To know the safety profile of the 4CMenB vaccine in adults in special situations.
MethodSecurity prospective study of phase IV. Inclusion criteria and some vaccination conditions were applied. The adverse reactions described in the data sheet were collected. The adverse reactions evaluation was performed 24 hours after vaccination (“requested”) and during the first seven days (“not requested”).
Results72 patients were included (54.2% men, mean age 52.5 years, 81.9% anatomic asplenia). The frequency of fever > 38 °C in the first 24 hours of vaccination was higher than the observed in the summary of product characteristics for the group of adults (12.5% vs. not known). More than 75% of the patients reported local pain in the first hours [average of the Analog Visual Scale score 3.22 (95% CI: 2.67-3.76) in the first dose and 3.23 (95% CI: 2.69-3.78) in the second dose]. There were no statistically significant differences. 97.22% registered symptoms until 7 days after vaccination.
Conclusions4CMenB shows a good safety profile in adults in special situations. The frequency of fever > 38 °C is higher than expected. Local pain is the most frequently recorded adverse reactions, but the intensity is low. These results suggest a review of the situation in order to suggest a possible modification of the summary of product characteristics of the vaccine.
Conocer el perfil de seguridad de la vacuna del meningococo B (4CMenB) en adultos en situaciones especiales.
MétodoEstudio prospectivo de seguridad de fase IV. Se aplicaron criterios de inclusión y ciertas condiciones de vacunación. Se recogieron las reacciones adversas descritas en la ficha técnica. La evaluación de las reacciones adversas se realizó a las 24 horas de la vacunación (“solicitadas”) y durante los siete primeros días (“no solicitadas”).
ResultadosSe incluyeron 72 pacientes (54,2% hombres; media de edad 52,5 años; 81,9% asplenia anatómica). La frecuencia de fiebre > 38 °C en las primeras 24 horas fue mayor de la observada en la ficha técnica para el grupo de adultos (12,5% versus no conocida). Más del 75% de los pacientes refirió dolor local en las primeras horas [media de la puntuación de la Escala Visual Analógica 3,22 (IC95%: 2,67-3,76) en la primera dosis y 3,23 (IC95%: 2,69-3,78) en la segunda dosis]. No hubo diferencias estadísticamente significativas. El 97,22% registró síntomas hasta los siete días postvacunación.
Conclusiones4CMenB muestra un buen perfil de seguridad en adultos en situaciones especiales. La frecuencia de fiebre > 38 °C es mayor que la esperada. El dolor local es la reacción adversa más frecuentemente registrada, pero la intensidad es baja. Estos resultados invitan a una revisión de la situación de cara a sugerir una posible modificación de la ficha técnica.
Neisseria meningitidis is an exclusively human pathogen, capable of causing severe infections such as invasive meningococcal disease (IMD)1. Age is an essential factor in the distribution of the risk associated with meningococcus, and therefore there is a higher risk of invasive disease in children, although there is a higher rate of carriers among adolescents and young adults2.
In 2014, 2,760 confirmed cases of IMD in Europe were reported to the European Centre for Disease Control and Prevention; this shows an annual incidence rate of 0.5 cases per 100,000 inhabitants. Of these cases, 64% were caused by a serogroup B meningococcus (MenB)3.
Currently there are six meningococcal vaccines marketed in Spain4: three monovalent vaccines against serogroup C (Menjugate®, Meningitec® and NeisVac-C®), two tetravalent vaccines against serogroups A, C, W and Y (Menveo® and Nimenrix®), and the four-component vaccine against the serogroup B identified with the acronym 4CMenB (Bexsero®). A new vaccine has been recently marketed against serogroup B, with the two antigenic variables of the FHbp protein (binding protein to factor H), known as MenB-FHbp (Trumenba®).
Currently, only the vaccine against serogroup C is included in the systematic child vaccination calendar in Spain, while the 4CMenB vaccine is only recommended and reimbursed under some special risk situations within the Spanish National Health System (SNS)5.
Vaccine safety and pharmacovigilance are two key elements in any vaccination program. Those clinical trials conducted on the 4CMenB vaccine show that the main adverse reactions (ARs) reported are pain and local erythema. Moreover, in the case of babies, fever >38 °C appears in 14% to 50% of the vaccinated group, and clearly higher figures will be reached (85%) if administered jointly with other vaccines6–11. It is worth highlighting that these clinical trials were conducted particularly with healthy children, adolescents and adults; however, and regardless of the clear indication of this vaccine for patients in special situations (anatomic and functional asplenia, and others), there are no studies so far dealing with safety in this group.
For this reason, the objective of this research is to understand the safety profile of 4CMenB vaccine in adult persons in special situations with recommendation of vaccination according to the Spanish NHS.
MethodsType of studyA post-marketing study for prospective follow-up associated with safety.
Study setting and timingThe study was conducted at the Vaccination Unit (VU) of a regional hospital of reference with 1,069 beds, from March 2015 to March 2017.
Inclusion criteriaThe following inclusion criteria were implemented:
- 1)
Patients with indication for vaccination with 4CMenB according to the Spanish SNS5:
- a.
Complement/properdin deficiency.
- b.
Treatment with eculizumab.
- c.
Asplenia or severe splenic dysfunction.
- d.
History of previous IMD.
- e.
Lab staff in potential contact with N. meningitidis.
- a.
- 2)
Age >18-year-old.
- 3)
No specific contraindications for the administration of the 4CMenB vaccine (Bexsero®) according to the product specifications.
- 4)
Having signed the Informed Consent provided by the VU.
The following vaccination conditions were taken into account:
- 1)
The vaccine was administered isolatedly, without the concomitant administration of any other vaccine at the same vaccination act.
- 2)
The vaccination dosing regimen for >11-year-old and adults was used, according to the indications in the product specifications (PS) for Bexsero®: two doses separated by at least 30 days. No booster vaccination has been established.
- 3)
There was a time interval of at least 30 days between other vaccines previously administered and any of the two doses of Bexsero®.
- 4)
There was a time interval of at least 30 days between the second dose of Bexsero® and any other subsequent vaccine.
- 5)
In patients with immunosuppresor and immunomodulator treatment, there was an interval of at least 1 week before and after taking this medication and the administration of any dose of Bexsero®.
- 6)
It was not recommended to take prophylactic oral paracetamol, unless the patient presented body temperature >37.5 °C after the first dose.
- 7)
It was not administered to patients with acute disease, suspected incubation period, or fever / feverishness > 37.5 °C as measured by thermometer at the time of evaluation. In these cases, vaccination was postponed until the acute condition was solved.
Sociodemographical variables were collected (gender, age, indication of vaccination), as well as the development of any of the adverse reactions described in the section “4.8. Adverse Reactions” of Bexsero®12 's product specifications (fever, headache, pain at the injection site, skin rash, somnolence, irritability, unusual crying, vomiting, convulsions, dry skin, paleness, Kawasaki disease, musculoskeletal pain and general malaise), the time between vaccination and the initiation of the adverse reaction symptoms, and the duration of said symptoms.
Procedure for information collectionAdverse reaction recording was conducted in the same way for the first and the second dose of vaccine administered.
It was structured into two phases: (1) early onset symptoms (within the first 24 hours), collected through telephone survey by trained nursing staff (“requested” adverse reactions), and (2) symptoms appearing within the first seven days after vaccination, collected by the patient through a record sheet designed for this purpose (“not requested” adverse reactions), including the clinical sign or symptom, the start date and its duration. Similarly, patients were asked to report if they needed to take paracetamol or any other medication in order to alleviate said symptom.
For the evaluation of “pain at the injection site”, each patient was trained on the use of the Analogue Visual Scale (AVS), where 1 means “no pain” and 10 means “unbearable pain”.
Reporting to the Spanish Pharmacovigilance SystemAdverse reactions or severe mistakes associated with vaccination were reported to the Spanish Pharmacovigilance System (SEFV-H) through www.notificaram.es. The adverse reactions were classified into severe and nonsevere, according to the terminology of the European Medicines Agency and the SEFV-H. A severe adverse reaction was defined as: (1) causing death; (2) threatening the patient's life; (3) leading to patient's hospitalization; (4) causing inability to work; (5) causing congenital defects, or (6) being clinically relevant.
Type of analysisFor the analysis of variables associated with the report at 24 hours after vaccination, descriptive statistics was conducted for each variable (univariate analysis), exploring the absolute and relative frequencies of the qualitative variables under research. Mean values were calculated (quantitative variables) as well as percentages (qualitative variables), and their 95% Confidence Intervals (CI 95%). Bivariate analysis was conducted to find out if the selected study variables were associated or not. Chi square test was used for dichotomous qualitative variables, and Student's T test was used for paired samples. A p value <0.05 was considered statistically significant. The PASW program (previously known as SPSS), version 18, was used for all this.
On the other hand, qualitative methodology based on the biographical method was used for the record of adverse reactions conducted by each patient during the first seven days after vaccination13.
Ethical aspectsThe study received a favourable report by the Clinical Research Ethics Committee (Ref 119/15), and was classified by the Spanish Agency of Medicines and Medical Devices as a post-marketing study for prospective follow-up (Ref MFC-BEX-2016-01).
ResultsIn total, the study included 72 patients who met the inclusion criteria. Of these, 54.2% (39) were male and 45.8% (33) were women. Their mean age was 52.5 years, with a ±18.0-year standard deviation.
Regarding the medical indication for vaccination against 4CMenB, the sample was distributed into: 81.9% (59) with anatomic asplenia, 12.5% (9) with previous IMD, 2.8% (2) on treatment with eculizumab, 1.4% (1) with common variable immunodeficiency in a lab staff in contact with N. meningitidis, and 1.4% (1) with functional asplenia due to graft-versus-host disease in a patient with allogeneic transplant of haematopoietic progenitors. On the other hand, 80.6% (58) were receiving treatment for some chronic disease at the time of vaccination, while 19.4% (14) were receiving no treatment.
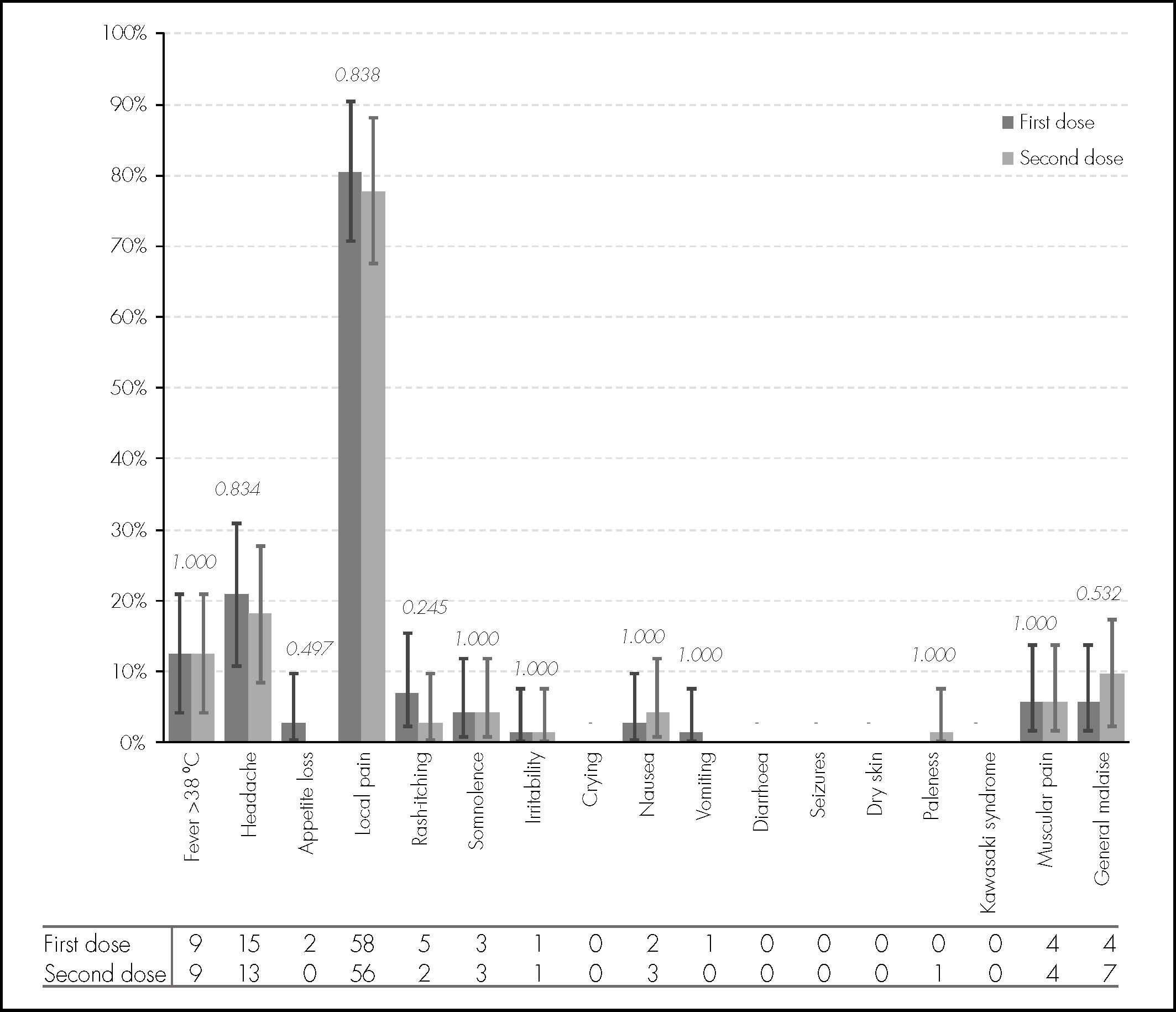
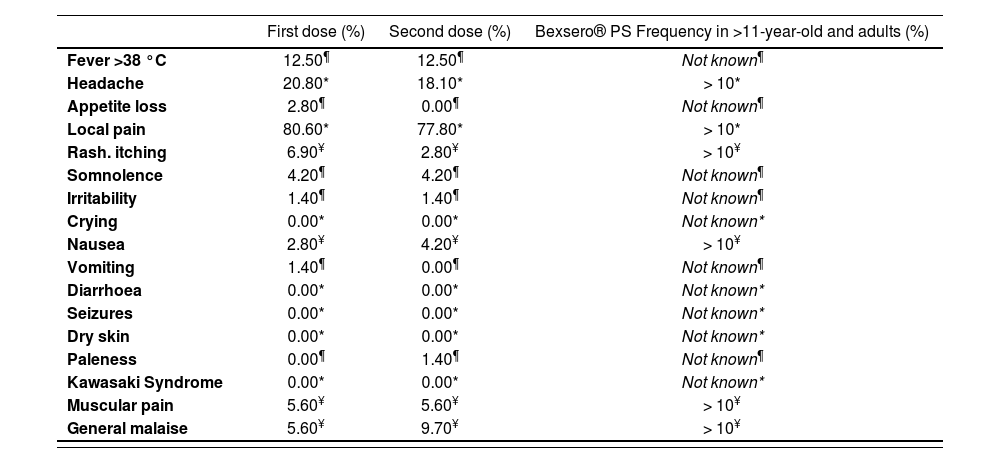
The most frequent adverse reactions, appearing within the first 24 hours after vaccination with the first and second doses of Bexsero® were: local pain [80.6% (58) after the first dose and 77.8% (56) after the second dose], headache [20.8% (15) after the first dose and 18.10% (13) after the second dose] and fever >38°C [12.5% (9) after the first and second doses]. Figure 1 shows the frequency rate of all adverse reactions described at Bexsero®‘s product specifications for the first and second doses of the vaccine. No statistically significant differences were found between any of the variables studied. However, when comparing the adverse reactions identified in this study with those shown in Bexsero®‘s PS, some noteworthy differences can be observed. Thus, we can highlight the development of six adverse reactions that are not described in the PS of the vaccine for the >11-year-old and adult group, such as: fever >38 °C, somnolence, loss of appetite, irritability, vomiting and paleness (Table 1).
Proportion of requested adverse reactions after the first and second doses of Bexsero®, with their 95% confidence interval, and statistical analysis of the differences between them (p values in italics). The number of patients who presented each one of the adverse reactions is shown in the table below the graph.
Frequency of adverse reactions after the first and second doses in comparison with the frequencies described in the product specifications of Bexsero®
| First dose (%) | Second dose (%) | Bexsero® PS Frequency in >11-year-old and adults (%) | |
|---|---|---|---|
| Fever >38 °C | 12.50¶ | 12.50¶ | Not known¶ |
| Headache | 20.80* | 18.10* | > 10* |
| Appetite loss | 2.80¶ | 0.00¶ | Not known¶ |
| Local pain | 80.60* | 77.80* | > 10* |
| Rash. itching | 6.90¥ | 2.80¥ | > 10¥ |
| Somnolence | 4.20¶ | 4.20¶ | Not known¶ |
| Irritability | 1.40¶ | 1.40¶ | Not known¶ |
| Crying | 0.00* | 0.00* | Not known* |
| Nausea | 2.80¥ | 4.20¥ | > 10¥ |
| Vomiting | 1.40¶ | 0.00¶ | Not known¶ |
| Diarrhoea | 0.00* | 0.00* | Not known* |
| Seizures | 0.00* | 0.00* | Not known* |
| Dry skin | 0.00* | 0.00* | Not known* |
| Paleness | 0.00¶ | 1.40¶ | Not known¶ |
| Kawasaki Syndrome | 0.00* | 0.00* | Not known* |
| Muscular pain | 5.60¥ | 5.60¥ | > 10¥ |
| General malaise | 5.60¥ | 9.70¥ | > 10¥ |
1 All data coinciding with those collected in the Product Specifications for the group of “>11-year-old children and adult persons” appear with (*); data not stated in the PS but found in this study appear with (¶), and finally, those data where a lower rate vs. that stated in the PS has been found appear with (¥).
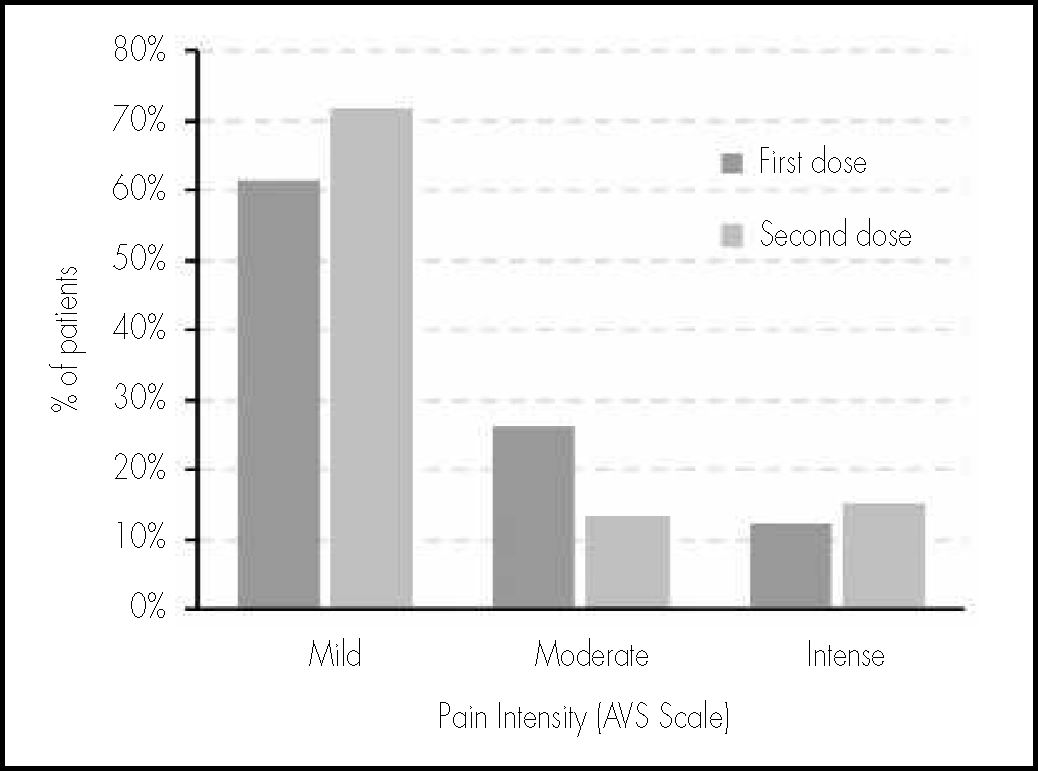
Pain at the site of injection was evaluated at 24 hours of vaccination through an AVS on which each patient had been previously trained; 80.6% (58) and 77.8% (56) of patients reported local pain within the first 24 hours after vaccination with the first and second doses. The mean AVS score with the first dose was 3.22 (CI95%: 2.67-3.76) and 3.23 with the second dose (CI95%: 2.69-3.78). There were no statistically significant differences (p=0.979); however, there was a higher frequency of moderate pain with the first dose (AVS score 4-6), and of mild pain with the second dose (AVS score 1-3), as shown in Figure 2. It is worth highlighting that only two patients assigned a value >8 in the AVS to their pain both for the first and the second dose of the vaccine.
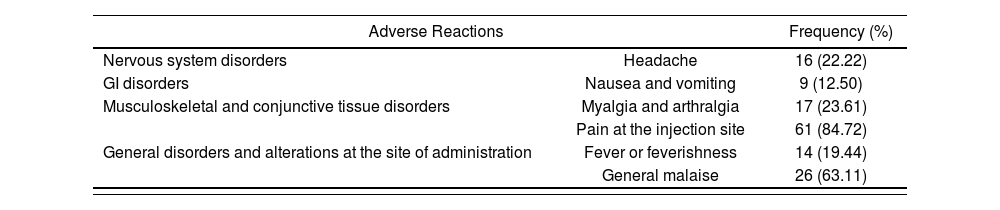
Adverse reactions in the first seven days after vaccination (“not requested”) were analyzed with qualitative methodology. Of those 72 persons included in the research, only 2 reported no sign or symptom within the seven days after vaccination. On the contrary, the remaining 70 persons reported some adverse reaction, including: local pain [84.72% (61)], general malaise [63.11% (26)] and fever [22.22% (16)] within the first seven days after Bexsero®. The “latency” and “duration” variables could not be analyzed due to lack of record by patients. Table 2 shows the grouped outcomes.
Frequency of adverse reactions described in the PS for Bexsero® and reported by participants within the first seven days after vaccination (“not requested”)
| Adverse Reactions | Frequency (%) | |
|---|---|---|
| Nervous system disorders | Headache | 16 (22.22) |
| GI disorders | Nausea and vomiting | 9 (12.50) |
| Musculoskeletal and conjunctive tissue disorders | Myalgia and arthralgia | 17 (23.61) |
| Pain at the injection site | 61 (84.72) | |
| General disorders and alterations at the site of administration | Fever or feverishness | 14 (19.44) |
| General malaise | 26 (63.11) | |
It is worth noting that even though 80.6% of patients in the study were receiving chronic treatment due to their basal condition, the development of both local and systemic adverse reactions was attributed to vaccination, because symptoms were compatible both clinically and epidemiologically, and moreover, these were solved without any treatment interruption.
The only adverse reaction classified as severe, according to the clinically relevant criterion, was reported to the SEFV-H (Record No. 600167). This adverse reaction consisted in general malaise with nausea, dizziness, and unspecific abdominal discomfort; it lasted over three days and then disappeared.
DiscussionPhase IV studies are essential in order to improve the detection of adverse pharmacological reactions. In most occasions, infrequent adverse reactions, or those appearing in specific patient subpopulations, are not identified in pre-marketing stages; therefore, post-marketing monitoring becomes specially relevant14,15. Many studies have been conducted with the 4CMenB vaccine in paediatric population, but not so many in the adult population, beyond those conducted in specific closed populations, such as university campuses or labs with staff at risk10,16,17. Therefore, we have a lack of clinical trials, not only in the adult population but specifically for the group of adults in special situations. For this reason, this present research represents a novelty in terms of studying the safety of this vaccine in the adult population in special situations.
The frequency of adverse reactions on the sample under study has been compared with the one stated in the product specifications12. It is worth highlighting that the PS states that the frequency rate of fever for the >11-year-old and adult group is unknown, while this study has recorded a frequency >10% for both doses. These results coincide with what other authors have described: they have also found, although with great variability, that the frequency of developing fever is higher than expected6,9.
A recent systematic review of the safety of the 4CMenB vaccine vs. Trumenba® (a recently marketed vaccine against meningococcus B) and vaccines routinely administered in children and adolescents, reached the conclusion that the most frequently reported severe adverse reactions were febrile seizures18. These results cannot be compared with this study that has been conducted in adults in special situations; besides, only one severe case was identified. It is worth noting that the same review found a 74% prevalence of pain at the injection site, and a 24% prevalence of fever18; both results are higher than those found in our research, although matching the Product Specifications of the vaccine for this group12. On the other hand, the quantitative evaluation of local pain through the AVS represents an innovation regarding previous studies on the safety of the 4CMenB vaccine. So far, authors such as Santolaya and Grossner used qualitative scales where pain was rated according to its intensity: mild, moderate or severe8,9. However, using a quantitative scale allows more accurate comparisons of local pain intensity, and this scale is the most frequently used in research associated with pain measurement19,20. No significant differences have been found in terms of pain intensity between the first and the second doses. Unlike what other authors have done, i.e. administering Bexsero® and placebo, or Bexsero® and other vaccines9, in this case both doses were administered in the same conditions, and therefore these results cannot be compared.
Finally, the main limitation in our study is sample size. In this sense, the implementation of inclusion criteria and vaccination conditions, as well as the target population chosen to conduct this research, made it difficult to obtain a large sample within a reasonable period of time; in the future, it could be considered to conduct multicentre studies.
Summing up, vaccination against meningococcus B with Bexsero® in adults in special situations shows a good safety profile; however, there is limited information available, given the low number of patients studied. The frequency of fever in this population is higher than expected for the same age group according to the Product Specifications. Local pain is the adverse reaction most frequently reported for both vaccine doses; however, pain intensity is low.
FundingNo funding.
Conflict of interestsNo conflict of interests.
Contribution to the scientific literature.
The great majority of studies so far regarding the four-component vaccine against meningococcus B (4CMenB) have been conducted in paediatric population; only a minority of studies have been conducted in adults specifically healthy. This study is innovative not only in terms of its subjects, i.e. patients in special situations, but also for the use of the AVS pain scale unlike other studies for vaccine safety, where qualitative scales were used. It is important to highlight that the results shown by this study seem to differ with the information contained in the product specifications for the vaccine, mostly regarding fever >38°C, because this reaction is not described in clinical trials for the >11-year-old children and adults group. For this reason, it could be justified to extend this research, and to review the product specifications of the 4CMenB vaccine.