To describe the effectiveness and safety of remdesivir in patients with SARS-CoV-2 pneumonia in real-world clinical practice conditions.
MethodRetrospective observational study that included all adults with SARS-CoV-2 pneumonia admitted at the Moisés Broggi Hospital and treated with remdesivir between July 1st and November 7th, 2020. Efficacy outcomes were time to recovery, 28-day mortality, length of hospital stay, and the need of mechanical ventilation after treatment. The main safetyrelated endpoint was elevation of transaminases after treatment.
ResultsA total of 111 patients were included of whom 97 (87.4%) were receiving low-flow oxygen therapy. Median time to recovery was 9 days [6-14]. Seven patients (6.3%) died at 28 days’ follow-up. Median length of hospital stay was 12 days [9-22] and 15 patients (13.5%) needed mechanical ventilation after treatment with remdesivir. Severe hypertransaminasemia was observed in 4 patients (4%).
ConclusionsClinical outcomes of patients with SARS-CoV-2 pneumonia on low-flow oxygen therapy treated with remdesivir were similar to those published in clinical trials, both in terms of time to recovery and 28-day mortality.
Describir la efectividad y seguridad de remdesivir en pacientes con neumonía por SARS-CoV-2 en condiciones de práctica clínica real.
MétodoEstudio observacional retrospectivo que incluyó a todos los pacientes tratados con remdesivir en el Hospital Moisès Broggi entre el 1 de julio y el 7 de noviembre de 2020. Como variables de efectividad se registraron el tiempo hasta la recuperación, la mortalidad a los 28 días, la estancia hospitalaria y la proporción de pacientes que requirió ventilación mecánica invasiva tras el tratamiento. Como variable de seguridad se registró la alteración en las transaminasas tras el tratamiento.
ResultadosSSe incluyeron 111 pacientes, 97 (87,4%) con oxigenoterapia de bajo flujo. El tiempo hasta la recuperación fue de 9 días [6-14] de mediana y 7 pacientes (6,3%) habían fallecido a los 28 días de seguimiento. La estancia hospitalaria fue de 12 días [9-22] de mediana. Un total de 15 pacientes (13,5%) requirió ventilación mecánica invasiva tras el tratamiento y 4 pacientes (4%) presentaron una alteración grave de las transaminasas.
ConclusionesEl tratamiento con remdesivir en la práctica clínica habitual presenta resultados similares a los publicados en los ensayos clínicos en el subgrupo de pacientes con oxigenoterapia de bajo flujo, tanto en el tiempo hasta la recuperación como en la mortalidad a los 28 días.
Several drugs have been analyzed to determine their efficacy against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) since the first few cases of SARS-CoV-2 pneumonia were diagnosed in December 2019 in Wuhan1. The results of such analyses have been rather disheartening as most of the drugs tested did not prove effective2-4. The only drugs that have so far been shown to reduce mortality in patients with severe SARS-CoV-2 pneumonia are dexamethasone5 and, more recently, tocilizumab6.
Remdesivir is a prodrug of an adenosine nucleotide that is metabolized within the host cells into its active adenosine triphosphate analog form, which inhibits the virus’ RNA polymerase. Remdesivir has demonstrated in vitro antiviral activity against SARS-CoV-27. It has also achieved a shorter time-to-recovery (TTR) vs. placebo8, with one study even reporting a reduction in 14-day mortality, which was not maintained at 28 days8-10.
Since the end of June 2020, Spanish hospitals have been able to use remdesivir as a compassionate treatment through the Spanish Drug Agency (AEMPS)'s expanded access program. Treatments under the program require AEMPS’ authorization which is granted on the basis of a series of clinical criteria that have evolved with time11.
The purpose of this study is to describe the efficacy and safety of remdesivir in patients with severe SARS-CoV-2 pneumonia hospitalized in the Moisès Broggi Hospital.
MethodsDesign and data collectionThis was a retrospective observational study that included all the patients with SARS-CoV-2 pneumonia who were treated with remdesivir in the Moisès Broggi Hospital, a 380-bed second-level hospital in Sant Joan Despí, Barcelona. The study period extended from 1 July to 7 November 2020. The data was obtained from the patients’ electronic medical records.
The study was approved by the Research Ethics Committee of Fundació Institut d'Investigació Biomèdica de Bellvitge (IDIBELL), which the Moisès Broggi hospital is affiliated to.
Patients and treatmentAll patients treated with remdesivir in accordance with the criteria defined by AEMPS were included in the study. All the applications were filed by the Pharmacy Department once the patients had signed the relevant informed consent forms. Treatments had to be authorized by AEMPS before they could be administered.
The treatment comprised an intravenous dose of 200 mg on the first day and of 100 mg daily between the second and the fifth day in patients on low-flow oxygen therapy, high-flow oxygen (HFO) therapy, or noninvasive mechanical ventilation (NIMV). In patients on invasive mechanical ventilation (IMV) or extracorporeal membrane oxygenation (ECMO) the treatment could last up to 10 days. However, as a result of the limited supply of remdesivir available and the publication of new evidence, in August 2020 AEMPS established the maximum duration of treatment at 5 days for all patients11-13.
In September 2020, the AEMPS published the last modifications to the conditions under which remdesivir had to be used11. According to these, administration of remdesivir came to be restricted to adult patients with severe SARS-CoV-2 pneumonia who required low-flow oxygen therapy and who presented in the first seven days from symptoms’ onset. Patients were required to meet the following criteria: respiratory rate ≥ 24, baseline oxygen saturation (SpO2) ≤ 94%, or partial pressure arterial oxygen/fraction of inspired oxygen (PaO2/FiO2) ratio < 300.
Exclusion criteria to receive remdesivir were: need of NIMV, HFO, IMV or ECMO; alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) levels ≥ 5 times the upper limit of normal (ULN); glomerular filtration rate ≤ 30 mL/min, hemodialysis or peritoneal dialysis treatment; pregnancy or breastfeeding; evidence of multiorgan failure; and need of inotropic drugs to maintain blood pressure.
Variables analyzedThe following baseline variables were collected: age, sex, comorbidities, age-adjusted Charlson index, body mass index (BMI) and oxygen saturation/fraction of inspired oxygen ratio (SpO2/FiO2) at initiation of remdesivir treatment. Correlating the value of the latter with the PaO2/FiO2 ratio allows an assessment of the presence of initial respiratory distress14.
The treatment-related variables considered included: duration of treatment with remdesivir, days from symptoms's onset to initiation of remdesivir, and concomitant treatments (dexamethasone and tocilizumab).
The effectiveness variables recorded were: TTR, 28-day mortality, length of hospital stay and need of IMV after initiation of remdesivir. Following previously published studies7, recovery was defined as the first day during follow-up that the patient met the criteria laid down in items 1, 2 or 3 of the following 8-item ordinal scale: 1) not hospitalized with no limitations on activities; 2) not hospitalized, with limitations on activities and/or requiring home oxygen; 3) hospitalized, not requiring oxygen or ongoing medical care (used when hospitalization is longer than expected for non-medical reasons); 4) hospitalized, not requiring supplemental oxygen but requiring medical care in connection with SARS-CoV-2 or other reasons; 5) hospitalized, requiring supplemental oxygen; 6) hospitalized, requiring NIMV or HFO therapy; 7) hospitalized, requiring IMV and/or ECMO; and 8) death.
Safety was evaluated by analyzing any alterations in AST and/or ALT levels. Toxicity was rated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 515. CTCAE uses the following AST/ALT levels to assess severity: grade 1 (mild, AST/ALT 3 times > ULN; grade 2 (moderate, AST/ALT 3-5 times > ULN); grade 3 (severe, AST/ALT 6-20 times > ULT); and grade 4 (life-threatening, AST/ALT more than 20 times > ULN).
Statistical analysisCategorical variables were expressed as counts and percentages while continuous variables were expressed as medians and first and third quartiles, unless mean and standard deviation (SD) were specifically specified. Differences across groups were evaluated using the Chi-squared test in the case of categorical variables and the Mann-Whitney U test for continuous variables. Student's t test was used to compare the means of paired samples, indicating the difference and its 95% confidence interval (95% CI). Differences were considered statistically significant if p value was < 0.05. The Stata 15.1 software package (Stata Corp., College Station, TX, USA) was used to carry out the statistical analysis and prepare the graphs included in the study.
ResultsDuring the study period, 111 patients were administered remdesivir in our hospital. None of the applications filed by our hospital under the expanded access program was rejected by the AEMPS.
The subjects’ baseline characteristics are shown in table 1. Median age was 56.8 years [43.6-67.8] and 81 (73%) subjects were male. Mean score on the age-adjusted Charlson index was 1 [0-4]. A total of 51 patients (45.9%) presented obesity (BMI > 30 kg/m2). The most common comorbidities were hypertension [27 patients (24.3%)] and diabetes [25 patients (22.5%)]. At the time of initiation of remdesivir, 97 patients (874%) required low-flow oxygen therapy (score 5 on the ordinal scale), 8 patients (7.2%) required HFO or VMNI (score 6), 5 patients (4.5%) required IMV (score 7) and only one patient (0.9%) did not need oxygen therapy (score 4). As regards concomitant treatments, 88 patients (79.3%) received dexamethasone and 15 (13.5%) tocilizumab.
Baseline characteristics of patients treated with remdesivir
| Age (years) [interquartile range] | 56.8 [43.6-67.8] |
|---|---|
| < 50 years | 41 (36.9) |
| 50-69 years | 49 (44.1) |
| > 69 years | 21 (18.9) |
| Male sex, n (%) | 81 (73) |
| Age-adjusted Charlson score [interquartile range] | 1.0 [0.0-4.0] |
| BMI > 30 kg/m2, n (%) | 51 (45.9) |
| Time from onset of symptoms to initiation of remdesivir, days [interquartile range] | 6 [4-8] |
| Initial SpO2/FiO2 [interquartile range] | 294.7 [276.6-360.5] |
| Patients with initial respiratory distress (SpO2/FiO2 < 315), n (%) | 81 (73.0) |
| Comorbidities, n (%) | |
| Hypertension | 27 (24.3) |
| Diabetes | 25 (22.5) |
| Chronic heart disease | 15 (13.5) |
| Chronic renal disease | 6 (5.4) |
| Cancer | 5 (4.5) |
| Chronic pulmonary disease | 4 (3.6) |
| Chronic liver disease | 3 (2.7) |
| Initial score on the ordinal scale, n (%) | |
| 4. Hospitalized patient, not requiring oxygen | 1 (0.9) |
| 5. Hospitalized patient, requiring low-flow oxygen | 97 (87.4) |
| 6. Hospitalized patient, requiring high flow oxygen or NIMV | 8 (7.2) |
| 7. Hospitalized patient, requiring IMV or ECMO | 5 (4.5) |
BMI: body mass index; ECMO: extracorporeal membrane oxygenation; IMV: invasive mechanical ventilation; NIMV: noninvasive mechanical ventilation; SpO2/FiO2: oxygen saturation-fraction of inspired oxygen ratio.
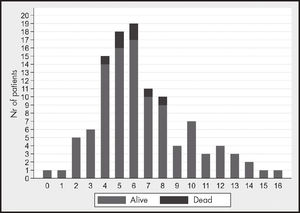
The data on treatment and its efficacy are presented in table 2. Median length of treatment was 5 days [5-5]. Remdesivir had to be discontinued in 10 patients for the following reasons: clinical deterioration (8 cases), hepatic toxicity (2 cases) and death of the patient (1 case). Mean time from symptoms's onset to initiation of remdesivir was 6 days [4-8], with 76 patients (68.5%) started on remdesivir within the first 7 days from symptoms's onset. Figure 1 shows the distribution of patients according to the time of initiation of remdesivir. Once treatment was initiated, 29 patients (26.1%) required admission to the ICU and 20 patients (18%) required initiation of IMV. Median TTR among patients who received the treatment was 9 days [6-14] and 7 patients (6.3%) had died at 28 days’ follow-up.
Variables related with remdesivir and its efficacy
| Length of treatment with remdesivir, days [interquartile range] | 5 [5-5] |
| Time from onset of symptoms to initiation of treatment, days [interquartile range] | 6 [4-8] |
| Patients who initiated remdesivir “early”, n (%) | 76 (68.5) |
| Time to recovery, days [interquartile range] | 9 [6-14] |
| 28-day mortality, n (%) | 7 (6.3) |
| Length of hospital stay, days [interquartile range] | 12 [9-22] |
| Patients admitted to ICU following initiation of remdesivir, n (%) | 29 (26.1) |
| Patients initiating IMV following initiation of remdesivir, n (%) | 15 (13.5) |
ICU: intensive care unit; IMV: invasive mechanical ventilation.
The patients who died were significantly older (median of 69.8 years [52.1-85.3] vs. 54.9 years [42.9-66.2] of age, p = 0.024) and presented with more comorbidities (median Charlson index of 3 [1-6] vs. 1 [0-3]), although this difference did not reach statistical significance (p = 0.0667).
No significant differences in mortality were found when comparing patients who received dexamethasone with those who did not; 7 of the 88 (8%) who received dexamethasone died vs. 0 of the 23 (0%) who did not (p = 0.1623). Nor were any mortality-related statistically significant differences found between patients who received tocilizumab and those who did not: one of the 14 (6.7%) patients on tocilizumab died vs. 6 of the 96 patients (6.3%) who were not on the drug (p = 0.9508).
As regards adverse events, 51 patients (46%) presented with higher-than-normal AST and/or ALT concentrations. Elevations were mild in 37 cases (33%) (grade 1 on the CTCAE scale), moderate in 10 cases (9%) (grade 2 on the CTCAE scale) and severe in 4 cases (4%) (grade 3). No patients presented with a grade 4 alteration. The initial mean AST value was 52.8 U/l (SD 34.8) and the final mean value was 64.7 U/l (SD 58.9), with the difference between final and initial mean AST values not reaching statistical significance (11.9 U/L, 95% CI: -2 to 25.9, p = 0.0928). A statistically significant difference of 45.2 U/L (95% CI: 26.4 to 64.0. p < 0.0001) was however found between initial and final mean values for ALT (55.2 U/L, SD 56.7 vs. 100.4 U/L, SD 85.1). Changes in AST and/or ALT could not be determined in 13 patients (11%) because of the lack of follow-up data.
DiscussionThis study reports on the clinical results of remdesivir in patients with severe SARS-CoV-2 pneumonia in real-world clinical practice with respect to AEMPS’ access criteria.
TTR in our study was 9 days. This contrasts with the findings of Wang et al.9, who reported a TTR of 21 days, which was similar to that in patients on in the placebo group. This difference can be explained by several factors. For a start, mean time from onset of symptoms to initiation of treatment in Wang et al. was 11 days, whereas in our study it was nearly half (6 days). According to these authors, earlier administration of treatment could result in a more efficient inhibition of viral replication, as shown in some animal studies16. Secondly, Wang et al.'s definition of the TTR parameter was different. Finally, patients included in that study were older (65 years or older) and exhibited a higher incidence of hypertension (46%) than patients in this study. Age, hypertension, diabetes and obesity are, among others, negative prognostic factors for patients with SARS-CoV-2 pneumonia17-19.
Beigel et al.8 reported a TTR of 10 days with statistically significant differences with respect to subjects treated with placebo where TTR was 15 days. Those authors used the same TTR definition as in this study, which means that their data is comparable to the one obtained here. The subgroup analysis of this study resulted in a TTR for patients on low-flow oxygen therapy of 7 days. It is important to note that in our study almost 90% of the patients required low-flow oxygen therapy at the time on initiation of remdesivir. Although our results are similar to Beigel et al.‘s (median TTR was 9 days), it must be considered that the incidence of hypertension and diabetes in that study was considerably higher (50.7% and 30.6% respectively) that in our population. In contrast, the incidence of obesity was similar (45.4%) in both studies. These considerations, added to the fact that few of our patients received IMV as initial treatment, could indicate that our population had less severe initial SARS-CoV-2 pneumonia than Beigel et al.‘s8 and, for that reason, it would be expected to find a shorter TTR in our study.
Twenty-eight-day mortality was 6.3% in our population. This rate is lower than that reported for patients treated with remdesivir in Beigel et al.8 (11.4%), Wang et al.9 (14%) or the SOLIDARITY trial4 (11%), none of which were able to demonstrate a reduction in mortality with respect to the control group. Beigel et al.'s8 subgroup analysis found a 4% 28-day mortality in patients on initial low-flow oxygen therapy (score 5 on the CTCAE scale) who received remdesivir, with statistically significant differences with respect to placebo, which should be confirmed by randomized clinical trials20. This mortality rate is similar and rightfully comparable to the one found in this study (6.3%) as most of our patients exhibited the same profile at the initiation of treatment. Nonetheless, patients in our study were initiated on remdesivir earlier than those in Beigel et al.8, had a lower incidence of hypertension and diabetes, and mostly received dexamethasone. It is therefore difficult to claim that remdesivir had a beneficial effect on mortality in our study given that the variable could have been affected by all the factors mentioned.
As regards the potential effect of dexamethasone on the results of our cohort, although no statistically significant mortality differences were found between the patients treated with dexamethasone and those who were not, the numerical difference was certainly striking (8 vs. 0 deaths, respectively). This might be explained by the fact that our hospital's clinical protocol only recommended dexamethasone to be administered to patients with SpO2 ≤ 94% from the seventh day following the onset of symptoms. These are therefore more severely ill patients than those who do not receive dexamethasone. Regarding the potential effect of tocilizumab, only 14 patients (13%) in our cohort were treated with tocilizumab. Although no significant differences were found between patients who received tocilizumab and those who did not, it is not possible to draw any conclusions in this respect given the low number of patients treated with this drug.
García-Vidal et al.21 recently published real world results of the use of remdesivir in 123 COVID-19 patients in a third-level hospital in Spain. The authors found similar mortality results among patients treated with remdesivir as those reported in this study (4.1% vs. 6.3%, respectively), However, ICU admissions and the need of IMV were higher in our cohort (26.1% vs. 19.5% and 18% vs. 7.3%, respectively). Patients in that study exhibited similar baseline characteristics as those in presented here in terms of median age (58 years) and median time of initiation of remdesivir (7 days from symptoms’ onset). However, patients in the García-Vidal et al. presented more comorbidity than those in this study.
According to the SEMI-COVID-19 Register22 mortality among people between 50 and 69 years (which was the majority age group patients in this study belonged to) stood between 4.7% and 10.5% during the first wave of the pandemic. Although the proportion of patients receiving remdesivir in the register was low (0.5%), the mortality data reported is similar to that in this study. This is in correspondence with the evidence published to date, which indicates that remdesivir has not as yet been able to demonstrate an ability to reduce 28-day mortality in patients with SARS-CoV-2 pneumonia8-10,20.
As regard the treatment's toxicity, nearly half of the patients exhibited increased transaminase levels, although only 4% presented grade 3 toxicity. This is in line with the findings of Beigel et al8, where elevation of AST occurred in 3.4% of patients and that of ALT in 2.3%. As the authors state in the appendix to the study, these elevations resulted mainly from grade 3 or 4 adverse events. Although the ALT elevation observed in this cohort was statistically significant, it must be taken into consideration that in spite of the time relation existing between initiation of remdesivir and the appearance of hypertransaminasemia, SARS-CoV-2 infection can cause elevated transaminase levels due to liver inflammation.
No other adverse events were observed that could help establish the toxicity profile of remdesivir treatment. This could have been due to the retrospective nature of the study, which could have led to an underestimation of adverse events.
The main strength of this study is that it closely reflects real-world clinical practice in Spain. However, it does present a few limitations. Firstly, it is a retrospective observational study with no control group, which precludes the establishment of a direct cause-effect relationship between the intervention carried out and the results obtained. Secondly, the fact that the criteria for accessing remdesivir changed during the course of the study made it impossible to divide the patient sample into different subgroups and analyze the effect the treatment had on each of them. In spite of that, the majority of patients were receiving low-flow oxygen therapy which, according to some authors8, could be the group of patients deriving the greatest benefit from remdesivir. This hypothetical benefit should be demonstrated in future clinical trials that focus exclusively on those patients20.
In conclusion, treatment with remdesivir in our hospital yields similar results to those published for patients with low-flow oxygen therapy both in terms of TTR and 28-day mortality.
FundingNo funding.
Conflict of interestsNo conflict of interests.
Contribution to the scientific literature
The present study is intended to describe the authors’ experience of the use of remdesivir for treating patients with SARS-CoV-2 pneumonia in clinical practice. The majority of patients treated with remdesivir presented with respiratory failure and required low-flow oxygen therapy at the time of initiation of the drug. They all received early treatment (≤ 7 days from onset of symptoms). According to the literature, this subgroup of patients is the one that could obtain the greatest benefit from treatment with remdesivir.
Although the results of our study in line with those of clinical trials, such findings must be taken with care as no control group was used. It is therefore necessary to carry out a placebo-controlled clinical trial on COVID-19 patients on low-flow oxygen therapy in order to determine the real efficacy of this treatment.
Early Access date (08/02/2021).