The Intensive Care Unit (ICU) of the University Hospital of Fuenlabrada was forced to critically increase its capacity in the COVID-19 pandemic. The objective of this work is to describe the activities promoted by the pharmacist in the care of the critically ill patient in this context.
A new organizational structure was designed, analyzing the tasks necessary to make the processes profitable. Two pharmacists joined the critical patient care to help the pharmacist who was already integrated in the ICU team.
The development of the operational structure was carried out on three levels.
The healthcare activity highlights the daily participation of pharmacists in the two clinical sessions in which the ICU teams evaluated all cases and made decisions. This in turn facilitated the pharmaceutical validation that was carried out in the critical units themselves. In addition, one of the pharmacists created the Immuno-COVID Committee, in which they participated together with different specialists for therapeutic decision-making in the most complex cases.
On the other hand, the availability of human and material resources allowed the implantation of centralized elaboration in the Pharmacy Service of many intravenous mixtures, including antibiotics elastomers Pumps for continuous infusion, and non-sterile elaborations.
In logistics management, in addition to the acquisition of COVID-19 therapies, the reconciliation with nursing activity stands out. The physical presence of the pharmacist favored the detection of needs, the availability in time of medications in the unit, including sterile and non-sterile preparations, and coordination with the central pharmacy.
In knowledge management, the participation of the pharmacist in the working group for the development of the hospital management protocol COVID-19 stands out.
The daily presence in the unit and the joint work with the entire multidisciplinary team demonstrate the value that the pharmacist can bring. In addition to efficient resource management, support for clinical decision-making and improvement actions, it provides the climate of inter-professional trust necessary to respond to the complexity of the critical patient and promote joint projects.
La Unidad de Cuidados Intensivos del Hospital Universitario de Fuenlabrada se vio obligada a aumentar de manera crítica su capacidad en la pandemia por COVID-19. El objetivo de este trabajo es describir las actividades impulsadas por el farmacéutico en la atención del paciente crítico en este contexto.
Se diseñó una estructura organizativa nueva, analizando las tareas necesarias para rentabilizar los procesos. Dos farmacéuticos se incorporaron a la atención del paciente crítico para ayudar al farmacéutico que ya estaba integrado en el equipo de la Unidad de Cuidados Intensivos.
El desarrollo de la estructura operativa se llevó a cabo en tres niveles.
En la actividad asistencial destaca la participación diaria de los farmacéuticos en las dos sesiones clínicas en las que los equipos de la Unidad de Cuidados Intensivos valoraban todos los casos y tomaban las decisiones. Esto, a su vez, facilitaba la validación farmacéutica que se realizaba en las propias unidades de críticos. Además, uno de los farmacéuticos ideó el Comité Inmuno-COVID, en el que participaban junto a diferentes especialistas para la toma de decisiones terapéuticas en los casos más complejos.
Por otro lado, la disponibilidad de recursos humanos y materiales permitió implantar la elaboración de forma centralizada en el Servicio de Farmacia de muchas mezclas intravenosas, incluyendo elastómeros de anti-bioterapia en perfusión continua, y de elaboraciones no estériles.
En la gestión logística, además de la adquisición de las terapias COVID-19, destaca la conciliación con la actividad de enfermería. La presencia física del farmacéutico favorecía la detección de necesidades, la disponibilidad en tiempo de medicamentos en la unidad, incluyendo las elaboraciones estériles y no estériles, y la coordinación con la Farmacia central.
En la gestión del conocimiento destaca la participación del farmacéutico en el grupo de trabajo para desarrollo del protocolo hospitalario de manejo de la COVID-19.
La presencia diaria en la unidad y el trabajo conjunto con todo el equipo multidisciplinar ponen de manifiesto el valor que el farmacéutico puede aportar. Además de una gestión eficiente de los recursos, soporte en la toma de decisiones clínicas y acciones de mejora, proporciona el clima de confianza interprofesional necesario para dar respuesta a la complejidad del paciente crítico y promover proyectos conjuntos.
The integration of hospital pharmacists (HP) in Intensive Care Units (ICU) is established based on criteria of efficacy1,2. The benefits of such integration are the reduction of side events and medication errors, the improvement of outcomes in infectious diseases and thromboembolic events and the adoption of the ABCDEF bundle (pain, analgesia and sedation, awakening and breathing, delirium monitoring and early mobility)1.
Interdisciplinary ICU teams must create a favorable physical, emotional and communication climate to generate an environment of confidence that facilitates decision-making and the management of potential errors3.
Additionally, in the recent years, we have gained a better understanding of the post-ICU syndrome4. This syndrome is associated with higher morbidity and mortality and poorer mental, physical and cognitive health status after ICU admission. A battery of strategies such as post-ICU syndrome visits, have been developed to improve the quality of life of ICU survivors and prevent relapse and readmission. This strategy provides an opportunity of collaboration to intensive care specialists and HP5.
Challenges: The COVID-19 pandemic forced the transformation of the ICU ecosystem in Hospital Universitario de Fuenlabrada.
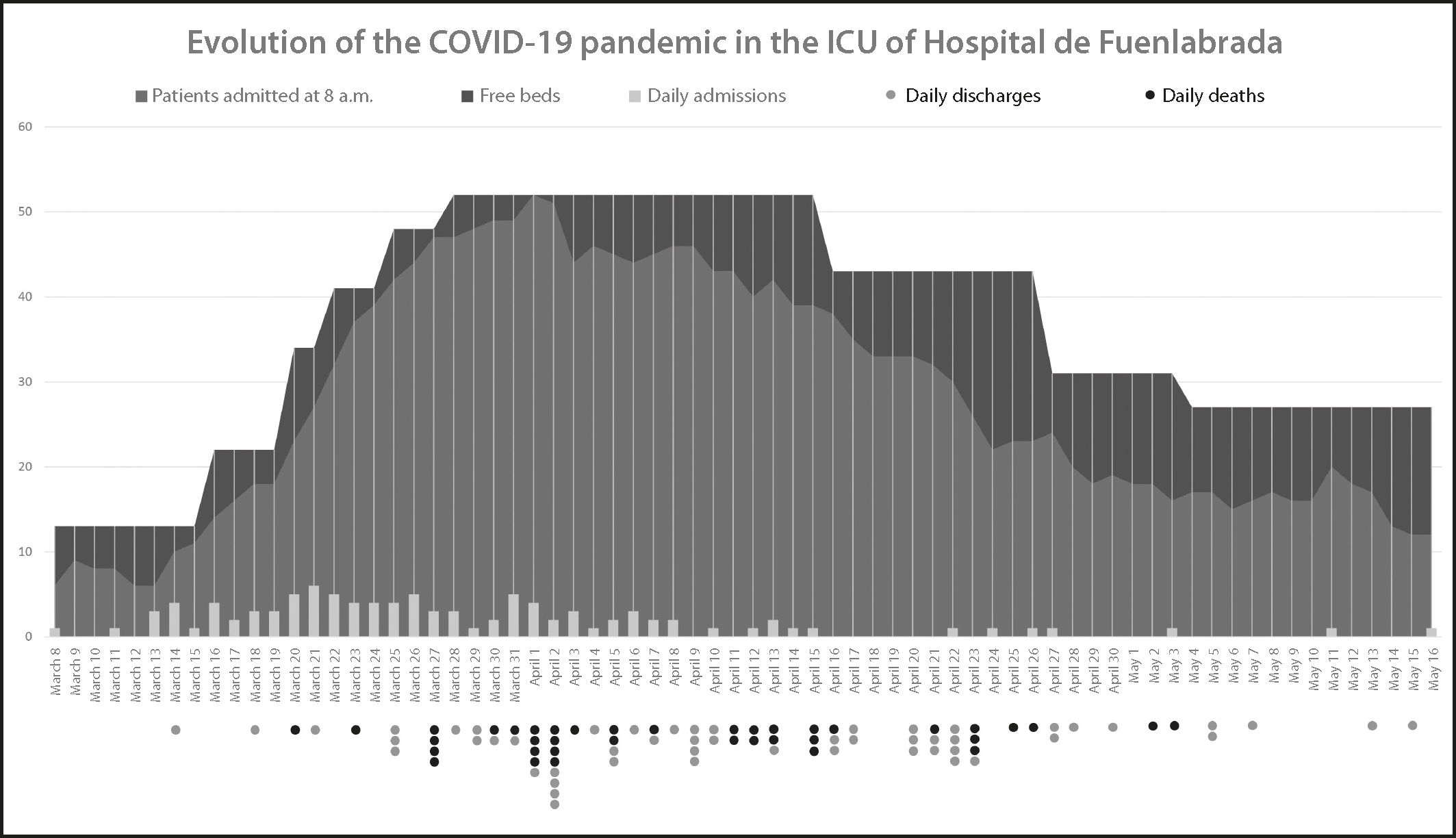
Between March 1st and May 15th, 2020, a total of 1,612 patients were admitted to our hospital, of whom 100 (6.2%) were admitted to the ICU. Our hospital is equipped with 10 ICU beds, with 350-380 admissions per year. To meet the dramatic increase in the demand for care, the number of beds was increased to 13 and two wards were repurposed as ICUs (post-anesthesia recovery unit and Surgical Day Hospital), wich reached a total of 48 ICU beds and four intermediate-care beds.
Figure 1 shows the evolution of the COVID-19 pandemic in the ICU. Data on inpatients, available beds, admissions, discharges to the ward and deaths were collected on a daily basis over the period detailed above.
Three multidisciplinary teams were set up to staff the three ICUs, which included intensive care specialists, anesthesists, a cardiologist trained in critical care, and a specialist in pediatric intensive care. The nursing staff was reinforced with personnel from other units with ICU experience, with the participation of nurses from the post-anesthesia unit, OR, and other wards.
Objective: To describe the COVID-19 action plan implemented to guarantee a quality pharmacy care of critical patients in the context of a pandemic and ensure an effective management of resources.
Developed strategyDesign of the organizational structureAn analysis of HP tasks was performed to ensure an effective process and time management. To such purpose, the human resources of these units were increased.
HP tasks include validating the medication of critical patients, providing pharmaceutical advice, solving cross-consultations from intensive-care specialists, and managing ICU drug cabinets.
The COVID-19 action plan involved the integration of two more PH to the critical care units: the head of the Service, as he had previous experience in the ICU, and a HP with experience in pharmaceutical compounding, who provided support to the area of compounding. The focus was placed on three operating areas:
- •
Healthcare activity.
- •
Logistic management.
- •
Knowledge management and protocol implementation.
Operational tasks were correlated with organizational tasks. While some are carried out on a daily basis, others are performed as needed. A description of the most relevant tasks is provided below:
Healthcare activity- –
Case evaluation and follow-up: two PH were allocated to the three critical care units to participate in the two daily clinical sessions where each particular case was discussed by a multidisciplinary team. In the 08:15 h session, a report is provided of the events occurred during the night shift and new admissions. The action plan for the morning shift Iis designed (complementary studies and treatment changes mainly). In the 14:00 h session, an update of the evolution of patients is provided, the outcomes of clinical and therapeutic decisions are reported, and an action plan is established for the night shift.
- –
On-site pharmaceutic validation: two PH used the pharmacy computer program installed in the ICUs to validate medications during the period between the two clinical sessions described above, which was facilitated by the information obtained in the morning session.
- –
Immuno-COVID Committee: the most complex cases requiring antiviral and anti-inflammatory therapy were discussed by two intensive-care specialists, two specialists in infectious diseases, two pharmacists and a microbiologist. Cases of patients who were likely to be transferred to the ICU shortly were also discussed in these sessions. This Committee also suggested changes to follow-up or treatment protocols for COVID-19 patients.
- –
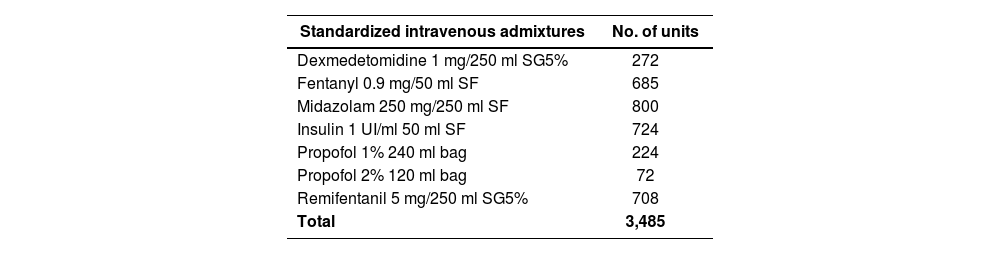
Pharmaceutical compounding: Standardized intravenous admixtures are used in the ICU. These intravenous admixtures are defined in the ICU computer application, which provides data on the dosage, dilutant and volume to facilitate prescription by the intensive care physician. Thus, medicines are compounded extemporaneously by the Unit staff. Finally, it was agreed with the nursing management to centralize compounding in the Pharmacy Service (PS) for the following reasons:
- •
Alleviate the huge workload of the nursing staff.
- •
Ensure the correct intravenous admixtures compounding in quantitative and qualitative terms, in the light of the climate of stress and incorporation of new personnel. To guarantee asepsis.
- •
Ensure an efficient use of medicines subject to potential stockout events.
- •
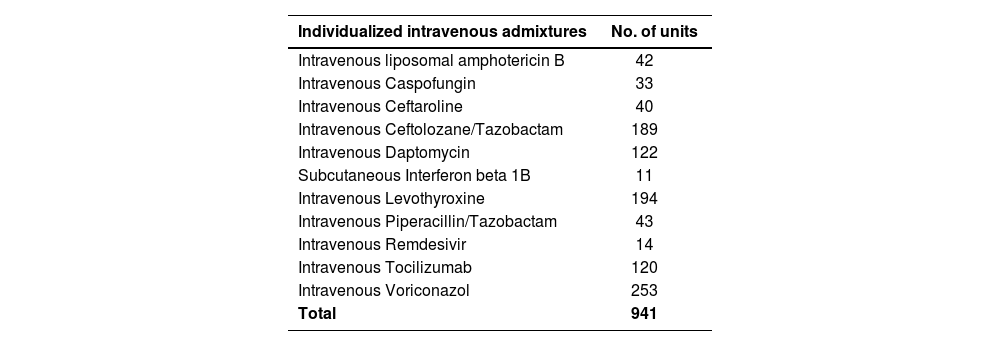
In the case of individualized intravenous mixtures, the preparation of antibiotic elastomeric pumps for continuous infusion used in home hospitalization was considered.
In this sense, the Intravenous Admixture Unit of the PS could meet the demand thanks to the incorporation of pharmacy technicians and the fitting out of two white rooms equipped with two horizontal laminar flow cabinets. The demand for standardized and individualized intravenous admixtures increased in type and number (Tables 1 and 2).
Standard intravenous fluids
| Standardized intravenous admixtures | No. of units |
|---|---|
| Dexmedetomidine 1 mg/250 ml SG5% | 272 |
| Fentanyl 0.9 mg/50 ml SF | 685 |
| Midazolam 250 mg/250 ml SF | 800 |
| Insulin 1 UI/ml 50 ml SF | 724 |
| Propofol 1% 240 ml bag | 224 |
| Propofol 2% 120 ml bag | 72 |
| Remifentanil 5 mg/250 ml SG5% | 708 |
| Total | 3,485 |
Personalized intravenous fluids
| Individualized intravenous admixtures | No. of units |
|---|---|
| Intravenous liposomal amphotericin B | 42 |
| Intravenous Caspofungin | 33 |
| Intravenous Ceftaroline | 40 |
| Intravenous Ceftolozane/Tazobactam | 189 |
| Intravenous Daptomycin | 122 |
| Subcutaneous Interferon beta 1B | 11 |
| Intravenous Levothyroxine | 194 |
| Intravenous Piperacillin/Tazobactam | 43 |
| Intravenous Remdesivir | 14 |
| Intravenous Tocilizumab | 120 |
| Intravenous Voriconazol | 253 |
| Total | 941 |
COVID-19 therapies were prepared in the area of non-sterile compounding of the PS: oral syringes of hydroxychloroquine, lopinavir/ritonavir, imatinib and baricitinib for administration through a nasogastric tube or in case of dysphagia.
Logistics management- •
Drug procurement: the regulations of the Spanish Agency of Medicines and Medical Devices addressed the management of COVID-19 in its platform, and required the provision of clinical data on the case of interest and coordination with prescribers.
- •
Drug stock management: the physical presence of the pharmacist in the Unit facilitated first-person detection of logistic needs, which helped coordinate and accelerate drug procurement through urgent delivery of drugs to solve shortages of supply in the Unit medicine, stockouts, unit dose discrepancies, and provide information on medicines in shortage to anticipate drug replacement. Coordination with the central hospital pharmacy favored an effective drug distribution.
- •
Management of the stock of sterilized and non-sterilized drugs prepared in the HP: in the PS: the needs for intravenous infusions for patients were reviwed daily in the PS. The pharmacy technician reviewed the stock of intravenous admixtures available in the Units. Then, the bags needed to meet the daily demand were prepared. When stability data were available, the preparation of non-sterile solutions was centralized. The PS prepared in the Unit the solutions that required extemporaneous compounding. To such purpose, Units were equipped with laboratory equipment.
- •
A team was set up including specialists in infectious diseases, emergency care physicians, pulmonologists, intensive-care physicians and pharmacists to design a local protocol for the management of COVID-19. A specialist pharmacist in infectious diseases and a specialist pharmacist in intensive care were integrated in this team. The latter was involved in the development of the protocol for the inflammatory stage of the infection and conceived the creation of the immune-COVID committee for the follow-up of particular cases not considered in the protocol.
- •
Provision of scientific evidence, especially in relation to the inflammatory stage of the disease. For example, pharmacists focused on the management of patients’ needs such as delirium after weaning and sought a safe alternative with less drug-interactions among atypical antipsychotic drugs.
- •
Pharmacy advice: the presence of the HP in the Unit facilitated consultations from both, physicians and nurses. The most frequent enquiries were about the availability of sedation and analgesia drugs, therapies for infectious diseases, immunotherapy, drug interactions, incompatibility, and stability of drugs and routes of administration.
Lessons learned. Future applicability in pharmacy services
We have observed and confirmed that hospital pharmacists should spend more time in the ICU to detect needs, take part in decision-making and implement logistic and organizational improvement actions aimed at guaranteeing patient safety.
In relation to healthcare activity, the participation of the HP in the multidisciplinary team is of special relevance. In the morning session, the HP identifies daily needs, design actions to ensure an effective validation process, and anticipate logistic needs. In the second session, the multidisciplinary team favors a shared decision-making to ensure the efficiency of processes and creates a climate of confidence, which is essential to manage the needs of critical patients.
In logistics, the HP coordinates with the nursing staff. Centralized sterile and non-sterile compounding in the Pharmacy Service is an essential improvement action that allows to:
- •
Minimize medication errors.
- •
Guarantee that preparations are adequately prepared in quantitative and qualitative terms, as they are prepared in batches.
- •
Compound under aseptic conditions.
- •
Alleviate the workload of the nursing staff.
Knowledge management is essential to support healthcare activity and logistics. The COVID-19 management protocol and the Immuno-COVID Committee ensured an efficient resource management and supported clinical decision-making, where ICU hospital pharmacists were involved.
After the COVID-19 experience in the ICUs, the improvement actions to be implemented are:
- •
Routine participation of the hospital pharmacist in ICU team sessions in the design of therapeutic approaches.
- •
Preparation of personalized sterile preparations identified with a bar code for a safe administration.
- •
Participation and implementation of protocols: pharmacological management of delirium, nutritional support, antibiotic therapy, sedation and analgesia, management of SARS-CoV-2 infection.
- •
Setting up of a pharmaceutical consultation for patients with post-ICU syndrome that is integrated in and complementary to the intensive care consultation in anticipation of the increase in the number of patients with post-ICU syndrome after the COVID-19 pandemic.