The literature has described the interaction between valproic acid and carbapenems. This interaction leads to decreases in plasma concentrations of valproic acid. The main objectives of this study were to assess its relevance in clinical practice, to identify variables associated with increased seizure episode rates, and to analyse the impact of pharmaceutical intervention on avoiding the effects of this interaction.
MethodAn observational retrospective study of inpatients with epilepsy admitted between 2016 and 2020. Their pharmacological treatment throughout admission was recorded, and the presence of other interactions leading to decreased plasma concentrations of valproic acid was reviewed. The seizure rate during the year prior to admission was compared to that during the interaction period. For every episode in which the interaction was detected, an intervention was conducted by providing the prescriber with information on the interaction and suggesting a change of antibiotherapy as well as the pharmacokinetic monitoring of valproic acid.
Results37 episodes were included. 58.1% of the patients were male and median age was 70 years. In total, 56.8% of the patients received meropenem and 43.2% received ertapenem. The median duration of concomitant treatment with valproic acid and carbapenem was 4 days. The incidence rate ratio was 2.60 (95% confidence interval: 1.61-4.21). Thus, this interaction was associated with a higher seizure rate. A statistically significant association was found between higher seizure rates and patients treated with more than one anti-epileptic drug. Hospital pharmacists detected 24 episodes (64.9%). In total, 17 interventions (70.8%) were accepted and 13 combinations were discontinued. Pharmacokinetic monitoring was conducted in 13 episodes (35.1%) and infratherapeutic levels were found in all of them.
ConclusionsThe interaction between valproic acid and meropenem or ertapenem is clinically relevant. It is recommended that this combination should be avoided provided that a viable alternative is available. Pharmaceutical intervention may contribute to preventing seizures associated with this combination.
La interacción entre ácido valproico y carbapenems está descrita en la literatura y conlleva una disminución de los niveles plasmáticos de ácido valproico. Los objetivos son evaluar su relevancia en la práctica clínica, conocer las variables que se asocian a un incremento de crisis epilépticas y analizar el impacto de la intervención farmacéutica para evitar las consecuencias de dicha interacción.
MétodoEn este estudio observacional retrospectivo se estudiaron pacientes con epilepsia hospitalizados entre 2016 y 2020. Se registró el tratamiento farmacológico prescrito en el ingreso y se revisó la presencia de otras interacciones que redujeran la concentración plasmática de ácido valproico. La frecuencia de crisis epilépticas durante el año previo al ingreso se comparó con la correspondiente al periodo de interacción. Se realizó una intervención en todos los episodios con la interacción detectada informando al prescriptor sobre la interacción y proponiendo sustitución de la antibioterapia, así como monitorización farmacocinética de ácido valproico.
ResultadosSe incluyeron 37 episodios. El 58,1% eran varones y la mediana de edad fue de 70 años. El 56,8% de los pacientes recibió meropenem y el 43,2% restante, ertapenem. Para la duración del tratamiento concomitante entre ácido valproico y el carbapenem prescrito se obtuvo una mediana de 4 días. Se halló una razón de tasas de incidencia de 2,60 (intervalo de confianza del 95%: 1,61-4,21), por lo que esta interacción se asocia a una mayor frecuencia de crisis epilépticas. Se asoció una mayor frecuencia de crisis estadísticamente significativa en los pacientes tratados con más de un fármaco antiepiléptico. Los farmacéuticos hospitalarios detectaron 24 episodios (64,9%). Se aceptaron 17 intervenciones farmacéuticas (70,8%) y se suprimieron 13 combinaciones. Se realizó monitorización farmacocinética en 13 episodios (35,1%) y en todos se hallaron niveles infraterapéuticos.
ConclusionesLa interacción entre ácido valproico y meropenem o ertapenem es clínicamente relevante y se recomienda evitarla siempre que existan alternativas viables. La intervención farmacéutica puede contribuir a prevenir las crisis epilépticas favorecidas por esta combinación.
Valproic acid (VPA) is a first-generation antiepileptic drug (AED). Its effect is directly related to its plasma levels (current reference range: 50-100 μg/mL). Regarding distribution, VPA exhibits saturable plasma protein binding even in the therapeutic range, which may explain the lack of correlation between dose and plasma concentrations1,2. The biotransformation of VPA mainly occurs in the liver and is excreted in urine as glucuronide conjugate. This metabolic pathway is reversible by acyl-peptide hydrolase (APEH), which induces the deconjugation reaction. Carbapenem antibiotics inhibit APEH, promoting the glucuronidation of VPA and its renal clearance. Thus, concomitant treatment with a carbapenem and VPA leads to a rapid decrease in plasma VPA levels and compromises its antiepileptic effectiveness. In fact, previous studies have reported reductions in its plasma concentrations of more than 80%2-7. This interaction is classified by UpTo-Date as risk D (i.e. it is generally recommended that therapy modification be considered)8.
Despite theoretical knowledge of this interaction, there are few published studies analysing its relevance in routine clinical practice7,9.10. However, these studies have not addressed factors inherent to antiepileptic treatment that, in practice, could influence seizure control. These factors include the number of AEDs prescribed for each patient, potential alterations in liver function, or the form of administration of VPA.
Hospital pharmacists are highly competent in the correct management of pharmacotherapy and thus have a relevant professional role in the review of inpatient treatment. Within the pharmaceutical care of inpatients, the review of interactions is included as one of the actions to guarantee the pharmacotherapeutic safety of patients11.
The main objective of this study was to assess the relevance of this interaction in routine clinical practice by quantifying differences in the frequency of seizures experienced by epileptic patients under treatment with VPA when exposed to any carbapenem marketed in Spain. The secondary objectives were to determine which variables are associated with a higher incidence of epileptic seizures and to analyse the impact of pharmaceutical intervention in the prevention of this interaction.
MethodsA retrospective observational study conducted in a Group 5 hospital graded according to the Spanish Ministry of Health 2008 DRG classification12. The study was approved by the ethics committee of the hospital in which the study was conducted.
We assessed all patients admitted between January 2016 and July 2020 who had received concomitant treatment with VPA and a carbapenem marketed in Spain (i.e. imipenem/cilastatin, meropenem, or ertapenem). Inclusion criteria were as follows: 18 years or older, a diagnosis of epilepsy with chronic treatment with VPA (i.e. for at least 3 months prior to admission), and the absence of infections or neoplasms involving the central nervous system.
We recorded concomitant pharmacotherapeutic treatment for each patient during the VPA-carbapenem interaction period, and the presence or absence of other interactions that decreased VPA plasma concentrations. These were determined using Lexi-Interact®, with special attention placed on the detection of interactions widely described in the literature2,8.
In order to differentiate interaction-free seizures and interaction-based seizures, two periods were defined: the year prior to admission and the admission period itself, during which period patients were prescribed concomitant treatment with VPA and a carbapenem.
Hospital and primary care electronic medical records were used to determine the pharmacological treatment prescribed and the frequency of seizures during the admission period under study and during the previous year.
If concomitant treatment was detected, pharmaceutical intervention was conducted in writing via the electronic prescription and by telephone to the prescribing physician. Information was provided on the available evidence on the interaction, pharmacokinetic monitoring of VPA was recommended, and, if feasible, changeover from the antibiotic to a safer alternative was proposed10,13. In our centre, the pharmacokinetic monitoring of VPA levels is measured as total plasma concentrations.
Data were recorded and analysed in a pseudoanonymised data collection notebook. Regarding the descriptive analysis, quantitative variables are expressed as medians (interquartile range) and qualitative variables are expressed frequency distributions (%).
The main study objective was assessed by calculating the seizure incidence rates (IR) during the year prior to admission and during the VPA-carbapenem interaction period. The seizure incidence rates were used to calculate the incidence rate ratios (IRR) for the total sample with their respective 95% confidence intervals (95% CI) and compared by calculating the absolute risk difference14. The number needed to harm was also calculated15.
Associations between different pharmacotherapeutic variables and seizure rates were also assessed by calculating the IRs and the IRRs with their respective 95%CIs. To this end, patients were grouped by the carbapenem prescribed and the number of antiepileptic drugs administered. We calculated the IRRs for each subgroup and compared the IRRs associated with the same variable with each other. Cases in which the confidence intervals did not overlap were considered to be statistically significantly different.
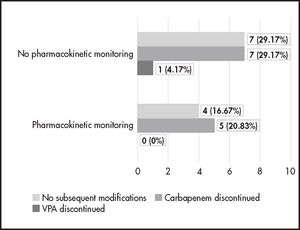
We assessed the acceptance and impact of the pharmaceutical intervention by recording the actions taken by the prescribers after they received information and recommendations from the pharmacists. Episodes were distributed according to whether VPA pharmacokinetic monitoring had been initiated or not. For each group, we counted the number of episodes where carbapenem or VPA had been discontinued and the number of episodes in which both prescriptions remained unchanged.
ResultsWe included 37 episodes in 31 patients (see Table 1 for the main data). None of our patients were treated with imipenem/cilastatin.
Main data of the patients comprising the total number of episodes and their respective pharmacological prescriptions during hospital admission. Qualitative variables are expressed as absolute values (%) and quantitative variables as medians (interquartile range).
| Sex | |
|---|---|
|
|
| Age, years | 70 (50.5-79.0) |
| Liver function | |
| 1 or no liver enzyme > 3 times ULN | 35 (94.6%) |
| 2 or more liver enzymes > 3 times ULN | 2 (5.4%) |
| Carbapenem prescribed | |
| Meropenem | 21 (56.8%) |
| Ertapenem | 16 (43.2%) |
| Carbapenem dose | |
| Adjusted to renal function | 36 (97.3%) |
| Higher than recommended for renal function | 1 (2.7%) |
| Duration of VPA-carbapenem combination, days | 4 (1-6) |
| VPA treatment modality | |
| Antiepileptic monotherapy | 15 (40.5%) |
| Antiepileptic polytherapy | 22 (59.5%) |
| VPA administration route | |
| Oral | 33 (89.2%) |
| Immediate-release tablets or oral solution | 31 (83.8%) |
| Delayed-release tablets | 2 (5.4%) |
| Intravenous | 4 (10.8%) |
ULN, upper limit of normal; VPA, valproic acid.
It is noteworthy that during the search for interactions other than VPA-carbapenem, we also found one episode involving VPA-darunavir and VPA-ritonavir interactions that may have potentially decreased the plasma concentrations of the antiepileptic. Both could be implicated in a potential reduction of antiepileptic drug levels and are classified by UpToDate as risk C: thus, monitoring is recommended. However, the patient did not experience an increase in the seizure rate during this period.
In 13 episodes (35.1%), pharmacokinetic monitoring of VPA was performed during the drug-combination period. During all episodes, sub-therapeutic levels of VPA were found with a mean total plasma drug concentration of 15.5 ± 12.1 μg/mL.
During the VPA-carbapenem interaction period, the seizure incident rate was 8.56% (8.56 seizures per 100 patient-days), whereas in the previous year, without interaction, it was 3.28%. Thus, the IRR was 2.60 (95% CI: 1.61-4.21), the absolute risk difference was 5.28%, and the number needed to harm was 19 combinations.
In the subgroup analysis, no statistically significant differences were found between patients receiving meropenem and those receiving ertapenem. In contrast, a statistically significant increase in the IRR was found for patients treated with antiepileptic polytherapy (PAE) versus patients treated with VPA as antiepileptic monotherapy (MAE) (Table 2).
Incidence rates and incident rate ratios for the study groups, calculated as number of seizures per 100 patient-days
| Study group | IR during the non-interaction period | IR during the interaction period | IRR (95% CI) | |
|---|---|---|---|---|
| Total sample (n = 37) | 3.28 | 8.56 | 2.60 (1.61-4.21) | |
| Concomitant carbapenem | Meropenem (n = 21) | 2.10 | 5.51 | 2.62 (1.27-5.44) |
| Ertapenem (n = 16) | 4.86 | 15.00 | 3.10 (1.65-5.81) | |
| Number of AEDs prescribed |
|
|
|
|
AED: antiepileptic drugs; AEM: antiepileptic monotherapy; AEP: antiepileptic polytherapy; 95% CI: 95% confidence interval; IR: incident rate; IRR: incident rate ratio.
The Pharmaceutical Care department for inpatients detected 24 episodes (64.9%) and conducted interventions in all of them. These pharmaceutical interventions prompted clinical decisions that led to the discontinuation of 13 combinations (Figure 1).
DiscussionThis study obtained pharmacokinetic results that are in line with those described in the previous literature. We found that the interaction between VPA and meropenem or ertapenem is clinically relevant and potentially severe. These results were obtained not only because the study patients exposed to meropenem or ertapenem had sub-therapeutic plasma concentrations of VPA, but also because there was a significant increase in the seizure episode rate compared to that during the period in which VPA was prescribed without associated carbapenem therapy. Moreover, strategies based on increasing the dose of VPA have been shown to be ineffective in restoring plasma VPA levels7,10,16.
The relevance of the interaction is such that studies are currently underway on the clinical applicability of the use of carbapenems in VPA poisoning17,18. However, more information is needed on their efficacy and safety in this setting.
We found a marked difference in seizure rates between the antiepileptic monotherapy and antiepileptic polytherapy groups. This finding may have been obtained because patients requiring more than one AED have a history of poorer seizure control and their condition is more resistant to pharmacological treatment1,19. This factor is also a limitation since we did not estimate what proportion of the increase in seizures could be related to it. Given that antiepileptic polytherapy is associated with increased seizure rates, we recommend that patients receiving this type of treatment should be closely monitored.
Due to the significant predominance of the use of immediate-release oral forms in the sample, no analysis was performed of the different forms of VPA administration in these patients. It would be of interest to include this variable in future studies, especially to understand the impact of the interaction in patients receiving continuous infusion VPA.
This study was conducted in a single centre with a small sample, which might explain the lack of significant differences by carbapenem administered. Previous studies have found this type of difference and shown that the reduction in VPA levels was less in patients receiving imipenem than in patients receiving meropenem or ertapenem7.
This study is also limited by its retrospective design: for example, the potential omission of seizure episodes prior to admission that may not have been recorded in the clinical history.
This study has demonstrated the relevance of this interaction and its pharmacokinetic impact. Based on these results, we recommend that the use of meropenem or ertapenem in epileptic patients receiving VPA therapy should be avoided unless there is no viable alternative. Furthermore, given the available evidence on interactions with imipenem, we extend this recommendation to the carbapenem group marketed in Spain7,9,16. Previous studies have proposed the use of levofloxacin or piperacillin/tazobactam as an alternative to the use of carbapenems10, although antibiotherapy should always be individualised. If patients need carbapenem therapy, it has been suggested that another antiepileptic is used instead of VPA9,16.
In addition, when the concomitant use of these drugs is detected, it is well established that pharmaceutical intervention should be a priority because it contributes to the discontinuation of the VPA-meropenem or VPAertapenem combination in a considerable proportion of cases.
To maximise the impact of the intervention, we recommend the implementation of early warning systems to assist pharmaceutical validation to avoid inaction in unnoticed cases. However, the optimal approach to this interaction is to avoid the concomitant prescription of potentially interacting drugs, rather than conducting interventions when this situation is detected during pharmaceutical validation20,21. To this end, pharmacists should be integrated in health care teams.
FundingNo funding.
Conflict of interestNo conflict of interests.
Contribution to the scientific literature
This study found no statistically significant differences between meropenem and ertapenem in their interactions with valproic acid. The relevance of antiepileptic polytherapy as a predisposing factor for the increased risk of epileptic seizures is emphasised.
Concomitant treatment with these carbapenems and valproic acid may cause severe interactions. Their prevention should be prioritised.
Early Access date (09/27/2021).