Pyoderma gangrenosum (PG) is an inflammatory neutrophilic dermatosis. The incidence of the condition is 3-10 cases per million population a year and is most common among adults between 30 and 50 years of age1. There are several kinds of PG, the best known one being ulcerative PG, occurring mostly in the lower limbs. Ulcerative PG is typically accompanied by a rapid progression of ulcerative lesions with purple margins1.
Colorectal cancer (CRC) accounts for 10% of all cancers diagnosed every year. Systemic treatment of the metastatic disease consists in a combination of chemotherapy with targeted therapies (vascular endothelial growth factor [VEGF] and epidermal growth factor receptor [EGFR] inhibitors)2.
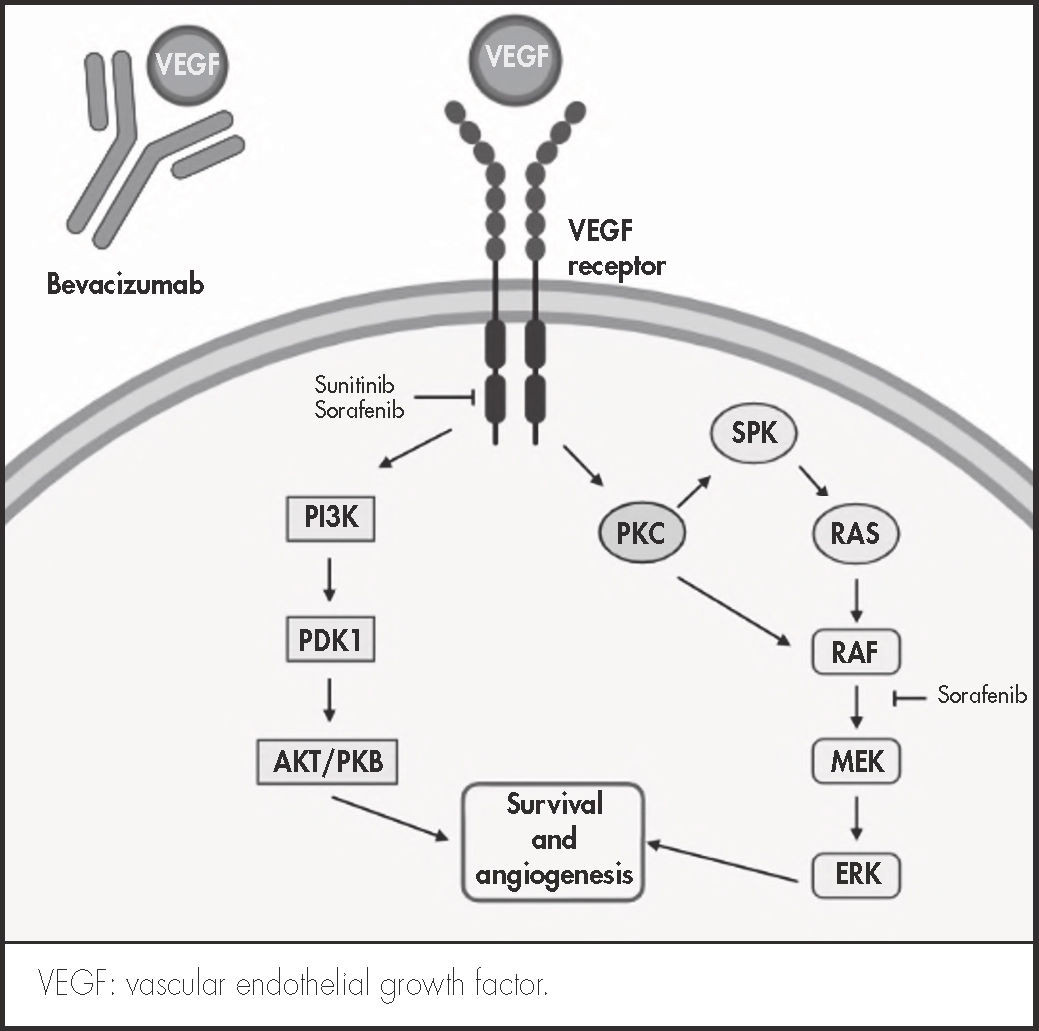
Bevacizumab belongs in the angiogenic drug class. It is a humanized monoclonal antibody that binds to VEGF-A, preventing its interaction with the VEGF-e receptor; inhibiting the cell signaling pathway, which would result in neovascularization of the tumor; and inducing apoptosis of the cancer cells. Adverse events caused by bevacizumab include hypertension, proteinuria, gastrointestinal perforation, hemorrhage, and thrombosis. It may also hinder wound healing and may induce the formation of lesions.
Although there are no reports in the literature describing the relationship between the appearance of PG and angiogenesis, PG has been diagnosed in patients treated with inhibitors of tyrosine kinase, an enzyme that is closely linked to angiogenesis3 (Figure 1).
Eleven reports have to date been published on the EudraVigilance website on the occurrence of PG that implicate bevacizumab as the potential culprit4. This paper is, to the best of our knowledge, the first comprehensive description of a case of PG in a patient treated with bevacizumab for CCR.
Description of the caseThe patient was a 69-year-old male, with no significant medical history, who had been operated in 2013 for a stage II (T4, N0, M0) sigmoid colon adenocarcinoma. In October 2018, he was reoperated for a local recurrence. In the face of the persistence of the disease, in December 2018 it was decided that the patient be administered palliative systemic treatment with fluorouracil + oxaliplatin (FOLFOX6) + panitumumab. In June 2019, in view of the progression of the disease, a second line of chemotherapy was indicated with capecitabine + bevacizumab at a dose of 1,000 mg/m2 and 7.5 mg/kg, respectively.
The patient underwent six cycles, which were well tolerated and succeeded in keeping the disease at bay. However, in the course of the sixth cycle he reported atrophic, ulcerative lesions in both lower limbs, which had been developing for 3-4 weeks and did not improve despite treatment with corticosteroids and topical antibiotics. Faced with a potential problem of toxicity, it was decided to discontinue chemotherapy and refer the patient for dermatological assessment.
Dermatological examination revealed multiple ill-smelling, painful ulcers with purple edges in the thighs, knees, elbows, and feet. A biopsy was performed for diagnosis, cultures were obtained, and treatment was started with topical antibiotics and corticosteroids, together with ciprofloxacin and a tapering schedule of oral corticosteroids.
The culture of the ulcers showed growth of Staphylococcus aureus and Enterococcus faecalis; the material collected by biopsy was not enough for diagnosis. In view of a suspicion of PG, the treatment was supplemented by colchicine/papaverine and dapsone, following the recommendations of the clinical guidelines. The biopsy was repeated1.
The results of the pathological examination confirmed the diagnosis of PG, the sample showing necrosis and inflammatory cells, with superficial lymphohistiocytic infiltrate and intense neutrophilic infiltrate. Seven months after initiation of immunomodulating treatment, the ulcers healed satisfactorily.
Naranjo's causality algorithm was applied to evaluate the relationship between the adverse reaction and bevacizumab. A score of 3 points (possible association)5 was obtained, which led to a decision not to resume treatment with bevacizumab. Capecitabine was reintroduced at one months from appearance of the lesions and was maintained until progression four cycles later, without reappearance or worsening of PG.
The adverse reaction was reported to the regional pharmacovigilance unit (identification #NR10590).
DiscussionAlthough the etiology and physiopathology of PG are not clearly understood, several factors about the pathogenesis of PG have been elucidated such as an alteration in the patients’ immune system, excessive chemotaxis of proinflammatory cytokines, and an abnormal migration of neutrophiles1. As regards the origin of PG, it has been shown that over half of patients with PG present with a concomitant systemic disease, among them solid tumors, with an incidence of 7.4-18%. A systematic review found 8 cases of PG associated to CRC6.
Patients with advanced cancer may present with neutrophilia or increased production of interleukin 1, both of them typically found in PG lesions7. The studied patient had a neutrophil count of 9,190/mL three weeks before the appearance of the ulcerative lesions.
Chua et al. found a relationship between PG and VEFG such that lesions caused by PC usually exhibit an overexpression of VEGF, of hypoxiainducible factor-2 alpha, and of angiopoetin-28. These are proangiogenic mediators whose synthesis could have been accelerated in response to bevacizumab.
On the other hand, PG cases have been reported in connection with TKIs, particularly sunitinib, imatinib and gefitinib. TKIs inhibit the cell signaling mechanisms involved in tumor growth, among them VEGF-mediated angiogenesis3 (Figure 1). A recent review applied Naranjo's causality algorithm5 to all published cases of TKI-related PG and found a probable correlation between TKIs and PG9.
The relationship between PG and bevacizumab has only been reported on one occasion10. This case report described a case of PG where several factors came together: the presence of metastatic CRC, which may lead to PG as a result of a paraneoplastic mechanism, and concomitant treatment with bevacizumab, which interacts with cell signaling mediators, often overexpressed in the lesions that accompany PG.
Although a paraneoplastic syndrome could explain the occurrence of PG, the fact that some of the cases described have been associated with drugs sharing a similar mechanism of action; that overexpression of VEGF has been shown to occur in cases of PG; and that a similar case to ours has been reported in the literature, seems to indicate that bevacizumab could play a role in the development of PG.
FundingNo funding.
Conflict of interestNo conflict of interests.
Early Access date (09/16/2021).