores the complexity of HRQoL in KRT patients.
Contribution to the scientific literatureThis study contributes to the existing body of knowledge on health-related quality of life and negative outcomes associated with medications in nephrology.
Recognizing the health-related quality of life among patients undergoing kidney replacement therapies and identifying the predominant influencing factors could pave the way for recommending healthy dietary practices, lifestyle adjustments, and educational interventions. Additionally, evaluating health-related quality of Life in individuals with end-stage renal disease is a valuable tool for healthcare professionals, integrating seamlessly into patient-centered care.
Funding sourcesFinancial support received as a Doctoral Grant [reference number OAICE-143-2020] from the Office of International Affairs and External Cooperation, University of Costa Rica.
Presentation at CongressesCongress of the Andalucian Society of Nephrology, 2024. Organised by the Sociedad Andaluza de Nefrología. Location: Sevilla, Spain.
Data AccessFor Data supporting reported results contact corresponding author.
CRediT authorship contribution statementAlfonso Pereira-Céspedes: Writing – review & editing, Writing – original draft, Visualization, Validation, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Alberto Jiménez-Morales: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization. Aurora Polo-Moyano: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Conceptualization. Elizabeth Spruce-Esparza: Writing – review & editing, Writing – original draft, Investigation, Formal analysis. Magdalena Palomares-Bayo: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Project administration, Methodology, Formal analysis. Fernando Martínez-Martínez: Writing – original draft, Supervision, Resources, Project administration, Methodology, Investigation, Formal analysis, Conceptualization. Miguel Ángel Calleja-Hernández: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization.
This publication is part of the results derived from the doctoral thesis titled “Seguimiento farmacoterapéutico de personas con enfermedad renal crónica estadio 5 en tratamiento renal sustitutivo: impacto clínico y humanístico” [Ph.D. in Pharmacy Program, University of Granada].