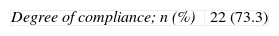To design a therapeutic exchange protocol for antidepressants and clinically assess variables, such as compliance level, frequency of cases with clinically significant increase on the Udvalg-für-Kliniske-Undersogelser (UKU) psychopharmacological scale, adverse effects analysis, overall analysis of UKU rating development and patients’ level of acceptance. Secondary objectives were to correlate psychopharmacological treatment aspects with the pharmacological morbidity level, and evaluate the clinical impact of pharmacotherapeutic optimisation measures.
MethodThe protocol is designed in accordance with a bibliographical review, which was approved by the Pharmacy and Therapeutics Commission. Sequential study was carried out with a sample of 30 patients. Three measurements were taken (base line, at 48–72h and at 1–3weeks) to calculate the pharmacotherapeutic morbidity with the UKU rating scale and the Global Clinical Impression. Pharmacotherapeutic optimisation measures were used for those patients with high pharmacotherapeutic morbidity levels.
ResultsThe compliance level was 73.3%. One patient experienced ≥25% increase on the UKU rating scale and another patient suffered from an adverse effect. The final UKU rating reached statistical significance compared with the measurements taken at 48–72h (P=.032) and with the base line measurement (P=.007). Patient acceptance was 90%. The impact of optimisation measurements on the pharmacotherapeutic morbidity level was clinically and statistically significant (P<.001).
ConclusionsThe proposed protocol has been widely accepted and it is quite certain that it is to be introduced in at a general hospital level.
Diseñar un protocolo de intercambio terapéutico de antidepresivos y evaluarlo clínicamente a través de variables como: grado de cumplimiento, frecuencia de casos con aumento clínicamente significativo en la escala de morbilidad psicofarmacológica Udvalg-für-Kliniske-Undersogelser (UKU), análisis de eventos adversos, análisis de la evolución global de la puntuación en la UKU y grado de aceptación de los pacientes; objetivos secundarios fueron correlacionar aspectos del tratamiento psicofarmacológico con el grado de morbilidad farmacoterapéutica y evaluar el impacto clínico de medidas de optimización farmacoterapéuticas.
MétodoEl protocolo se diseñó de acuerdo con una revisión bibliográfica y fue aprobado por la Comisión de Farmacia y Terapéutica. Se realizaron, sobre una muestra de 30 pacientes seleccionados secuencialmente, 3 mediciones (basal, a las 48-72h y a las 1-3 semanas) en las que se cuantificó la morbilidad farmacoterapéutica mediante la escala UKU y la Impresión Clínica Global, implantando medidas de optimización farmacoterapéutica en aquellos sujetos con niveles de morbilidad farmacoterapéutica elevada.
ResultadosEl grado de cumplimiento fue del 73,3%. Un paciente experimentó un aumento ≥ 25% en la escala UKU y otro paciente experimentó un evento adverso. La puntuación final en la escala UKU alcanzó la significación estadística en comparación con las medidas realizadas a las 48-72h (p=0,032) y con la medida basal (p=0,007). El grado de aceptación de los pacientes fue del 90%. El impacto de las medidas de optimización sobre el nivel de morbilidad farmacoterapéutica fue clínica y estadísticamente significativo (p<0,001).
ConclusionesEl protocolo propuesto presenta una amplia aceptación y puede considerarse seguro para su implementación en un hospital general.
Drug selection is not an austerity measure, but rather an exercise in clinical intelligence. Joan Ramon Laporte
The health system is often faced with questions that were not answered by the clinical trials which are fundamental to a new drug's commercialisation process. One of the most important questions has to do with creating a systemic perspective on pharmacotherapy. A substantial number of second-generation antidepressants are currently in circulation, and their indications include general anxiety1 or eating disorders.2 As a result, a significant number of patients admitted to hospitals are on antidepressant treatments, and it therefore seems necessary to establish a treatment protocol for second-generation antidepressants (SGAD) in a general hospital environment.
The literature1–17 reviewed by the working group formed with the purpose of elaborating a therapeutic exchange protocol (TEP) show that in general, the different molecules included in the analysis5,6 show no significant differences in terms of efficacy, effectiveness and quality of life in the treatment of a major depressive episode that manifests in any form. Patient response to second-generation antidepressants is variable and limited. More than 50% do not enter remission, which is the main clinical objective of treatment; furthermore, nearly 40% of patients show no clinical response, which is a less stringent parameter. Prediction factors for response are currently unknown.6 We must also consider the fact that antidepressants are rarely used in emergency situations (for example, risk of suicide) because other types of treatment are more effective. Differences in the safety profile,5,6,17 however, are in fact documented and this was the fundamental determining factor in the drug selection process. We placed special emphasis on SGAD discontinuation syndrome (DS) given the TEP's context of use (a general hospital) and the assumption of the relative frequency with which oral treatment is interrupted due to medical and surgical processes. The incidence of SGAD DS is higher for those drugs with a shorter elimination half-life (paroxetine and venlafaxine); the DS incidence rate for fluoxetine is estimated to be 100 times lower than that of paroxetine.17
Our main objective is to establish the bases for a SGAD selection process by creating a therapeutic exchange protocol (TEP) and subjecting it to clinical evaluation by means of the following variables:
- –
Degree of compliance with TEP.
- –
Frequency of cases for which there is a clinically significant increase on the psychopharmacological side effect rating scale Udvalg-für-Kliniske-Undersogelser18 (UKU) (≥25%) associated with the TEP.
- –
Analysis of adverse effects related to the TEP.
- –
Analysis of overall changes in the UKU score throughout the study.
- –
Changes in the Clinical Global Impression scale19 (CGI).
- –
Degree of acceptance by patients.
- –
Mean duration of the therapeutic exchange.
- –
Impact of TEP on SGAD selection upon discharge.
The secondary objectives are as follows:
- –
Correlate certain aspects of psychopharmacological treatment (total anticholinergic load, number of sedative drugs, defined daily doses, etc.) with the degree of pharmacotherapeutic morbidity.
- –
Analyse the effect of pharmacotherapeutic optimisation measures on psychopharmacological iatrogenesis.
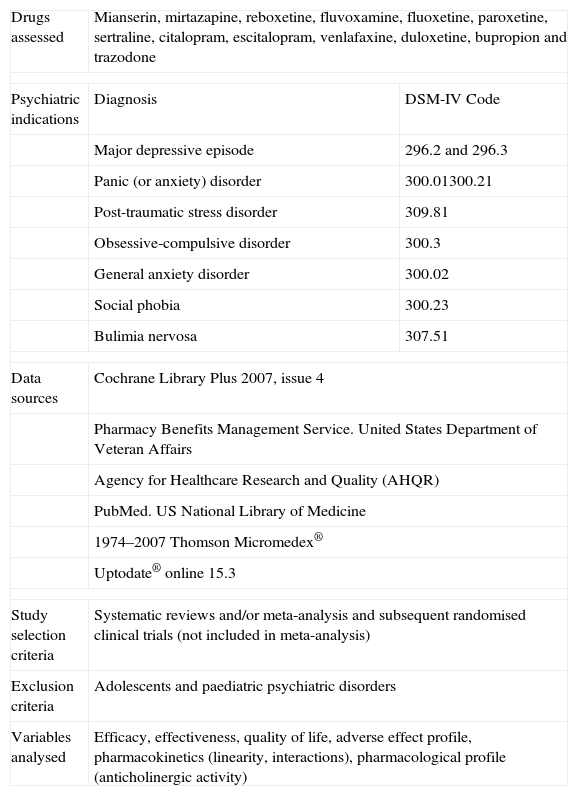
The TEP for SGAD was prepared by a group of experts (those listed in the “acknowledgements” section plus the authors of this study) and based on a literature review described fully in Table 1. The Pharmacy and Therapeutics Commission approved the final version (Table 2) for preliminary use and indicated that it was viable for a clinical evaluation study.
Methodology Used to Create Treatment Exchange Protocol.
| Drugs assessed | Mianserin, mirtazapine, reboxetine, fluvoxamine, fluoxetine, paroxetine, sertraline, citalopram, escitalopram, venlafaxine, duloxetine, bupropion and trazodone | |
| Psychiatric indications | Diagnosis | DSM-IV Code |
| Major depressive episode | 296.2 and 296.3 | |
| Panic (or anxiety) disorder | 300.01300.21 | |
| Post-traumatic stress disorder | 309.81 | |
| Obsessive-compulsive disorder | 300.3 | |
| General anxiety disorder | 300.02 | |
| Social phobia | 300.23 | |
| Bulimia nervosa | 307.51 | |
| Data sources | Cochrane Library Plus 2007, issue 4 | |
| Pharmacy Benefits Management Service. United States Department of Veteran Affairs | ||
| Agency for Healthcare Research and Quality (AHQR) | ||
| PubMed. US National Library of Medicine | ||
| 1974–2007 Thomson Micromedex® | ||
| Uptodate® online 15.3 | ||
| Study selection criteria | Systematic reviews and/or meta-analysis and subsequent randomised clinical trials (not included in meta-analysis) | |
| Exclusion criteria | Adolescents and paediatric psychiatric disorders | |
| Variables analysed | Efficacy, effectiveness, quality of life, adverse effect profile, pharmacokinetics (linearity, interactions), pharmacological profile (anticholinergic activity) | |
DSM-IV: The Diagnostic and Statistical Manual of Mental Disorders, fourth edition.
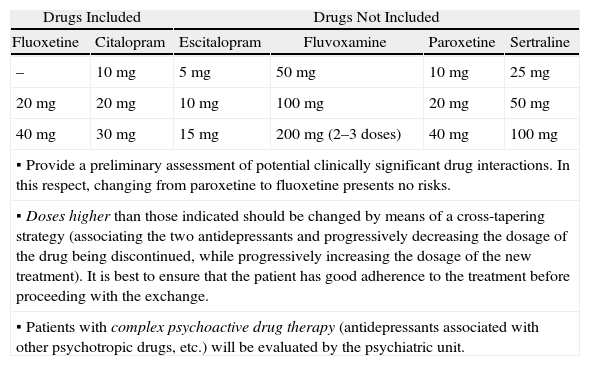
Treatment Exchange Protocol for SGAD at Hospital General Universitario de Alicante.
| Drugs Included | Drugs Not Included | ||||
| Fluoxetine | Citalopram | Escitalopram | Fluvoxamine | Paroxetine | Sertraline |
| – | 10mg | 5mg | 50mg | 10mg | 25mg |
| 20mg | 20mg | 10mg | 100mg | 20mg | 50mg |
| 40mg | 30mg | 15mg | 200mg (2–3 doses) | 40mg | 100mg |
▪ Provide a preliminary assessment of potential clinically significant drug interactions. In this respect, changing from paroxetine to fluoxetine presents no risks. | |||||
▪ Doses higher than those indicated should be changed by means of a cross-tapering strategy (associating the two antidepressants and progressively decreasing the dosage of the drug being discontinued, while progressively increasing the dosage of the new treatment). It is best to ensure that the patient has good adherence to the treatment before proceeding with the exchange. | |||||
▪ Patients with complex psychoactive drug therapy (antidepressants associated with other psychotropic drugs, etc.) will be evaluated by the psychiatric unit. | |||||
| Drug included (hypnotic/sedative) | Drugs not included | |
| Trazodone | Mianserin | Mirtazapine |
| 150mg (2–3 doses) | 30mg | 15mg |
| 300mg (2–3 doses) | 60mg | 30mg |
| Always begin the exchange with trazodone 50mg in single nightly doses with progressive dosage increases every 3–4 days | ||
| Drug included (SNRI) | Drugs not included |
| Venlafaxine | Duloxetine |
| 75mg | 30mg |
| 150mg | 60mg |
| Duloxetine: maintain when the indication is for diabetic peripheral neuropathy | |
The study took place in the period between March and July 2008. We included patients whose medical prescriptions included an antidepressant that was not included in the pharmacotherapeutic guidelines. Patient selection was sequential in the order in which prescriptions arrived. The first time this occurred for a hospitalised patient was considered to be the baseline time; another evaluation was made in a visit at 48–72h with another visit between the first and third weeks. Given the resources available to the study and the study characteristics (3 measurements at 3 different times per patient), a maximum of 1–2 patients were incorporated weekly. Our study sample was made up of a total of 30 patients.
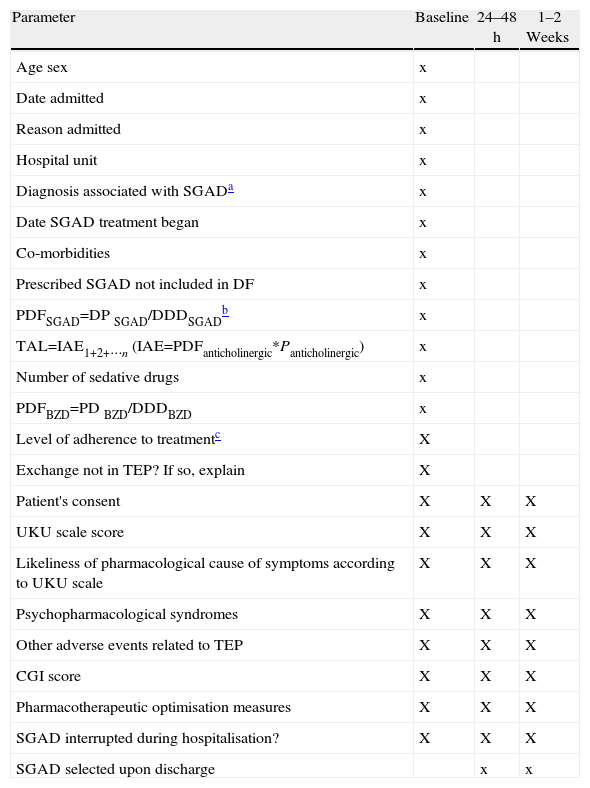
The baseline visit consisted in a review of the patient history and a 15–20min interview between the pharmacist and the patient, followed by an interview with the lead doctor on the case. Parameters recorded at each event are listed in Table 3. We used a reduced version of the UKU scale adapted through consensus to include a smaller number of items in the “other symptoms” field.
Parameters Recorded During Visits (Baseline, 24–48h and 1–3 Weeks).
| Parameter | Baseline | 24–48h | 1–2 Weeks |
| Age sex | x | ||
| Date admitted | x | ||
| Reason admitted | x | ||
| Hospital unit | x | ||
| Diagnosis associated with SGADa | x | ||
| Date SGAD treatment began | x | ||
| Co-morbidities | x | ||
| Prescribed SGAD not included in DF | x | ||
| PDFSGAD=DP SGAD/DDDSGADb | x | ||
| TAL=IAE1+2+⋯n (IAE=PDFanticholinergic*Panticholinergic) | x | ||
| Number of sedative drugs | x | ||
| PDFBZD=PD BZD/DDDBZD | x | ||
| Level of adherence to treatmentc | X | ||
| Exchange not in TEP? If so, explain | X | ||
| Patient's consent | X | X | X |
| UKU scale score | X | X | X |
| Likeliness of pharmacological cause of symptoms according to UKU scale | X | X | X |
| Psychopharmacological syndromes | X | X | X |
| Other adverse events related to TEP | X | X | X |
| CGI score | X | X | X |
| Pharmacotherapeutic optimisation measures | X | X | X |
| SGAD interrupted during hospitalisation? | X | X | X |
| SGAD selected upon discharge | x | x |
SGAD: second-generation antidepressant; BZD: benzodiazepines; TAL: total anticholinergic load; DDD: defined daily (maintenance) dose; PD: prescribed dose; EAI1+2+…n: intrinsic anticholinergic effect (summation for each drug); PDF: prescribed dose factor; DF: drug formulary; GCI: Global Clinical Impression scale; Panticholinergic: drug's anticholinergic potency (see Table 4). TEP: therapeutic exchange protocol; UKU: Udvalg-für-Kliniske-Undersogelser.
At all times, patients and/or doctor were able to express their preferences or recommendations for discontinuing the TEP, in which case the pharmacy would arrange to dispense the original antidepressant.
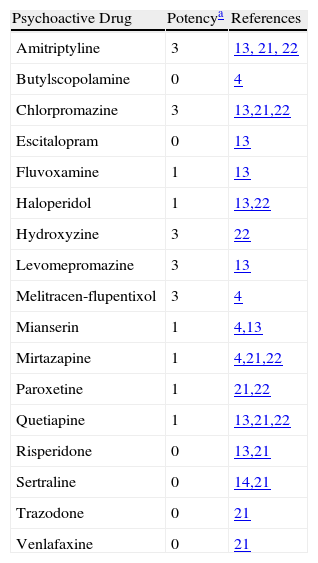
The total anticholinergic load (ACL) was calculated as described in Table 3; anticholinergic potency was graded between 0 and 3 in accordance with 4 bibliographic references.4,13,21,22 However, since there was no concordance between those references, the working group determined the anticholinergic potency (Table 4) for those cases in which there were discrepancies among the listed sources.
Anticholinergic Potency Determined by Committee Review of Cited Literature.
| Psychoactive Drug | Potencya | References |
| Amitriptyline | 3 | 13, 21, 22 |
| Butylscopolamine | 0 | 4 |
| Chlorpromazine | 3 | 13,21,22 |
| Escitalopram | 0 | 13 |
| Fluvoxamine | 1 | 13 |
| Haloperidol | 1 | 13,22 |
| Hydroxyzine | 3 | 22 |
| Levomepromazine | 3 | 13 |
| Melitracen-flupentixol | 3 | 4 |
| Mianserin | 1 | 4,13 |
| Mirtazapine | 1 | 4,21,22 |
| Paroxetine | 1 | 21,22 |
| Quetiapine | 1 | 13,21,22 |
| Risperidone | 0 | 13,21 |
| Sertraline | 0 | 14,21 |
| Trazodone | 0 | 21 |
| Venlafaxine | 0 | 21 |
The inferential statistics results were expressed as a mean and 95% confidence interval with a P-value. P was considered to be statistically significant for P<.05. For the comparison of the mean days of hospitalisation for our sample with respect to all hospital patients, we used an independent sample T-test. We used the Friedman ANOVA test to contrast the 3 UKU scores and contrasted them two-by-two using the Wilcoxon signed-rank test to compare 2 related samples. We used a test for 2 unrelated samples-the Mann–Whitney U test-to contrast variation on the UKU scale according to whether or not pharmacotherapeutic optimisation measures had been taken. We determined Pearson and Spearman correlation coefficients. Statistical analysis was carried out using the SPSS computer program, version 13.0 (SPSS Inc. Headquarters, USA).
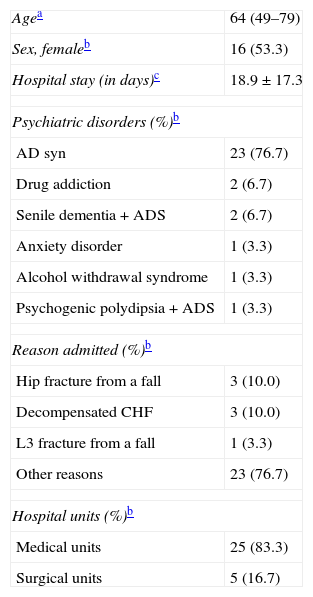
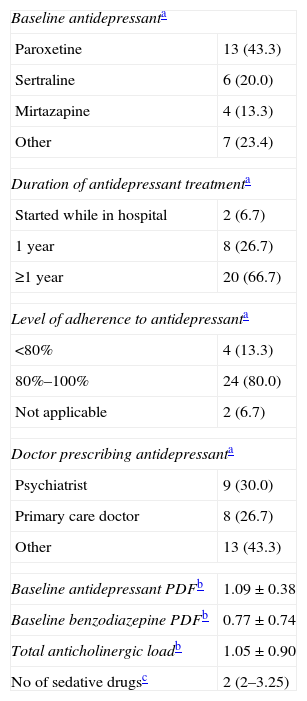
ResultsBaseline socio-demographical and clinical characteristics from our study sample are shown in Table 5. The study sample hospital stay is significantly longer than the mean length of stay for the general hospital sample (7.0 days) in the same period (P<.001). Characteristics of baseline antidepressant treatment are given in Table 6.
Socio-demographic Characteristics and Baseline Clinical Profiles of the Study Population (n=30).
| Agea | 64 (49–79) |
| Sex, femaleb | 16 (53.3) |
| Hospital stay (in days)c | 18.9±17.3 |
| Psychiatric disorders (%)b | |
| AD syn | 23 (76.7) |
| Drug addiction | 2 (6.7) |
| Senile dementia+ADS | 2 (6.7) |
| Anxiety disorder | 1 (3.3) |
| Alcohol withdrawal syndrome | 1 (3.3) |
| Psychogenic polydipsia+ADS | 1 (3.3) |
| Reason admitted (%)b | |
| Hip fracture from a fall | 3 (10.0) |
| Decompensated CHF | 3 (10.0) |
| L3 fracture from a fall | 1 (3.3) |
| Other reasons | 23 (76.7) |
| Hospital units (%)b | |
| Medical units | 25 (83.3) |
| Surgical units | 5 (16.7) |
ADS: anxiety-depression syndrome.
Characteristics of Baseline Antidepressant Treatment.
| Baseline antidepressanta | |
| Paroxetine | 13 (43.3) |
| Sertraline | 6 (20.0) |
| Mirtazapine | 4 (13.3) |
| Other | 7 (23.4) |
| Duration of antidepressant treatmenta | |
| Started while in hospital | 2 (6.7) |
| 1 year | 8 (26.7) |
| ≥1 year | 20 (66.7) |
| Level of adherence to antidepressanta | |
| <80% | 4 (13.3) |
| 80%–100% | 24 (80.0) |
| Not applicable | 2 (6.7) |
| Doctor prescribing antidepressanta | |
| Psychiatrist | 9 (30.0) |
| Primary care doctor | 8 (26.7) |
| Other | 13 (43.3) |
| Baseline antidepressant PDFb | 1.09±0.38 |
| Baseline benzodiazepine PDFb | 0.77±0.74 |
| Total anticholinergic loadb | 1.05±0.90 |
| No of sedative drugsc | 2 (2–3.25) |
| Baseline coadjuvant treatment | n (%) | Cumulative % |
| BZD | 12 (40.0) | 40.0 |
| BZD+opiates | 4 (13.3) | 53.3 |
| None (only AD) | 3 (10.0) | 63.3 |
| BZD+antipsychotic | 3 (10.0) | 73.3 |
| BZD+NMDA receptor antagonist | 2 (6.7) | 80.0 |
| Other | 6 (20.0) | 100.0 |
Cumulative %: cumulative frequency; PDF: prescribed dose factor.
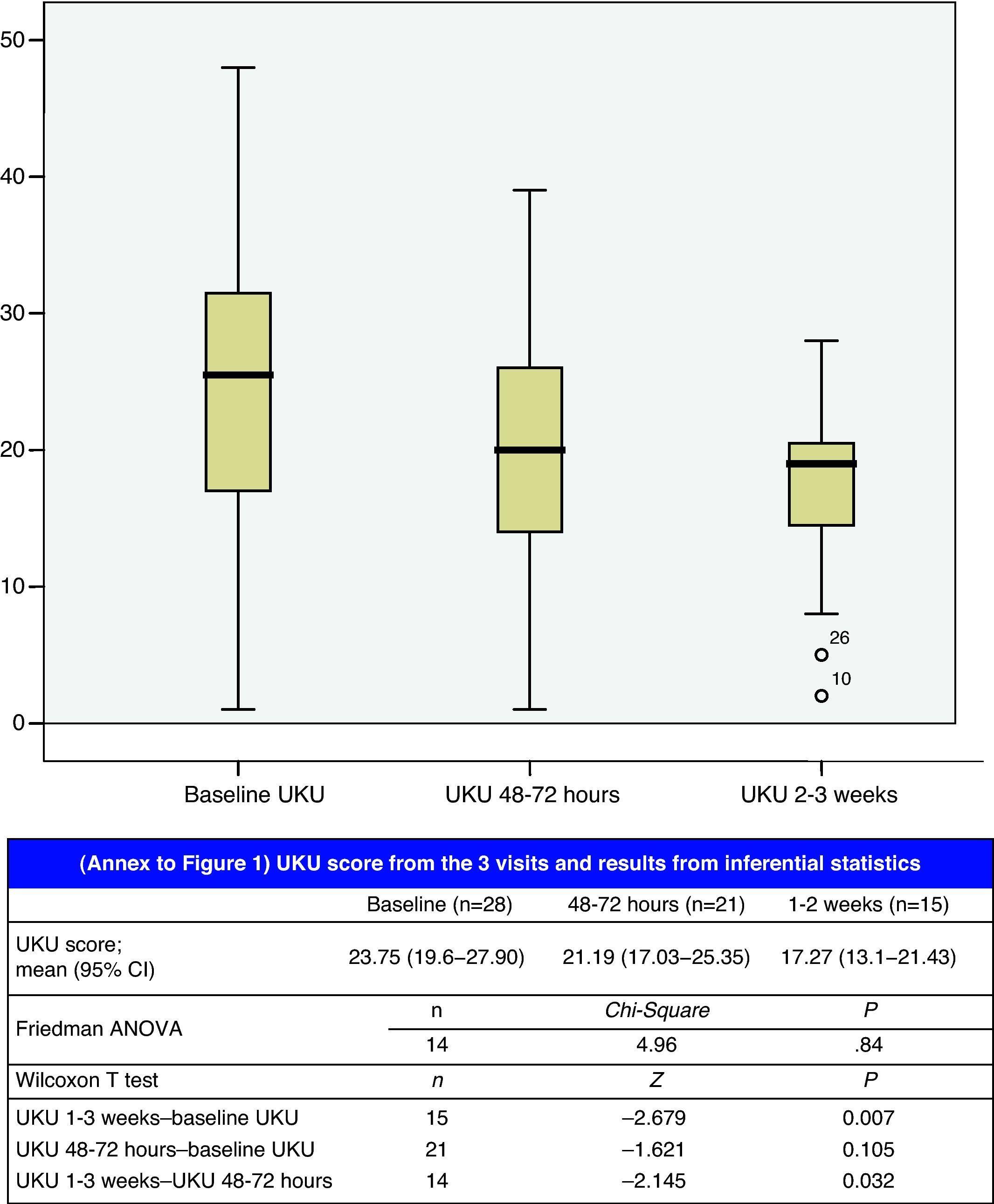
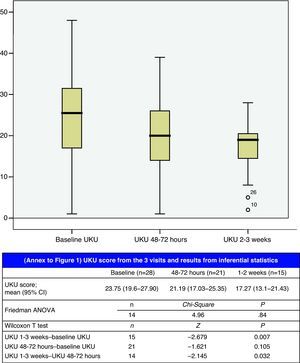
The degree of compliance with the TEP and the reasons for adapting the therapeutic exchange are given in Table 7. Adverse events and other variables used in evaluating the TEP are listed in Table 8. Variation in the UKU score throughout follow-up is shown in Fig. 1; the final measurement at 2–3 weeks was statistically significant compared with measurements recorded at 48–72h (P=.032) and with the baseline measurement (P=.007).
Degree of Compliance With the Treatment Exchange Protocol and Motives for Adapting It.
| Degree of compliance; n (%) | 22 (73.3) |
| Reasons for adapting the exchange | No of cases |
| Low level of adherence to antidepressant | 2 |
| Different medical indication from that in TEP | 2 |
| Association of 2 antidepressants | 1 |
| Association of 3 antidepressants | 1 |
| Antidepressant not matching indication | 1 |
| Antidepressant not included in protocol | 1 |
TEP: therapeutic exchange protocol.
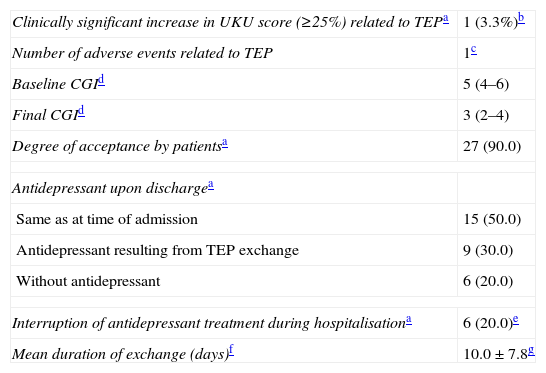
Other Measures for Evaluating the Therapeutic Exchange Protocol.
| Clinically significant increase in UKU score (≥25%) related to TEPa | 1 (3.3%)b |
| Number of adverse events related to TEP | 1c |
| Baseline CGId | 5 (4–6) |
| Final CGId | 3 (2–4) |
| Degree of acceptance by patientsa | 27 (90.0) |
| Antidepressant upon dischargea | |
| Same as at time of admission | 15 (50.0) |
| Antidepressant resulting from TEP exchange | 9 (30.0) |
| Without antidepressant | 6 (20.0) |
| Interruption of antidepressant treatment during hospitalisationa | 6 (20.0)e |
| Mean duration of exchange (days)f | 10.0±7.8g |
UKU: Udvalg für Kliniske Undersøgelser scale.
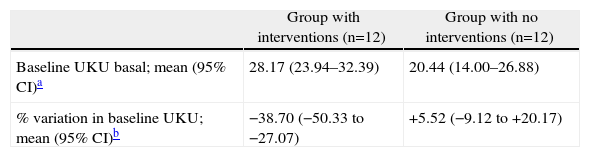
Baseline UKU score and the percentage of UKU variation by intervention group are shown in Table 9, along with the inferential statistical analysis results. Considering a variation above or below 25% as clinically significant, we can see that clinical relevance according to the UKU psychopharmacological side effect scale was reached in the group with pharmacotherapeutic optimisation measures; the score decreased by 38.7% for this group (95% CI, −50.33 to −27.07).
Baseline UKU Score and Percentage of Variation in UKU Between Intervention Groups.
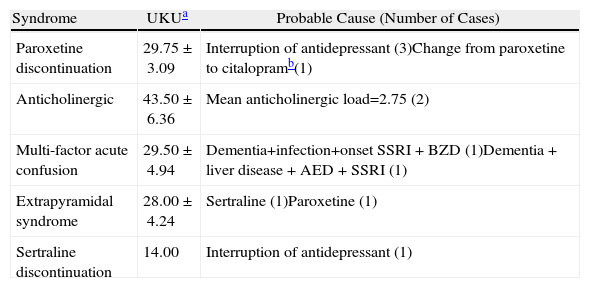
Of the patient total, 63.4% experienced at least one severe symptom (grade 3 on the UKU scale), which was probably related to psychotropic medication throughout the study. This degree of pharmacotherapeutic morbidity was apparent on a psychological level in 42.1% of the events; it was autonomic in 31.5% of the cases, neurological in 12.3% and of a different type in 14.0%. We detected 11 psychopharmacological syndromes (Table 10). The symptoms of DS tended to include frequent crying for no apparent cause, decrease in cognitive function, sleep cycle disturbances, nightmares and alternating states of anxiety and drowsiness. Autonomic effects included symptoms of diarrhoea, nausea and vomiting, urinary dysfunction and increased perspiration. Neurological effects observed included a certain degree of dystonia, stiffness, headaches and hypokinetic states; one patient experienced vertigo. Two patients developed an anticholinergic syndrome that manifested with symptoms including visual hallucinations, delirium, agitation, memory and cognitive disturbances and mucous membrane dryness (one patient who was also undergoing treatment with opiates developed paralytic ileus and severe urine retention). In addition, 2 patients developed an acute confusional state and underwent treatment with delirium-inducing psychoactive drugs.
Psychopharmacological Syndromes Detected During Follow-up.
| Syndrome | UKUa | Probable Cause (Number of Cases) |
| Paroxetine discontinuation | 29.75±3.09 | Interruption of antidepressant (3)Change from paroxetine to citalopramb(1) |
| Anticholinergic | 43.50±6.36 | Mean anticholinergic load=2.75 (2) |
| Multi-factor acute confusion | 29.50±4.94 | Dementia+infection+onset SSRI+BZD (1)Dementia+liver disease+AED+SSRI (1) |
| Extrapyramidal syndrome | 28.00±4.24 | Sertraline (1)Paroxetine (1) |
| Sertraline discontinuation | 14.00 | Interruption of antidepressant (1) |
TEP: therapeutic exchange protocol; UKU: Udvalg für Kliniske Undersøgelser scale; SSRI: selective serotonin reuptake inhibitor; BZD: benzodiazepines; AED: antiepileptic drugs.
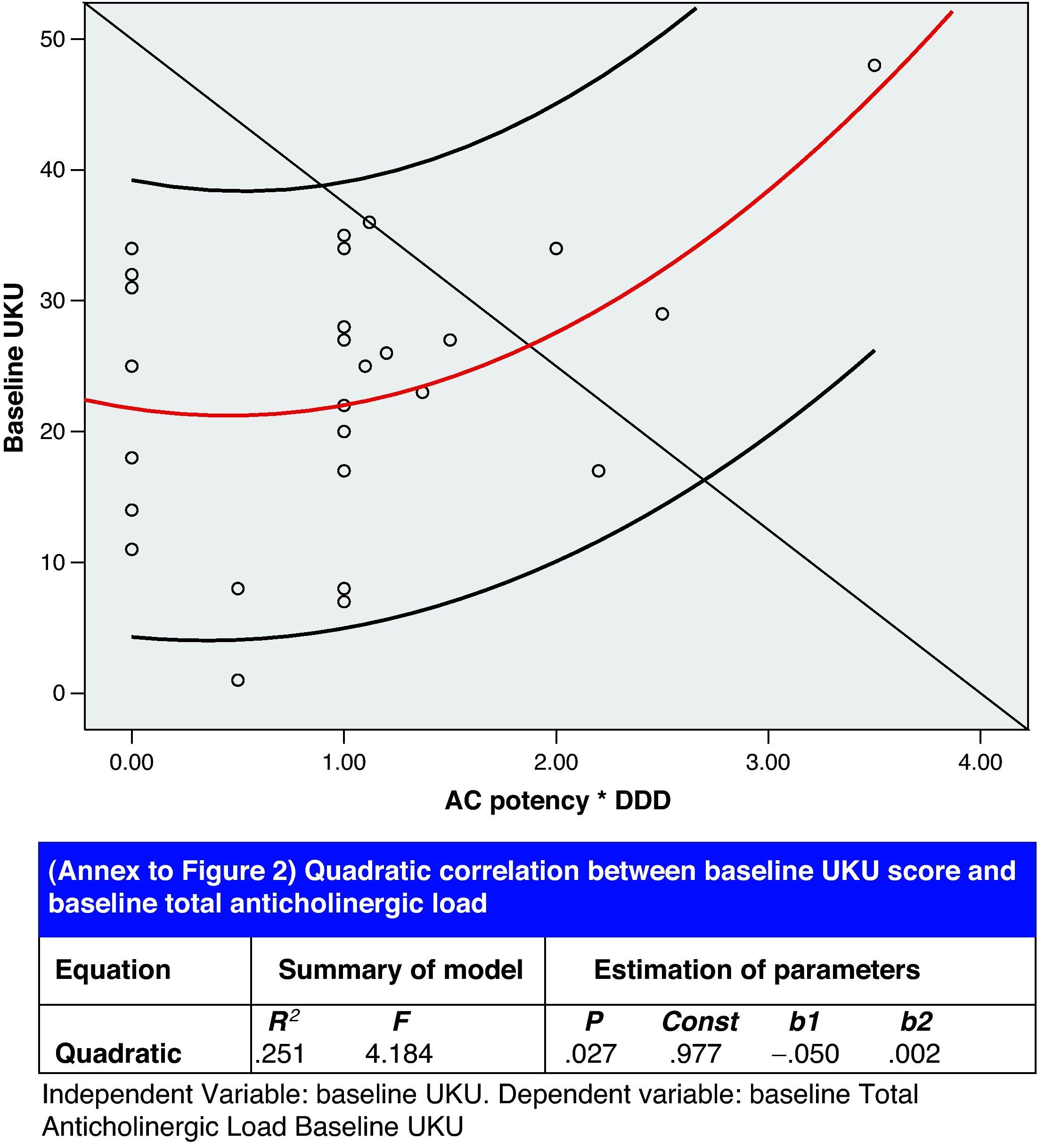
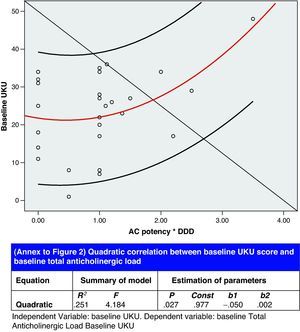
Lastly, results from the correlation analysis for the baseline UKU (independent variable) and the baseline ACL (dependent variable) are presented in Fig. 2. Other significant correlations between different aspects of psychopharmacological treatment were as follows:
- –
Number of sedative drugs – (R2=0.44; P<.001; Spearman's rho=0.704; P<.001).
- –
PDF for benzodiazepines – PDF for antidepressants (R2=0.19; P=.016).
After evaluating the cases that were monitored, we can state that the resulting TEP for SGADs is safe and widely accepted by both doctors and patients. It should be noted that 50% of the antidepressant treatments upon discharge are different from the baseline treatment due to doctor and patient preferences; however, this is a reflection of pharmacotherapeutic optimisation measures. One patient expressed a wish to resume the initial antidepressant after changing from mirtazapine to trazodone, due to feeling destabilised by the change; this case was subsequently evaluated by the psychiatrist who linked that episode to another medical cause (an infected defibrillator lead). Trazodone (the selected antidepressant sedative) exerts a hypnotic effect by behaving as an antagonist to 5-HT2a, 5HT2c and/or alpha-1-adrenergic receptors, while mirtazapine's hypnotic effect is attributed to a strong H1 antihistamine mechanism (like amitriptyline), although it is also an antagonist to alpha-1-adrenergic, 5-HT2a, 5HT2c, and 5-HT3 receptors. Unlike mirtazapine, trazodone has been studied in primary insomnia,23 in addition to having been evaluated in behaviour and psychiatric disorders in patients with dementia, which is a common indication in a general hospital setting. Although the systematic review14 did not show it to be effective (as with most psychoactive drugs studied in behaviour disorders associated with dementia), it did not show data that would contraindicate its use, and we value its low22 or non-existant21 anticholinergic potency. Our conclusion on this topic is that we may use trazodone as a sedative antidepressant, and should continue to evaluate its results.
We did not detect any other cases with a clinically significant increase in the UKU score for any of the drug changes applied, nor did we observe cases of serotonin syndrome, cholinergic rebound or DS associated with the change mandated by the TEP. All of the above are potential risks when changing antidepressants. However, we did detect 11 psychopharmacological syndromes associated with other causes.
The importance of recognising DS for SGAD lies in the following: (a) it sometimes requires hospitalisation; (b) the array of symptoms it causes are mostly non-specific, which implies a risk of diagnostic error and subjecting the patient to unnecessary diagnostic tests and/or procedures, and (c) patients who suffer from DS may abandon treatment24 (in our study, 2 of the 3 patients who did not accept the TEP made their decision due to fear of DS based on a previous episode). The potential problem of venlafaxine DS (high incidence rate and clinical importance) has not yet been completely resolved, since it is likely that treatment will be interrupted for the duration of the hospital stay. At the same time, we do not have experience with a duloxetine-venlafaxine treatment exchange, although it seems that it would not give rise to problems at the usual doses12 (up to 150mg of venlafaxine and 60mg of duloxetine).
The results from our study indicate that pharmacotherapeutic optimisation measures have resulted in a clinical and statistically significant improvement through decreasing pharmacotherapeutic morbidity. Naturally, both patients and doctors gave their consent for the measures that were implemented. They fundamentally consisted of decreasing psychopharmacological complexity by reducing the benzodiazepine, psychotropic-sedative and anticholinergic loads in cases in which the patient's overall condition so permitted.
Correlation analysis showed an association between the number of sedative drugs and TAL, which is a way of expressing a situation which we have observed: the use of non-benzodiazepine hypnotic psychoactive drugs (antipsychotics, antihistamines, antidepressants) causes the anticholinergic load to accumulate; in elderly patients, this may give rise to an anticholinergic syndrome known in the psychiatric field as anticholinergic delirium. Our study found a quadratic correlation between ACL and UKU score, which indicates potentially severe clinical repercussions (see Fig. 2). In fact, we detected two anticholinergic syndromes with a mean UKU score higher than that of other syndromes. Serum anticholinergic activity (SAA) can be measured in the laboratory and has been shown to be an independent risk factor for delirium. Furthermore, a large number of delirium symptoms are associated with higher SAA levels, which may be a more precise indication of the correlation our study found between anticholinergic load and pharmacotherapeutic morbidity level.25 SGAD-induced hyperkinetic movement is generally associated with selective serotonin reuptake inhibitors as a result of pharmacomodulation interaction between the serotonergic and dopaminergic neurotransmission systems.26 Our study recorded 2 cases, 1 associated with sertraline and the other with paroxetine.
Only 10% of patients were undergoing antidepressant monotherapy at the baseline time; the rest were receiving more complex treatments, mostly based on benzodiazepines with a median number of 2 sedative drugs (benzodiazepines and others). This seems to indicate that patients admitted while on antidepressants have problems with insomnia, anxiety and/or agitation, and may present behaviour disorders that require dispensing hypnotic psychoactive drugs. Studies indicate that difficulty sleeping does not correlate to age, but rather to psychosocial factors and health problems: 36% of subjects aged 65 years or older with no co-morbidities experienced sleep disorders, and this percentage increased to 69% in subjects with 4 or more co-morbidities. What is interesting is that the baseline medication itself and any drugs added to combat insomnia (sedative antidepressants, sedative antipsychotics, antihistamines, etc.) may lead to increased (and medication-induced) morbidity. As a result, the subject may experience worsened functionality, which is to be avoided when treating insomnia.27 On the other hand, depression is the medical condition most frequently associated with insomnia, and difficulty sleeping is a risk factor for depression; in our study, we observe a significant correlation between benzodiazepine PDF and antidepressant PDF, which provides the same conclusion from the perspective of drug practices. The meta-analysis by Glass et al.28 evaluating the benefits and risks of hypnotics use in subjects aged 60 or older shows a number needed to harm (NNH) of 6 (4.7–7.1) at the expense of increased cognitive deterioration, psychomotor skills deterioration (with fractures due to falling) and decreased daily function capacity. There is a correlation between the use of hypnotics and falls in hospitals, and the data seem to indicate that antidepressants are the psychoactive drugs associated with the greatest risk of falling.29 Further studies will be needed to analyse the clinical results of the hypnotics use observed in our sample.
We consider our proposed TEP for SGAD to be safe for implementation in general hospitals, and we recommend it be used from the admission date in order to reduce the risk of DS. Psychopharmacological treatment is subject to optimisation, especially measures that decrease the risk of drug-induced delirium. Doctor and patient acceptance rates are high for this type of proposal.
Conflict of InterestThe authors affirm that they have no conflict of interest.
To Dr Emilio Pol-Yanguas (Dr. Esquerdo Medical Centre), Dr Concepción Martín Serrano (Respiratory Medicine), Dr José Manuel Carratalá (Urgent Care Division/ICU), Dr. Fernando Quirce Andrés (Family and Community Medicine, Babel Specialty Centre), Dr Lucía Llanos (Clinical Pharmacology) and Dr Francisco Gracia Fleta (Neurology).
Please cite this article as: Martínez-Granados F, et al. Evaluación de un protocolo de intercambio terapéutico de antidepresivos de segunda generación: resultados clínicos. Farm Hosp. 2011;35:244–53.