To determine the effectiveness of a pharmaceutical care intervention based on the CMO methodology (Capacity, Motivation and Opportunity) in improving primary adherence to concomitant treatment in HIV+ patients on antiretroviral treatment.
MethodThis was a longitudinal prospective multicenter study carried out between September 2019 and September 2020, which included HIV+ patients older than 18 years who were on antiretroviral treatment and were taking concomitant medications. Demographic, clinical, and pharmacotherapeutic variables were collected. As required by the CMO methodology, all patients were followed for 6 months and stratified into three levels of care. Individualized pharmaceutical care was provided according to the interventions established for each level. At every consultation, a motivational interview was conducted based on each patient's alignment with and achievement of their pharmacotherapeutic objectives. A website was developed to deal with the opportunity pillar. The main variable was the percentage of patients considered primary adherents to the prescribed concomitant medication. Adherence over the six months prior to the study was compared to adherence at the end of the study. Additionally, the percentage of patients considered secondary adherents to concomitant treatment and antiretroviral treatment during the 6 months prior to the start of the study was compared to the percentage of such patients at the end of the study. Adherence was measured based on dispensation records and specific validated questionnaires. Patients were only considered adherent if they were deemed adherent by both methods.
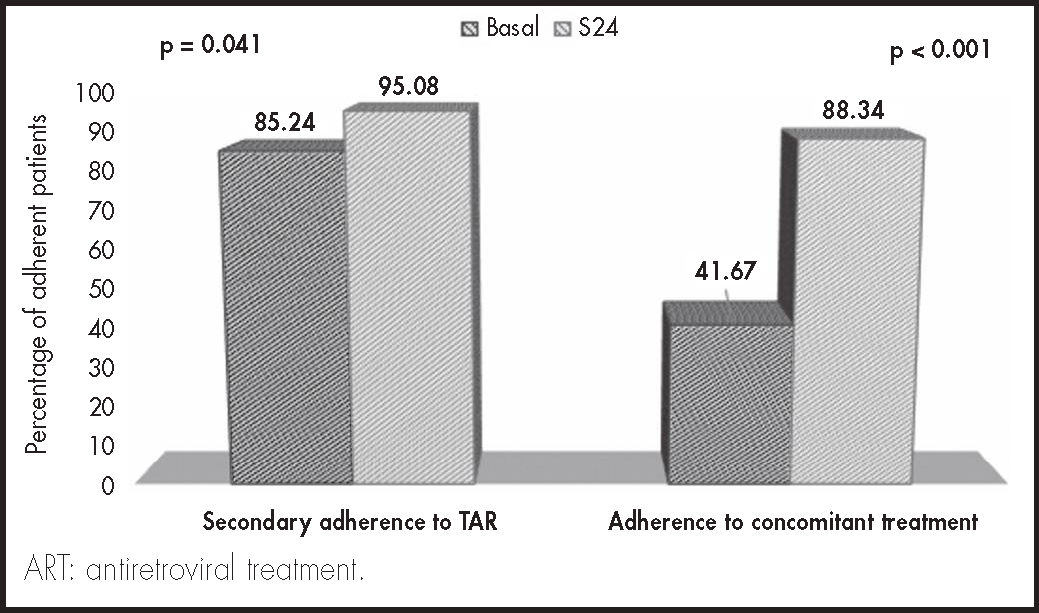
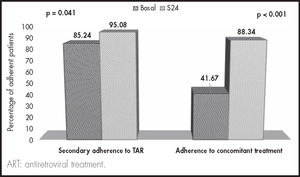
ResultsA total of 61 patients were included in the study, 72% male. Median age was 53 years and the median number of concomitant drugs prescribed was 7 A total of 60.6% of patients were polymedicated. The percentage of patients considered primary non-adherent was 52.5% at baseline (n = 32) and 4.9% (n = 3, p < 0.001) at the end of the study. Secondary adherence to both concomitant medication (41.6% vs 88.3%) and antiretroviral treatment (85.2% vs 95.1%) improved at the end of the study (p < 0.0001).
ConclusionsPharmaceutical care based on the CMO methodology significantly improved both primary and secondary adherence to concomitant drugs and to antiretroviral treatment.
Determinar la efectividad de una intervención farmacéutica, basada en la metodología CMO (Capacidad, Motivación, Oportunidad), para mejorar la adherencia primaria al tratamiento concomitante en pacientes VIH+ en tratamiento antirretroviral.
MétodoEstudio longitudinal, prospectivo, multicéntrico, realizado entre septiembre de 2019 y septiembre de 2020. Se incluyeron pacientes VIH+ mayores de 18 años, en tratamiento antirretroviral y prescripción de fármacos concomitantes. Se recogieron variables demográficas, clínicas y farmacoterapéuticas. Se realizó atención farmacéutica durante 6 meses según el modelo CMO en cada paciente, basado en su nivel de estratificación y las intervenciones establecidas para cada umbral. En cada consulta se realizó una entrevista motivacional basada en el alcance de los objetivos farmacoterapéuticos para cada paciente. Para desarrollar el pilar de oportunidad se creó y desarrolló la web: www.proyecto-pricmo.com. La variable principal fue el porcentaje de pacientes considerados adherentes primarios a la medicación concomitante prescrita, comparando los 6 meses previos al estudio, frente al mismo valor al finalizar el estudio. Adicionalmente, se comparó el porcentaje de pacientes adherentes secundarios al tratamiento concomitante y al tratamiento antirretroviral durante los 6 meses previos al inicio del estudio frente al mismo valor en los pacientes al finalizar el estudio. Para medir la adherencia se consideraron dos métodos: registros y cuestionarios validados específicos. Solo se consideraron adherentes si lo fueron a ambos métodos.
ResultadosSSe incluyeron 61 pacientes. El 72,0% fueron hombres, con una mediana de edad de 53 años. La mediana de fármacos concomitantes fue de 7 El 60,6% de los pacientes tenían presencia de polifarmacia. El porcentaje de pacientes considerados no adherentes primarios basalmente fue del 52,5% (n = 32), mientras que a la finalización fue del 4,9% (n = 3, p < 0,001). Tanto la adherencia secundaria a la medicación concomitante (41,6% versus 88,3%) como al tratamiento antirretroviral (85,2% versus 95,1%) mejoraron al finalizar el estudio (p < 0,001).
ConclusionesLa intervención farmacéutica basada en la metodología CMO mejoró significativamente tanto la adherencia primaria como secundaria a la medicación concomitante y la secundaria al tratamiento antirretroviral.
According to the ABC European Consensus, adherence to treatment involves active, cooperative, and voluntary involvement of a patient in following the recommendations made by the healthcare providers in charge of their care. The process comprises three steps: initiation, which is the moment the patient takes the first dose of the prescribed drug; implementation, which is related to the extent to which the prescription regimen is complied with; and discontinuation, which happens when the patient stops taking the prescribed medication for whatever reason1.
Adherence is a key factor for achieving successful treatment outcomes, especially in the case of chronic conditions. Generally speaking, the worse the adherence, the worse the health outcomes and, therefore, the patients’ quality of life. Adherence is also a critical determinant of healthcare costs.
Some authors have in the last few years taken a different view of adherence and the way it should be approached1. Against this background, the concept of primary adherence has come about as a complement to the classical form of adherence, which has now come to be called secondary adherence1. This means that primary non-adherence (PNA) can be defined as the failure on the part of the patient to collect the prescribed medication from the pharmacy shortly after it is prescribed. This constitutes a significant deviation from the expected pharmacological plan. Prevalence of PNA has been analyzed by various studies in the course of the last decade, especially in patients with chronic diseases (diabetes, hypertension, asthma, etc.)2-4. Borrego et al.5 showed that the prevalence of PNA among patients living with HIV (PLHIV) in Spain was of one-third. This negatively impacts the achievement of pharmacotherapeutic goals in these patients, particularly in this day and age when HIV+ individuals are getting to grow older, which makes them more prone to develop a higher number of comorbidities than observed in the non-HIV population6,7.
The new definition of pharmaceutical care (PC) advocates the participation of pharmacists in multidisciplinary teams to achieve the goals of pharmaceutical therapy through longitudinal follow-up and interventions guided by the clinical complexities shown by each individual patient. This methodology, inspired by the so-called CMO model, responds to the three basic pillars of PC: stratification (Capacity), pharmacotherapeutic goals (Motivation), and incorporation of new technologies to sustained patient follow-up (Opportunity)8,9.
Various studies have shown that application of this work methodology to these patients leads to improved health outcomes and an enhanced clinical experience. However, no study has as yet analyzed its potential effect in addressing PNA10-12.
The purpose of this study was to estimate the effectiveness of PC, as based on the CMO methodology in addressing PNA to concomitant treatment in PLHIVs receiving active antiretroviral treatment (ART). Secondary goals included an estimation of the effectiveness of PC in improving secondary adherence to ART and to its concomitant treatment.
MethodsThis was a longitudinal prospective multicenter study based on a structured healthcare intervention carried out between September 2019 and September 2020.
The study included PLHIVs older than 18 years of age who had been on ART for at least one year before inclusion. They were required to have been prescribed concomitant medication to ART by any doctor at least 6 months before initiation of the study and again at the beginning of the investigational period. All patients were required to give their informed consent to participate in the study. Patients participating in clinical trials that fully overlapped the follow-up period were excluded as were pregnant women and subjects refusing to sign the informed consent form.
The following demographic variables were recorded: age, sex, risk of contracting the disease and economic status (good, poor or very poor), relationship with healthcare providers (good, poor or very poor), understanding of the treatment and the condition (good, poor and very poor); clinical parameters (baseline parameters and those associated with HIV): plasma viral load (copies/mL), CD4 count (cells/mcL), CD4/CD8 ratio, cholesterol (mg/dL), HDL cholesterol (mg/dL), triglycerides (mg/dL), HbA1c (g/dL), blood pressure (mmHg); and pharmacotherapeutic variables such as ART prescribed during the study, type and number of concomitant medications, and presence or otherwise of polypharmacy (defined as the prescription of > 5 drugs a day)13.
Each subject was followed up for a mean of six months after inclusion. Patients who failed to show up for two consecutive pharmacotherapeutic follow-up appointments were removed from the study and classified as lost to follow-up, not being replaced by another subject.
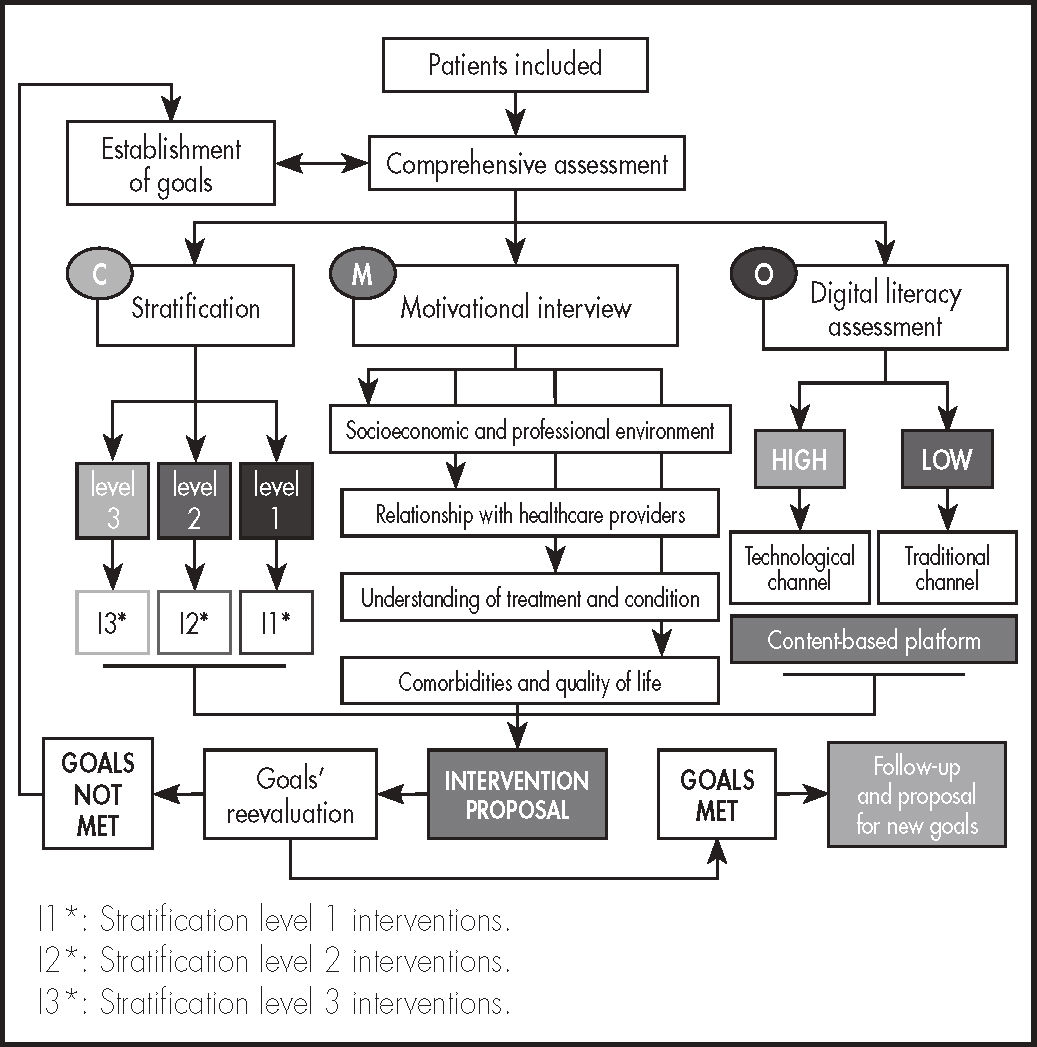
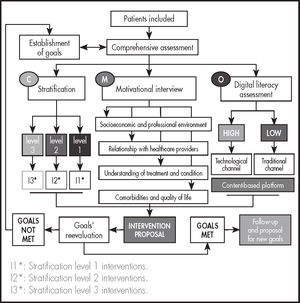
All patients were followed up using the CMO methodology (Figure 1). At the outset, patients were stratified into three levels, according to the criteria defined in the model developed by the Spanish Society of Hospital Pharmacists (SEFH) for PLHIVs14. The type of PC administered was more or less intensive as a function of the interventions defined for each level of care. At each face-to-face appointment at the hospital pharmacy department, a motivational interview was conducted to help patients achieve their individual pharmacotherapeutic goals, as a function of the evolution of their condition and their previous experience with the prescribed medications. The motivational interview was made up of two parts. The first part was devoted to identifying cases of potential resistance to treatment, helping patients identify any inappropriate behaviors, and reinforce their positive behaviors. In the second part patients were asked to reflect on their potential resistances with a view to helping them overcome them. At each interview, the patient's pharmacotherapeutic goals were (re)evaluated and, if appropriate, new goals were set by consensus between the patient and the medical team in charge of the case.
To develop the opportunity pillar, a specific (currently nonoperational) website was created (www.proyecto-pricmo.com) (Figure 2), which included a series of informative contents on the importance of adherence, with videos, infographics, diptychs, links to other websites, articles and other relevant contents. This tool was available and kept constantly updated throughout the project to ensure that patients could access the materials published at any time, according to their level of digital literacy.
Several ways of contacting their hospital pharmacists were made available to study participants (telephone, e-mail etc.) so that they could at any time clarify their doubts about their treatment.
The main variable was the percentage of patients considered to be primary adherents to the concomitant medication prescribed at baseline. Adherence over the previous six months was compared with adherence at the end of the study.
Primary adherence was defined as filling the prescribed medication at the community pharmacy within 14 days.
To achieve the secondary goal of the study, the percentage of patients considered secondary adherents to concomitant treatment over the six months prior to the initiation of the study was compared with the percentage of secondary adherents at the end of the study.
Secondary adherence to concomitant treatment was defined as the total number of days patients took the medication in accordance with the guidelines provided by the prescribing physician during the follow-up period15. To determine adherence, an analysis was made of the community pharmacy dispensation records (adherence was considered to occur only if the adherence/multiple dispensation interval ratio was higher than 90%) and of the score obtained on the e-ARMS questionnaire16.
A comparison was also made between the percentage of patients adhering to ART over the previous six months and at the end of the study. Adherence was evaluated by means of the outpatient dispensation records (adherence was considered to occur only if the adherence/multiple dispensation interval ratio was higher than 90% and the score on the validated SMAQ17 questionnaire was positive).
The data on the concomitant treatment as well as the corresponding dispensations at the community pharmacy were obtained from the IT systems of the different participating hospitals. The remaining variables were obtained in the course of the face-to-face interviews at the outpatient facilities of the participating hospital pharmacies and from the patients’ unified medical records.
As regards the statistical analysis of the data, quantitative variables were summarized with means and standard deviations, or with medians and P25 and P75 percentiles for asymmetrical distributions; quantitative variables were summarized with frequencies and percentages. Student's t test was used to compare the means of the quantitative variables and the Wilcoxon singed-rank test was used to compare related samples. Normality of the data had been previously determined using the Kolmogorov-Smirnov test to decide whether parametric or non-parametric tests should be used. McNemar's test was used to analyze the relationship between quantitative variables. The relationship between the different quantitative variables was established using Spearman's correlation coefficient; the Mann-Whitney U test was used for unrelated samples. The data was analyzed using the R studio v 1.1.456 software package.
The study was approved by the Research Ethics Committee of the Seville South Health Area (1841-N-17).
ResultsA total of 65 patients were recruited for the study, of which 61 were finally included in the statistical analysis as four were lost to follow up. The baseline characteristics of the study subjects are shown in table 1. Seventy-two percent of the sample were male, with a median age of 53 years (IQR: 51-58). Patients were receiving different drug combinations as ART: two reverse transcriptase inhibitors (RTIs) + an integrase inhibitor: 42.6%; two RTIs + a non-nucleoside reverse transcriptase inhibitor (NNRTI): 16.4%, two RTIs + a protease inhibitor: 11.5%; other single/ multiple therapies: 29.5%. During the follow-up period three clinically motivated changes were introduced in the drug combinations administered. The ART regimens administered thereinafter were as follows: two RTIs + an integrase inhibitor: 47.5%; two RTIs + an NNRTI: 11.5%, two RTIs + a protease inhibitor: 11.5%; other single/multiple therapies: 29.5%. No statistically significant differences were observed regarding the prescription percentages.
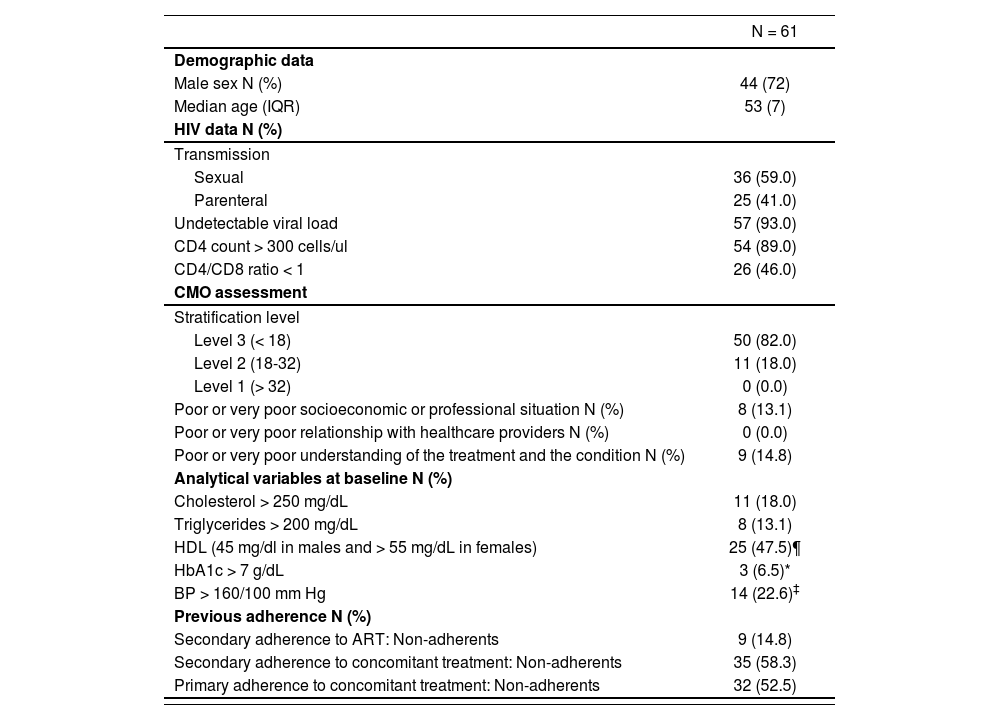
Baseline characteristics of patients included in the study
| N = 61 | |
|---|---|
| Demographic data | |
| Male sex N (%) | 44 (72) |
| Median age (IQR) | 53 (7) |
| HIV data N (%) | |
| Transmission | |
| Sexual | 36 (59.0) |
| Parenteral | 25 (41.0) |
| Undetectable viral load | 57 (93.0) |
| CD4 count > 300 cells/ul | 54 (89.0) |
| CD4/CD8 ratio < 1 | 26 (46.0) |
| CMO assessment | |
| Stratification level | |
| Level 3 (< 18) | 50 (82.0) |
| Level 2 (18-32) | 11 (18.0) |
| Level 1 (> 32) | 0 (0.0) |
| Poor or very poor socioeconomic or professional situation N (%) | 8 (13.1) |
| Poor or very poor relationship with healthcare providers N (%) | 0 (0.0) |
| Poor or very poor understanding of the treatment and the condition N (%) | 9 (14.8) |
| Analytical variables at baseline N (%) | |
| Cholesterol > 250 mg/dL | 11 (18.0) |
| Triglycerides > 200 mg/dL | 8 (13.1) |
| HDL (45 mg/dl in males and > 55 mg/dL in females) | 25 (47.5)¶ |
| HbA1c > 7 g/dL | 3 (6.5)* |
| BP > 160/100 mm Hg | 14 (22.6)‡ |
| Previous adherence N (%) | |
| Secondary adherence to ART: Non-adherents | 9 (14.8) |
| Secondary adherence to concomitant treatment: Non-adherents | 35 (58.3) |
| Primary adherence to concomitant treatment: Non-adherents | 32 (52.5) |
ART: antiretroviral treatment; BP: Blood Pressure; HDL: High Density Lipoprotein; IQR: interquartile range.
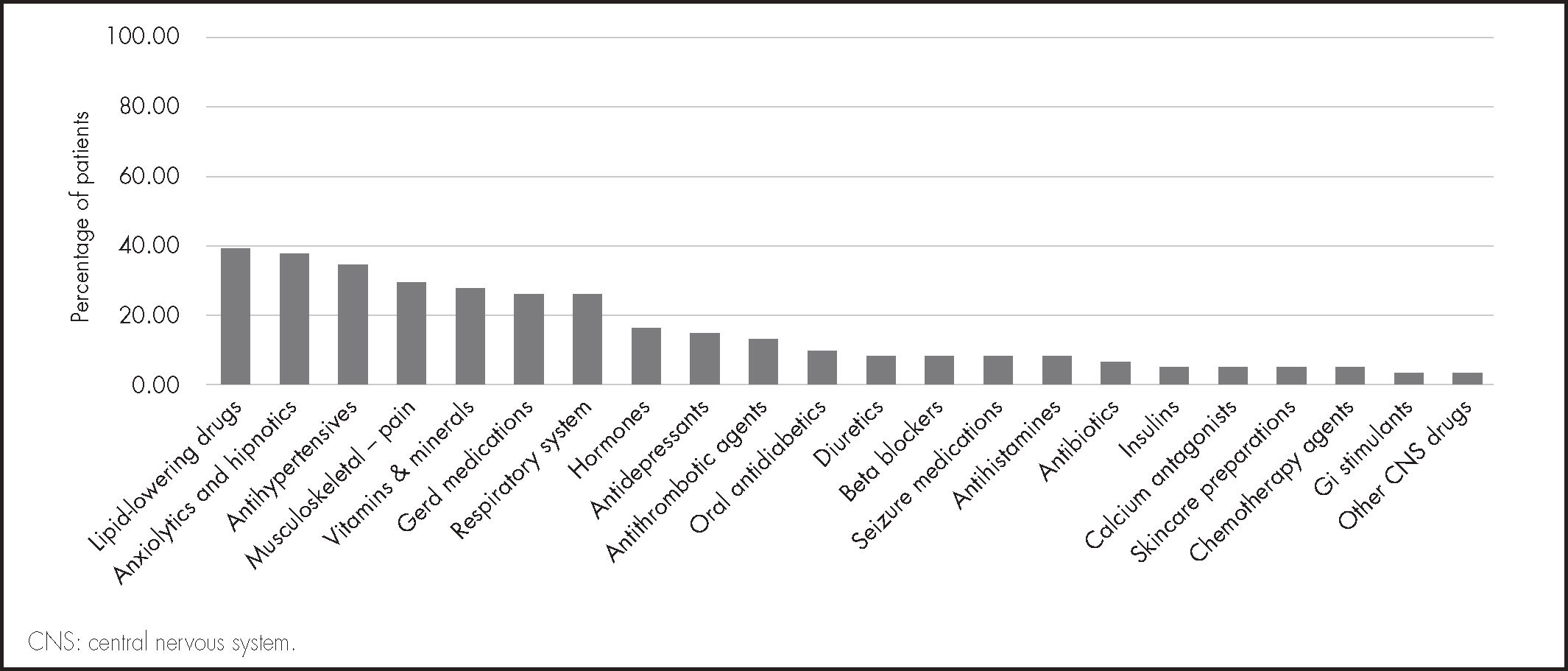
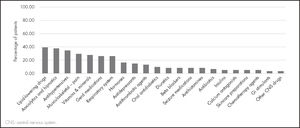
The median of concomitant drugs used was 7 (IQR: 5-8). The most common concomitant treatments included: lipid-lowering drugs, anxiolytics and hypnotics, and antihypertensives (Figure 3). A total of 60.6% of patients were polymedicated.
As regards the main variable of the study, the percentage of patients considered primary non-adherents at baseline was 52.5% (n = 32), while at the end of follow-up the percentage fell to 4.9% (n = 3, p < 0.001). The evolution of secondary variables related to adherence to concomitant treatment and to ART are shown in figure 4. Significant improvements were observed in the two cases at the end of follow-up (p < 0.001).
DiscussionThe present study showed that a pharmaceutical intervention based on a stratification of patients, a motivational interview, and the use of new technologies can improve adherence to concomitant medication in PLHIVs on active ART.
According to the literature, PNA in patients with chronic conditions may be associated with varying levels of prevalence depending on how PNA is defined, the context where it is applied and the methodology used, In spite of this, most authors seem to agree that it stands around 20%18. When looking at the most commonly used medications, PNA is around 25% for lipid-lowering, osteoporosis and antiasthmatic drugs; 16% for antihypertensives, 12% for antidepressants, and 10% for antidiabetics19,20. The majority of these drugs were part of the concomitant medication regimens prescribed for the population included in the present study, although PNA for these drugs was considerably higher at baseline, which is indicative of the complexity of addressing PAS in this patient population as well as the promise offered by the results obtained.
According to the literature, adherence in the chronic setting is influenced by several factors, including socioeconomic factors (e.g., being in work or unemployed), those related to the individual healthcare system (e.g., access to medications or relationship with healthcare providers), with the condition (e.g., presence of symptoms) or with the patient themselves (e.g., educational or cognitive aspects, beliefs and attitudes toward health). More specifically, in the case of PLHIVs -particularly those of advanced age- the growing dependance on multiple medications and the increasing complexity of drug treatments are frequently associated with non-adherence to concomitant medication21,22. Both those factors were observed in our study: median patient age exceeded the 50-year-old threshold set for successful treatment of older PLHIVs13, and the percentage of patients on polypharmacy (60%) was also higher than what is normal for Spain23.
Several authors have proposed specific interventions to avoid PNA in specific types of patients. In the last few years such interventions have focused on the incorporation of new technologies to patient follow-up (e.g., SMS reminders) even in the context of randomized clinical trials24. Other authors have resorted to cellphone calls or specific apps as tools to bring home the importance of an accurate diagnosis and timely initiation of the appropriate treatment in order to ensure primary adherence25,26. The role of the motivational interview has also been increasingly identified with significant benefits in patients with chronic conditions27-29.
Given the increasingly complex and multidimensional nature of PLHIV treatment, the present study resorted to a different approach, which was based not only on a multidisciplinary methodology or a specific therapeutic tool or enhancement such as new technologies or motivational interviews but, above all, on understanding the individual characteristics of each subject and designing individualized longitudinal interventions tailored to their complexity21,30. This was possible thanks to the stratification carried out, which took into account the influence of different variables broken down into specific health domains for each individual. This made it possible to provide patients with individualized care, whose intensity could be adjusted over time. This methodology had been used in previous studies, where it showed itself to lead to improved health outcomes as a result of enhanced secondary adherence to concomitant treatments10,11. To the best of our knowledge, this is the first study that used a specific web-based environment, with both written and visual materials, to reinforce the message even further. This has made it possible to focus all attention on the patient rather than on any problems related with their medication, the priority being the prompt achievement of the pharmacotherapeutic goals of each patient type in the study. The result has been a significant improvement in secondary adherence both to concomitant medication and to ART, where baseline adherence was already high.
This study presents with several limitations. Firstly, there was only an intervention group, without a control group. Given the growing expansion, understanding, and adoption of the CMO model by hospital pharmacists, and the results of the studies conducted in the last few years, it was thought that inclusion of a control group could lead to some degree of subject bias as interventions would be likened. For that reason, it was considered that the best way of analyzing the influence of the pharmaceutical intervention was a before-and-after design where each patient was their own control. In addition, the follow-up period used in the study (6 months) is a relatively short period in the lifetime of a PLHIV on chronic treatment. However, from a methodological point of view, 6 months is long enough to determine the impact of a structured healthcare intervention and to plan for and achieve the goals established for a specific period in the lifetime of these patients, as these are dynamic and are influenced by the different health domains they manifest.
In view of the lack of any classification to that effect in the literature, no investigation was made into the reasons why patients were not primary adherents to the medication (e.g., mistrust of diagnosis, high cost of treatment, incompatibility with lifestyle, etc.). As such information was unavailable for the period prior to the intervention, and there was no published classification to go by, a decision was made to focus exclusively on prevalence.
Future research should look into which patient characteristics (cognitive, socioeconomic, nutritional, self-care, discomfort, anxiety, etc.) are more prone to be influenced by the methodology applied, and whether there are polypharmacy or therapeutic complexity thresholds that may predict which patients will be more likely to be primary non-adherent. This would make it easier to approach them in a more individualized way, both through technologies based on the healthcare information systems and with the methodology proposed in this study. Moreover, longer-term investigations will allow identification of the health outcomes and clinical benefits (fewer additional appointments and hospital admissions etc.) that the strategies based on the proposed methodology may allow.
In short, pharmaceutical interventions based on the CMO PC model, which involve patient stratification, the establishment of pharmacotherapeutic goals, motivational interviews, and longitudinal follow-ups enabled by new technologies, are able improve primary and secondary adherence to concomitant medication and to ART.
FundingThe project was funded by a SEFH research grant in 2017.
AcknowledgementsThe authors would like to thank SEFH's pharmaceutical care of HIV+ patients and pharmacotherapeutic adherence (ADHEFAR) working groups for their support in creating, developing and disseminating the contents of this project.
Conflict of interestNo conflict of interest.
Contribution to the scientific literature
Primary non-adherence to concomitant medication is a serious problem in the context of HIV+ patient care.
The study shows that the CMO pharmaceutical care methodology, based on stratification, a motivational interview and new technologies, can improve both primary and secondary adherence in these patients.
Member of the research group of PRICMO Project:
María de las Aguas Robustillo-Cortés. Hospital Juan Ramón Jiménez. Huelva.
Ana Morillo Mora. Hospital Serranía de Ronda (Málaga).
Leticia Soriano Irigaray. Hospital de Elche (Alicante).
Ana García Monsalve. Hospital de Elche (Alicante).
Miguel Ángel Rodríguez Sagrado. Hospital Ramón y Cajal (Madrid).
Rosario Santolaya Perrin. Hospital Príncipe de Asturias. Alcalá de Henares (Madrid).
Sergio García Porto. Hospital Príncipe de Asturias. Alcalá de Henares (Madrid).
Rocío Díaz Acedo. Hospital Universitario de Valme. AGS Sur de Sevilla. Sevilla.
Early Access date (07/07/2021).