To analyse the differences in pharmacokinetic and clinical parameters (bleeding rates and joint health) before and after switching from standard half-life factor VIII (FVIII) to extended half-life pegylated FVIII in patients with severe/moderate haemophilia A on prophylaxis, 1 year before and after the switch in real-life.
MethodThis is a single-centre, comparative, observational, sequential, retrospective, and multidisciplinary study. Population pharmacokinetic models from the WAPPS-Hemo® application were used to calculate pharmacokinetic parameters and individualise prophylaxis. The annual rate of total and joint bleeds, joint health (Haemophilia Joint Health Score), plasma half-life and area under the curve ratios, FVIII consumption, administration frequency, and cost were analysed.
ResultsThirty-eight adult patients with haemophilia A who switched from standard half-life FVIII to extended half-life pegylated FVIII were analysed. Significant improvements (P < .05) were observed in all pharmacokinetic parameters, with plasma half-life and area under the curve improvement ratios of 1.5 and 1.9, respectively, as well as reductions in annual total and joint bleeding rates. A higher number of patients with zero total (16.0 vs. 29.0) and joint bleeds (23.0 vs. 33.0) was also observed. The median reductions in administration frequency and dose/kg/week were 30.0% and 19.7%, respectively, avoiding 44.3 infusions/patient/year, resulting in savings of 20,843 €/patient/year. Furthermore, joint health improved (23.0 vs. 21.0; P = .017), and target joints resolved after the switch.
ConclusionsThe pharmacokinetically guided switch from standard half-life FVIII to pegylated FVIII demonstrated significant clinical benefits with reduced bleeding rates and improvements in joint health. Additionally, improvements in pharmacokinetic parameters were observed, allowing for reduced treatment burden by decreasing administration frequency, as well as lower consumption and costs.
Analizar las diferencias en los parámetros farmacocinéticos y clínicos (tasas de sangrado y salud articular) antes y después del intercambio de factores VIII (FVIII) de vida media estándar a FVIII de vida media extendida pegilados en pacientes con hemofilia A grave/moderada en profilaxis, un año antes y después del intercambio en vida real.
MétodoEstudio unicéntrico comparativo, observacional, secuencial, retrospectivo y multidisciplinar. Se emplearon los modelos farmacocinéticos poblacionales de la aplicación WAPPS-Hemo® para calcular los parámetros farmacocinéticos e individualizar la profilaxis. Se analizó la tasa anual de sangrados totales y articulares, la salud articular (Haemophilia Joint Health Score), la ratio semivida plasmática y área bajo la curva, el consumo de FVIII, la frecuencia de administración y el coste.
ResultadosSe analizaron 38 pacientes adultos con hemofilia A que cambiaron de tratamiento de FVIII de vida media estándar a FVIII de vida media extendida pegilados. Mejoraron significativamente (p < 0,05) todos los parámetros farmacocinéticos, con ratios de mejora de semivida plasmática y área bajo la curva de 1,5 y 1,9, y se registraron reducciones en tasa anual de sangrados totales y articulares, y un mayor número de pacientes con cero sangrados totales (16,0 vs 29,0) y articulares (23,0 vs 33,0). Las medianas de reducción en frecuencia de administración y dosis/kg/semana fueron de un 30,0% y 19,7%, respectivamente, evitando 44,3 infusiones/paciente/año, lo que supuso un ahorro de 20.843 €/paciente/año. Además, se observaron mejoras en la salud articular (23,0 vs 21,0; p = 0,017) y se resolvieron las articulaciones diana tras el intercambio.
ConclusionesEl intercambio guiado por farmacocinética de FVIII de vida media estándar a FVIII pegilados ha demostrado un beneficio clínico significativo con tasas reducidas de sangrado y mejoras en la salud articular. Así mismo, también se observaron mejoras en los parámetros farmacocinéticos, lo que permitió disminuir tanto la carga de tratamiento, al reducir la frecuencia de administración en los pacientes, como el consumo y el coste.
Haemophilia A (HA) is an X chromosome-linked bleeding disorder characterised by a deficiency of coagulation factor VIII (FVIII). Patients with HA are at high risk of spontaneous bleeding episodes, which can lead to serious complications such as haemarthrosis, chronic joint damage, and other disabling effects.1
HA prophylaxis consists of the regular administration of treatments aimed at preventing bleeding by maintaining minimum FVIII levels ≥1%, although in recent years levels of 3%–5% or higher have been preferred. Extending the half-life (t1/2) of FVIII has been the purpose that led to the design of extended half-life FVIII (FVIII-EHL) using various strategies such as fusion to proteins (Fc region of IgG1 or albumin) or conjugation of FVIII to polyethylene glycol (PEG).2 The attachment of the hydrophilic polymer PEG to FVIII alters its physicochemical properties, thereby modifying the interaction between the protein and cellular clearance receptors, ultimately prolonging the circulation time of the factor in the bloodstream.3
FVIII-EHL has been shown to improve clinical outcomes and reduce treatment burden in HA patients.4,5 The primary objective of the present study was to analyse differences in pharmacokinetic (PK) and clinical parameters (bleeding rates and joint health) before and after switching from standard half-life (SHL) FVIII to pegylated FVIII-EHL (PEG-FVIII) in patients with severe/moderate HA on prophylaxis with WAPPS-Hemo®.6 Secondary objectives were to analyse the t1/2 ratio and area under the curve (AUC), frequency and consumption of FVIII, and economic costs avoided after switching, and to make an indirect comparison between the PEG-FVIII studied.
MethodsStudy design and populationSingle-centre, comparative, observational, sequential, retrospective, multidisciplinary, single-centre study approved by the local Clinical Research Ethics Committee on 26 January 2022 in accordance with the principles of the Declaration of Helsinki (HUF-PKFVIII-2022-01). This study was conducted according to standard clinical practice criteria from January 2022 to December 2023, and no special treatment intervention was required.
The inclusion criteria were as follows: (1) patients with severe (less than 1 IU/dL) or moderate (1–5 IU/dL) HA on prophylactic treatment with FVIII-SHL who switched to PEG-FVIII; (2) patients included in the WAPPS-Hemo®7 application database with a switch from FVIII-SHL to PEG-FVIII, with records of at least 3–4 samples of FVIII levels and individualised PK profile. Patients with follow-up data of less than 1 year before and after the switch or with no recorded data were excluded.
ProcedureTwo one-year periods were analysed: 1 year of FVIII-SHL treatment before the switch and 1 year after the switch to PEG-FVIII treatment. The aim of switching from FVIII-SHL to PEG-FVIII was to improve bleeding control and patient quality of life. The decision to switch from one concentrate to another was made by the clinician, taking into account several factors (availability, previous factor, clinical characteristics of the patient, storage conditions, and cost). The FVIII-SHL included were: moroctocog alfa, octocog alfa (3 commercial presentations), turoctocog alfa, lonoctocog alfa, and the FVIII/FVW combination. The PEG-FVIII factors included were: rurioctocog alfa pegol, turoctocog alfa pegol, and damoctocog alfa pegol.
FVIII levels were analysed using the one-stage clotting assay (COA) with Actin FS activator and Factor VIII-deficient plasma from Siemens®. WAPPS-Hemo® was used on 3–4 samples according to the International Society on Thrombosis and Haemostasis6 guidelines for Bayesian estimation of the individualised PK profile (pre-dose, 4–8 h, 16–28 h, and 40–60 h post-infusion for FVIII SHL, and the same samples with an additional level at 60–84 h for PEG-FVIII). The PK and clinical variables analysed were: t1/2, AUC, peak level (PL), trough level at 24, 48, and 72 h (TL24/TL48/TL72), target trough level (target TL), and time to reach FVIII levels of 5%, 2%, and 1% (T5%/T2%/T1%). The t1/2 and AUC ratios were calculated. AUC was obtained from data provided by McMaster University. All PK parameters were calculated with the WAPPS-Hemo®7 application, using the application's clinical calculator to obtain PL, TL, and target TL from the individualised PK profile.
The effectiveness of the treatment was assessed using the annualised bleeding rate (ABR) and annualised joint bleeding rate (AJBR). The percentage of patients with zero bleeds during the study period, the target joints and joint health score Haemophilia Joint Health Score (HJHS) were also evaluated. To estimate FVIII consumption, the number of weekly doses and the dose/kg/week were calculated, enabling the estimation of the cost per year per patient to provide economic data and assess resource utilisation with the use of PEGylated factors.
Statistical analysisStatistical analysis was conducted using R statistical software version 4.3.3 (29 February 2024). The Wilcoxon test was used to compare the variables between the 2 periods, and for the comparison between PEG-FVIII groups the Kruskal–Wallis test and the Wilcoxon post hoc test (2 to 2) were used to test the pairs between which differences existed. A value of P < .05 was considered statistically significant. Results were expressed as median and interquartile range (IQR).
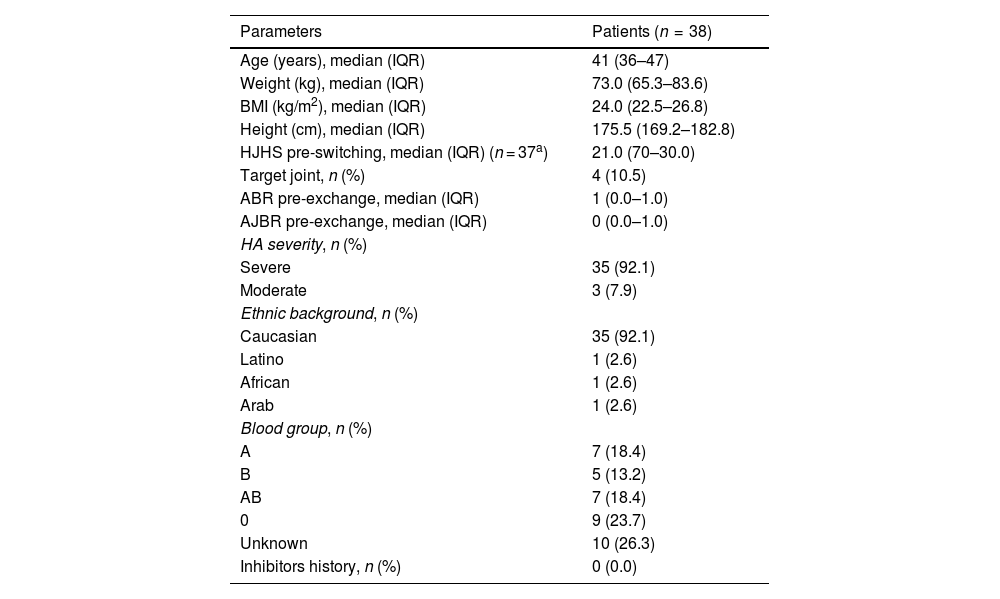
ResultsThirty-eight adult HA patients who were switched from FVIII-SHL treatment (moroctocog alfa [n = 5]; octocog alfa [n = 25]; turoctocog alfa [n = 5] lonoctocog alfa [n = 1]; FVIII/FVW combination [n = 2]) to PEG-FVIII (rurioctocog alfa pegol [n = 21]; turoctocog alfa pegol [n = 8]; damoctocog alfa pegol [n = 9]). The median age was 41 years (IQR: 36–47) and the median weight was 73.0 kg (IQR: 65.3–83.6), with no difference in weight between periods (P = .084). In terms of severity, 3 patients (7.9%) had moderate HA and 35 patients (92.1%) had severe HA. Table 1 provides the baseline characteristics of the patients.
Baseline characteristics of the patients included in the study.
| Parameters | Patients (n = 38) |
|---|---|
| Age (years), median (IQR) | 41 (36–47) |
| Weight (kg), median (IQR) | 73.0 (65.3–83.6) |
| BMI (kg/m2), median (IQR) | 24.0 (22.5–26.8) |
| Height (cm), median (IQR) | 175.5 (169.2–182.8) |
| HJHS pre-switching, median (IQR) (n = 37a) | 21.0 (70–30.0) |
| Target joint, n (%) | 4 (10.5) |
| ABR pre-exchange, median (IQR) | 1 (0.0–1.0) |
| AJBR pre-exchange, median (IQR) | 0 (0.0–1.0) |
| HA severity, n (%) | |
| Severe | 35 (92.1) |
| Moderate | 3 (7.9) |
| Ethnic background, n (%) | |
| Caucasian | 35 (92.1) |
| Latino | 1 (2.6) |
| African | 1 (2.6) |
| Arab | 1 (2.6) |
| Blood group, n (%) | |
| A | 7 (18.4) |
| B | 5 (13.2) |
| AB | 7 (18.4) |
| 0 | 9 (23.7) |
| Unknown | 10 (26.3) |
| Inhibitors history, n (%) | 0 (0.0) |
ABR: annualised bleeding rate; AJBR: annualised joint bleeding rate; HJHS: Haemophilia Joint Health Score; BMI: body mass index; IQR: interquartile range.
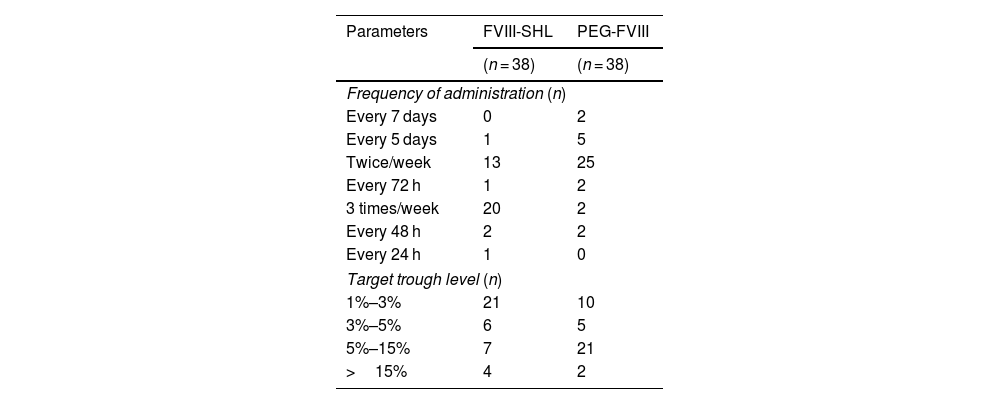
The median for administration frequency and the median FVIII dose per kilogramme per week (FVIII/kg/week) for SHL-FVIII were 3.0 (IQR: 2.0–3.0) and 70.2 (IQR: 55.1–82.7) IU/kg/week, respectively. For PEG-FVIII, the median frequency of administration was 2.0 (IQR: 2.0–2.0) and the median FVIII dose per kilogramme per week was 52.2 (IQR: 45.1–66.5) IU/kg/week. Following the switch, dosing frequency (P < .001) and dose per kilogramme per week (P < .001) were significantly reduced. The median reductions were 30.0% in frequency (IQR: 0.0%–33.3%) and 19.7% in dose per kilogramme per week (IQR: 4.1%–35.8%) IU/kg/week, avoiding 44.3 infusions per patient per year (IQR: 0.0–52.1). The prophylaxis regimens and target trough levels for SHL-FVIII and PEG-FVIII are detailed in Table 2.
Comparison between standard half-life factor VIII and pegylated extended half-life factor VIII on frequency of administration and target trough level achieved.
| Parameters | FVIII-SHL | PEG-FVIII |
|---|---|---|
| (n = 38) | (n = 38) | |
| Frequency of administration (n) | ||
| Every 7 days | 0 | 2 |
| Every 5 days | 1 | 5 |
| Twice/week | 13 | 25 |
| Every 72 h | 1 | 2 |
| 3 times/week | 20 | 2 |
| Every 48 h | 2 | 2 |
| Every 24 h | 1 | 0 |
| Target trough level (n) | ||
| 1%–3% | 21 | 10 |
| 3%–5% | 6 | 5 |
| 5%–15% | 7 | 21 |
| >15% | 4 | 2 |
FVIII-SHL; standard half-life factor VIII; PEG-FVIII: pegylated extended half-life factor VIII.
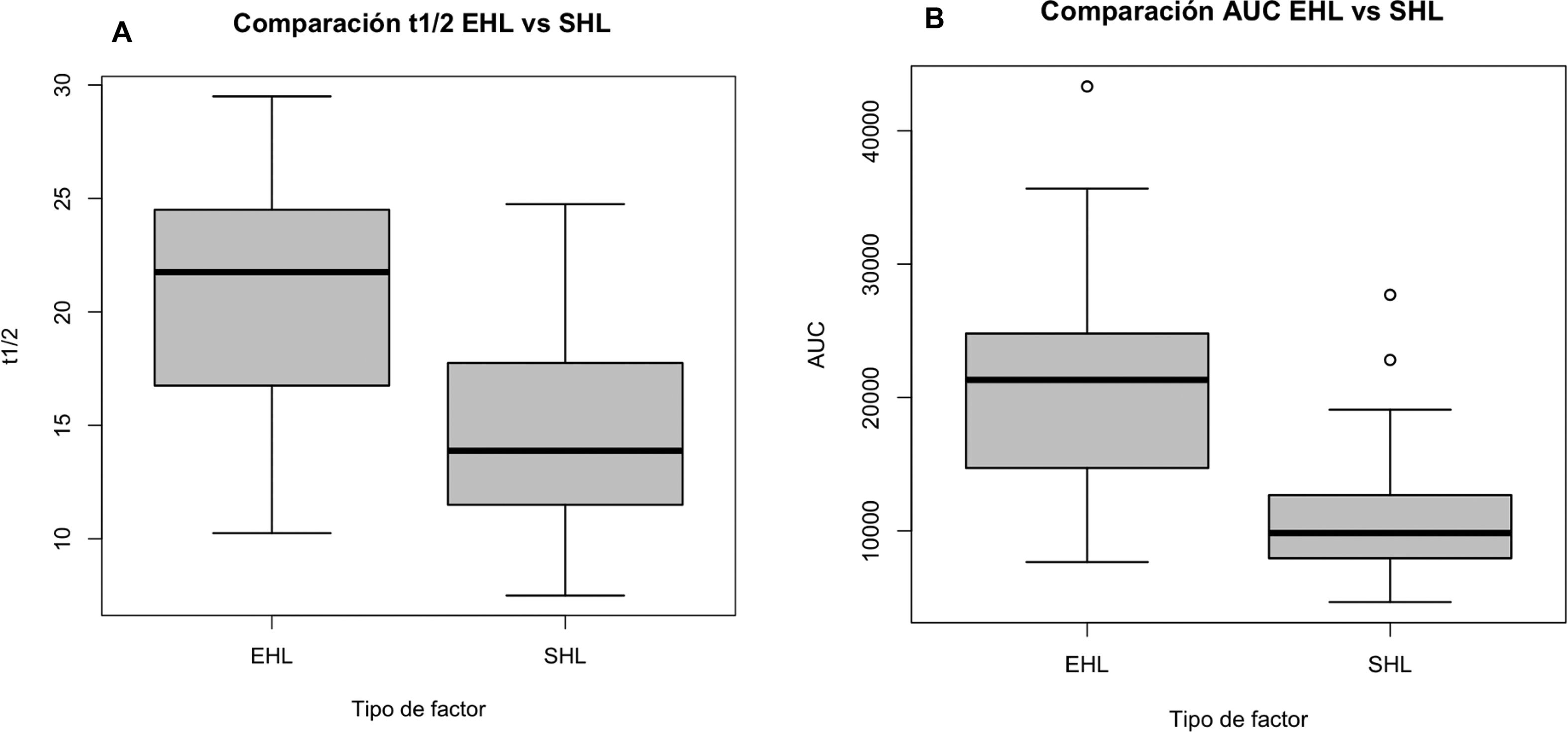
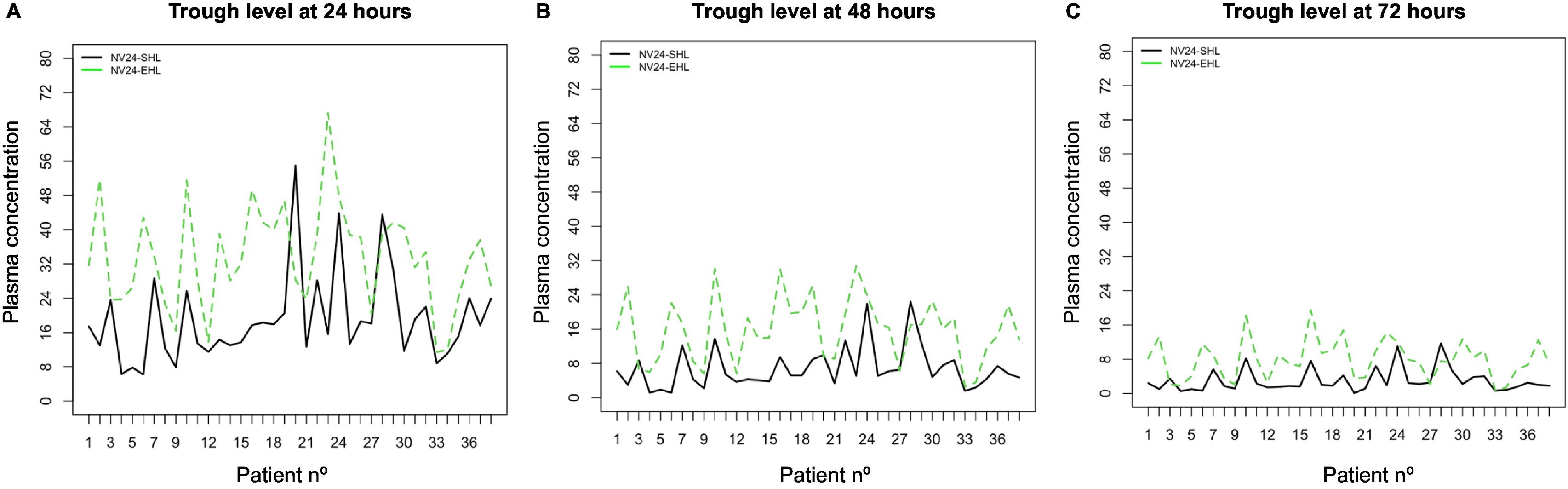
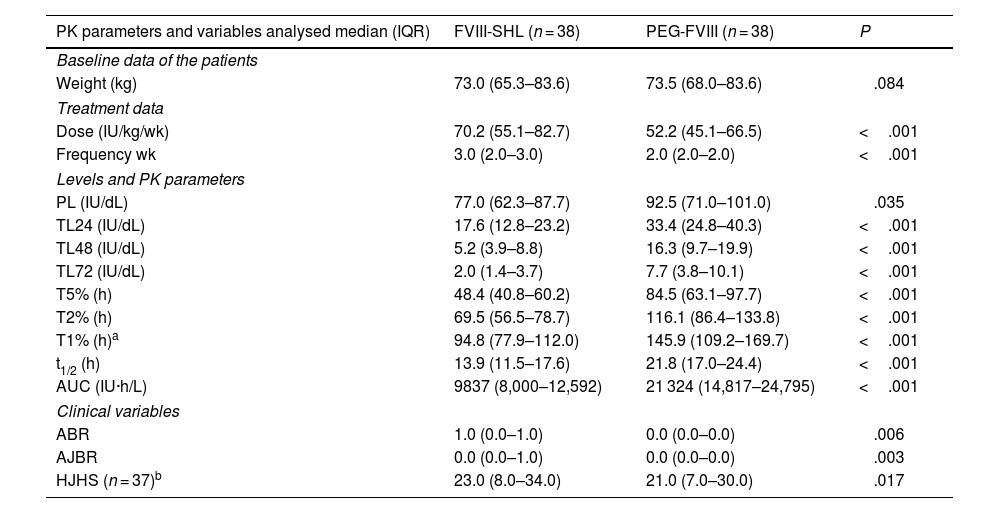
After switching to PEG-FVIII, all PK parameters improved significantly with median t1/2 and AUC ratios of 1.5 and 1.9, respectively (Table 3 and Figs. 1 and 2). Clinical improvements were also achieved with PEG-FVIII, with lower ABR (P = .006) and AJBR (P = .003), and more patients with zero total (42.1% vs. 76.3%) and joint (60.5% vs. 86.8%) bleeding. Improvements in joint health were seen in HJHS (23.0 vs. 21.0; P = .017) and patients with target joints (10.5%) resolved after the switch.
Comparison of pharmacokinetic parameters and variables analysed after switching from standard half-life factor VIII to pegylated extended half-life factor VIII.
| PK parameters and variables analysed median (IQR) | FVIII-SHL (n = 38) | PEG-FVIII (n = 38) | P |
|---|---|---|---|
| Baseline data of the patients | |||
| Weight (kg) | 73.0 (65.3–83.6) | 73.5 (68.0–83.6) | .084 |
| Treatment data | |||
| Dose (IU/kg/wk) | 70.2 (55.1–82.7) | 52.2 (45.1–66.5) | <.001 |
| Frequency wk | 3.0 (2.0–3.0) | 2.0 (2.0–2.0) | <.001 |
| Levels and PK parameters | |||
| PL (IU/dL) | 77.0 (62.3–87.7) | 92.5 (71.0–101.0) | .035 |
| TL24 (IU/dL) | 17.6 (12.8–23.2) | 33.4 (24.8–40.3) | <.001 |
| TL48 (IU/dL) | 5.2 (3.9–8.8) | 16.3 (9.7–19.9) | <.001 |
| TL72 (IU/dL) | 2.0 (1.4–3.7) | 7.7 (3.8–10.1) | <.001 |
| T5% (h) | 48.4 (40.8–60.2) | 84.5 (63.1–97.7) | <.001 |
| T2% (h) | 69.5 (56.5–78.7) | 116.1 (86.4–133.8) | <.001 |
| T1% (h)a | 94.8 (77.9–112.0) | 145.9 (109.2–169.7) | <.001 |
| t1/2 (h) | 13.9 (11.5–17.6) | 21.8 (17.0–24.4) | <.001 |
| AUC (IU·h/L) | 9837 (8,000–12,592) | 21 324 (14,817–24,795) | <.001 |
| Clinical variables | |||
| ABR | 1.0 (0.0–1.0) | 0.0 (0.0–0.0) | .006 |
| AJBR | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) | .003 |
| HJHS (n = 37)b | 23.0 (8.0–34.0) | 21.0 (7.0–30.0) | .017 |
ABR: annualised bleeding rate; AJBR: annualised joint bleeding rate; AUC: area under the curve; FVIII-SHL: standard half-life factor VIII; HJHS: Haemophilia Joint Health Score; PL: peak level; TL24, TL48, TL72: trough levels at 24, 48, and 72 h; PEG-FVIII: pegylated extended half-life factor VIII; PK: pharmacokinetics; IQR: interquartile range; wk.: week; t1/2: elimination half-life; T5%, T2%, T1%: time to reach FVIII levels of 5%, 2%, and 1%; IU: International units. Statistical test: Wilcoxon test; significance level: P < .05.
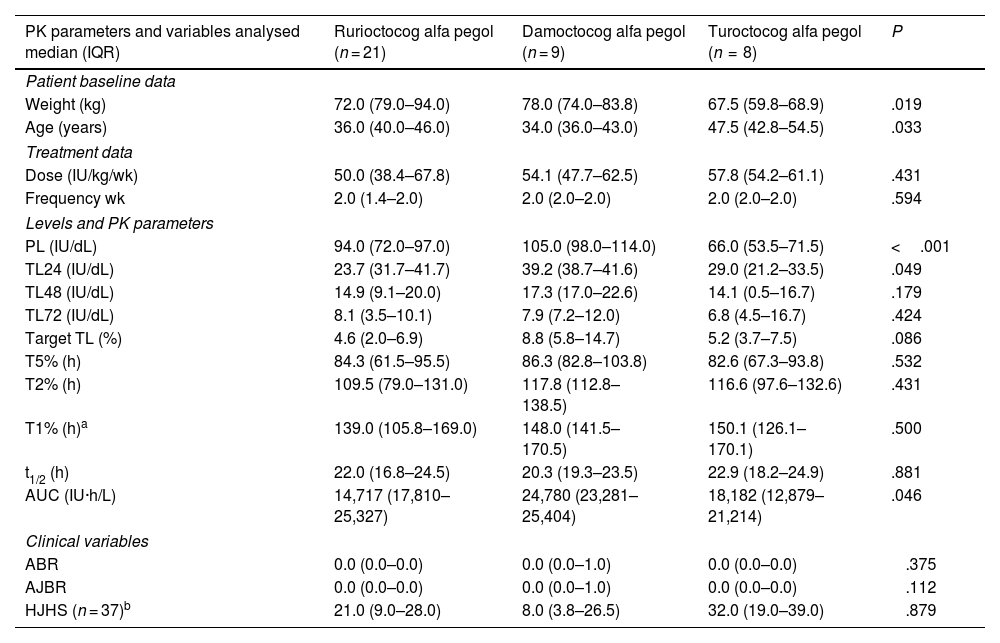
When analysing the results obtained with the different PEG-FVIII (Table 4), significant differences were observed between PEG-FVIII in some PK parameters, such as TL24 (P = .049), AUC (P = .046), and PL (P < .001), but no differences were found in t1/2, dosage (dose/kg/week and frequency) and effectiveness (ABR, AJBR).
Comparison between the PEG-FVIII administered a,b.
| PK parameters and variables analysed median (IQR) | Rurioctocog alfa pegol (n = 21) | Damoctocog alfa pegol (n = 9) | Turoctocog alfa pegol (n = 8) | P |
|---|---|---|---|---|
| Patient baseline data | ||||
| Weight (kg) | 72.0 (79.0–94.0) | 78.0 (74.0–83.8) | 67.5 (59.8–68.9) | .019 |
| Age (years) | 36.0 (40.0–46.0) | 34.0 (36.0–43.0) | 47.5 (42.8–54.5) | .033 |
| Treatment data | ||||
| Dose (IU/kg/wk) | 50.0 (38.4–67.8) | 54.1 (47.7–62.5) | 57.8 (54.2–61.1) | .431 |
| Frequency wk | 2.0 (1.4–2.0) | 2.0 (2.0–2.0) | 2.0 (2.0–2.0) | .594 |
| Levels and PK parameters | ||||
| PL (IU/dL) | 94.0 (72.0–97.0) | 105.0 (98.0–114.0) | 66.0 (53.5–71.5) | <.001 |
| TL24 (IU/dL) | 23.7 (31.7–41.7) | 39.2 (38.7–41.6) | 29.0 (21.2–33.5) | .049 |
| TL48 (IU/dL) | 14.9 (9.1–20.0) | 17.3 (17.0–22.6) | 14.1 (0.5–16.7) | .179 |
| TL72 (IU/dL) | 8.1 (3.5–10.1) | 7.9 (7.2–12.0) | 6.8 (4.5–16.7) | .424 |
| Target TL (%) | 4.6 (2.0–6.9) | 8.8 (5.8–14.7) | 5.2 (3.7–7.5) | .086 |
| T5% (h) | 84.3 (61.5–95.5) | 86.3 (82.8–103.8) | 82.6 (67.3–93.8) | .532 |
| T2% (h) | 109.5 (79.0–131.0) | 117.8 (112.8–138.5) | 116.6 (97.6–132.6) | .431 |
| T1% (h)a | 139.0 (105.8–169.0) | 148.0 (141.5–170.5) | 150.1 (126.1–170.1) | .500 |
| t1/2 (h) | 22.0 (16.8–24.5) | 20.3 (19.3–23.5) | 22.9 (18.2–24.9) | .881 |
| AUC (IU·h/L) | 14,717 (17,810–25,327) | 24,780 (23,281–25,404) | 18,182 (12,879–21,214) | .046 |
| Clinical variables | ||||
| ABR | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) | .375 |
| AJBR | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) | .112 |
| HJHS (n = 37)b | 21.0 (9.0–28.0) | 8.0 (3.8–26.5) | 32.0 (19.0–39.0) | .879 |
ABR: annualised bleeding rate; AJBR: annualised joint bleeding rate; AUC area under the curve; HJHS: Haemophilia Joint Health Score; PL: peak level; target TL: target trough level; TL24, TL48, TL72: trough levels at 24, 48, and 72 h; PK: pharmacokinetics; IQR: interquartile range; wk.: week; t1/2: elimination half-life; T5%, T2%, T1%: time to reach FVIII levels of FVIII del 5%, 2%, and 1%; IU: international units. Statistical test: Kruskal–Wallis test; significance level: P < .05.
In terms of costs, the reduction in FVIII administration (2,378 068.73 IU of FVIII avoided) resulted in a saving of €20,843/patient/year (IQR: 8,239–52,419), and a total of €1,822,460 avoided in the entire study cohort (calculated from the cost/IU of each factor). A cost saving of €37,967.9/bleed avoided was calculated (43 ABR bleeds averted in the 38 patients). The highest avoided cost/patient/year was with the switch to turoctocog alfa pegol (n = 8; 23,408 [IQR: 15,096–168,544] €) compared to rurioctocog alfa pegol (n = 8; 21,355.3 [IQR: 890.8–55,011.1] €) and damoctocog alfa pegol (n = 9; 11,018 [IQR: 7,762–37,015] €).
DiscussionThis exploratory analysis of real-world data has demonstrated the effectiveness of PEG-FVIII in clinical practice through improvements in both PK and clinical parameters, including lower ABR and AJBR, a higher proportion of patients with zero total and joint bleeding, and a reduction in treatment burden by reducing both the dose per kilogramme per week and the frequency of infusions.
PEG-FVIII has certain advantages over FVIII-SHL, such as more spaced administration.8 In our study, approximately half of the patients (n = 20, 52.6%) were on an SHL prophylaxis regimen of 3 times weekly (IQR: 2.0–3.0), whereas after the switch, the frequency of administration was reduced, with the main regimen (n = 25, 65.8%) being 2 times weekly (2.0 [IQR: 2.0–2.0]) (P < .001). In 5 similar cohorts that switched from FVIII-SHL to PEG-FVIII, a reduction in treatment burden was observed by reducing the frequency of administration and the dose/kg/week, in line with the results obtained in our cohort.9–13 A single-centre Canadian study including 25 patients reported a reduction in median infusions/week from 2.7 (IQR: 2.1–2.9) to 2.2 (IQR: 2.0–3.0) and a median reduction in annual FVIII consumption (IU/kg/year) of 12.2%.9 Clinical practice data from 3 USA centres reported a 19% reduction in weekly FVIII consumption and an 86.7% reduction in frequency.10 Nummi et al. reported a 29% reduction in median weekly infusions and a 17% reduction in factor consumption.11 A US study found a significant 25.2% reduction in frequency (2.8 vs. 2.1 days/week) and lower, but not significant, FVIII consumption.12 Similarly, in the multicentre study by Megías-Vericat et al., weekly infusion frequency and dose/kg/week decreased by 33.3% and 20.4%, respectively.13
Less intensive treatment regimens have been shown to maintain or improve protection against bleeding.9–15 In our study, a total of 44.3 infusions/patient/year were avoided by reducing the dose/kg/week and a higher target TL was achieved after switching, with more than half of the patients (55.2%) between 5% and 15% target TL. In contrast, with FVIII-SHL, the same percentage of patients were between 1% and 3% target TL, with higher levels associated with greater protection against bleeding (ABR; P = .006 and AJBR; P = .003). Similarly, bleeding was controlled and improvements in joint health were achieved in terms of HJHS (P = .017), replicating results previously observed in similar cohorts (5.5 vs. 5.0; P = .003)13 and a 2.5 point reduction,14 or 91% reduction in target joints15 after switching to PEG-FVIII.
With EHL factors, extended t1/2 has been achieved by different mechanisms; in the case of PEG-FVIII, the strategy is conjugation with PEG.2 With rurioctocog alfa pegol, t1/2 prolongation rates of 1.3–1.5 times over the reference SHL product (octocog alfa) have been reported in the literature.16 In our cohort, the ratio was 1.56 (IQR: 1.46–1.86) and the t1/2 was 22.0 (IQR: 16.8–24.5) hours. In the case of damoctocog alfa pegol, t1/2 increases of 1.4-fold are achieved,17 although in our cohort a lower ratio of 1.12 (IQR: 1.00–1.33) was obtained. As for turoctocog alfa pegol, it achieves a 1.61-fold increase in t1/2 compared with the standard comparator,5,18 and a ratio of 1.44 (IQR: 1.28–1.67) in our cohort. In the combined analysis of the 3 PEG-FVIII, a t1/2 ratio of 1.5 (IQR: 1.3–1.7) was achieved, which is the ratio required by the definition of FVIII to be considered EHL (greater than 1.3).19
The first-in-human trial testing turoctocog alfa pegol in 26 pre-treated patients with severe HA (84.6% Caucasian) obtained a T1% of 6.5 days (IQR: 3.6–7.9 days) and a t1/2 of 19.0 h (IQR: 11.6–27.3 h) for a dose of 50 IU/kg (IQR: 11.6–27.3 h).20 Similar results were obtained in our cohort, with doses of 57.8 IU/kg/week (IQR: 54.2–61.1), with a median T1% of 6.3 days (IQR: 5.3–7.1) and a t1/2 of 22.9 h (IQR: 18.2–24.9). It should be noted that the above assay results were determined using the chromogenic method rather than COA used in our study.
The extension study of the pivotal PATHFINDER 2 trial evaluated the safety and efficacy of prophylaxis with 75 IU/kg turoctocog alfa pegol administered weekly compared with 50 IU/kg every 4 days in the pivotal trial. The ABR was similar for both 0.0 (IQR: 0.0–2.4) and 0.0 (IQR: 0.0–2.2) and the number of patients experiencing bleeding was also similar for both regimens 47.1% vs. 42.1%, respectively.21 The turoctocog alfa pegol regimen for patients in our cohort was 57.8 (IQR: 54.2–61.1) IU/kg/week twice a week, with a median of 0.0 (IQR: 0.0–0.0) ABR and AJBR. Therefore, with the weekly prophylaxis regimen, similar bleeding rates are achieved while avoiding 43% of injections per year (from 91.3 to 52.0) in patients who had previously received turoctocog alfa pegol every 4 days, representing a clinical advantage and greater convenience for patients.21
In terms of efficacy, the factors damoctocog alfa pegol, rurioctocog alfa pegol, and turoctocog alfa pegol were studied in the pivotal trials PROTECT VIII (NCT01580293),22 PROLONG-ATE (NCT01736475),23 and PATHFINDER 2 (NCT01480180),21 respectively. The ABR reported in the studies was 1.3 (IQR: 0.0–4.6) for turoctocog alfa pegol (21), 1.9 (IQR: 0.0–5.8) for rurioctocog alfa pegol (23), and 1.9 (IQR: 0.0–4.2) for damoctocog alfa pegol.22 These data are comparable to those obtained in our cohort, with a median ABR of 0.0 (IQR: 0.0–0.0) for rurioctocog alfa pegol, turoctocog alfa pegol and 0.0 (IQR: 0.0–1.0) for damoctocog alfa pegol, resulting in less bleeding compared with pivotal trials. The differences observed between clinical trials and real life may be explained by the high percentage of patients with target joints in clinical trials, whereas in our study, only 4 patients (10.5%) had target joints, all of which resolved after treatment switching.24
Regarding the analysis between PEG-FVIII, our study showed significant differences between rurioctocog alfa pegol, damoctocog alfa pegol, and turoctocog alfa pegol in some PK variables, such as TL24, AUC, and PL. In the 2 to 2 post hoc analysis, differences were attributed to a greater extent to the damoctocog alpha pegol-turoctocog alpha pegol pair, although these differences could be explained by the disparity between the weight (P = .042) and age (P = .047) of the pair. Similarly, the rurioctocog alfa pegol–damoctocog alfa pegol pair also showed differences in PL, which could be attributed to differences in patient weight (P = .027). Solms et al. compared the PK of rurioctocog alfa pegol and damoctocog alfa pegol at doses similar to those used in our study, with a higher AUC associated with damoctocog alfa pegol compared with rurioctocog alfa pegol.25
In terms of costs, switching to PEG-FVIII resulted in savings of €20,843/patient/year and a total of €1,822,460 avoided in the entire cohort. An Italian study on the economic impact of using PK tools such as MyPKFit® in 13 HA patients on prophylaxis, proved to be a cost-effective strategy by significantly reducing ABR and AJBR, albeit at an increased cost of €7,394.8/bleed.26 However, it should be noted that there was no switch in this study, as there was in our cohort, where the PK-guided switch to FVIII-PEG resulted in savings of €37,967.9/bleed prevented.
Regarding the limitations of the study, firstly, the sample size was relatively small (especially for the turoctocog alfa pegol and damoctocog alfa pegol groups), but this size may be relevant considering other published studies and the fact that HA is a rare disease. Second, the external validity of the results of this single-centre study is limited and its applicability to other HA cohorts may not be appropriate. Third, despite the use of a sequential design in which each patient is his or her own control, the heterogeneity of the HA patients (age, weight, joint status, von Willebrand factor, etc.) may have influenced the bleeding scores and PK estimates, as well as the comparison of the PEG-FVIII factor, for which no sequential study was performed.
The results of the study show that switching to PEG-FVIII leads to a significant clinical benefit, with a reduction in total and joint bleeding rates, an increase in the percentage of patients with no bleeding and improved joint health. At the PK level, an improvement in t1/2 (>1.3) and AUC (>1.25) was achieved, complying with the ratios established for FVIII-EHL. The weekly frequency and dose/kg/week have been reduced, allowing infusions to be adapted to the needs and lifestyle of each patient, achieving in addition very significant savings in avoided costs. Consequently, PK monitoring of the drug switch has improved efficacy and reduced costs to the healthcare system.
Contribution to the scientific literatureThis study examines the pharmacokinetic-guided switch from conventional half-life to pegylated factor VIII in clinical practice, highlighting the benefits associated with this approach. Clinical trials have demonstrated the efficacy and safety of extended half-life factors, but few studies have analysed the impact of these factors in clinical practice, especially in Spanish cohorts. The results of this study confirm the clinical and economic advantages of pegylated factor VIII in haemophilia therapy.
FundingThe authors declare that this work was carried out without financial support.
Conference presentationsA poster related to the present work was sent to the Congress of the Spanish Society of Hospital Pharmacy (SEFH) 2024. The poster was accepted (reference mmf4381687). Organiser: Sociedad Española de Farmacia Hospitalaria (SEFH). Place: A Coruña. Date: 17–19 October 2024.
CRediT authorship contribution statementMaria Choví-Trull: Writing – review & editing, Writing – original draft, Validation, Software, Methodology, Formal analysis, Data curation, Conceptualization. Juan Eduardo Megías-Vericat: Writing – review & editing, Supervision, Methodology, Investigation, Data curation. Santiago Bonanad Boix: Writing – review & editing, Supervision, Methodology, Conceptualization. Saturnino Haya Guaita: Writing – review & editing, Supervision, Methodology, Data curation, Conceptualization. Ana Rosa Cid Haro: Writing – review & editing, Supervision, Methodology, Conceptualization. Marta Aguilar Rodriguez: Writing – review & editing, Supervision, Methodology, Data curation, Conceptualization. Jose Luis Poveda Andrés: Writing – review & editing, Supervision, Methodology, Data curation, Conceptualization.
We don't have any interest that could be perceived as a conflict or bias.
We are grateful for the collaboration of Emma Iserman and Alfonso Lorio of McMaster University, all the professionals of the Haemostasis and Thrombosis Unit of the Hospital Universitari i Politècnic La Fe, and all the patients included in the study.