Hospital pharmacy services have adapted to the COVID-19 pandemic. The aim of the study is to determine the economic consequences of replacing hospital pharmacy dispensation with other dispensing methods in the context of biological treatments for psoriasis in Spain.
MethodMultiple dispensation scenarios were evaluated, combining different dispensation frequencies and sites, and telepharmacy follow-up intervals. Self-injectable biological medicines for psoriasis (interleukin and tumour necrosis factor alpha inhibitors) were included. All costs (in 2020 euros) were considered from the perspective of the National Health System.
ResultsThe annual cost of hospital pharmacy-based dispensations every 4 weeks combined with telepharmacy monitoring at each administration ranged from €194.9 to €2,088.0 per patient. Across the different simulated scenarios, biological medicines associated with the lowest cost were those administered less frequently (every 12 weeks).
ConclusionsIn the post-COVID-19 era, new models of hospital pharmaceutical care that include changes in drug dispensation and telepharmacy strategies will have economic consequences for the National Health System that merit consideration.
Los servicios de farmacia hospitalaria se han adaptado a la pandemia de COVID-19. El objetivo del estudio es determinar las consecuencias económicas de sustituir la dispensación de medicamentos en el servicio de farmacia hospitalaria por otros métodos de dispensación en el contexto de los tratamientos biológicos para la psoriasis en España.
MétodoSe evaluaron múltiples escenarios de dispensación, combinando diferentes frecuencias y lugares de dispensación, y frecuencias del seguimiento de telefarmacia. Se incluyeron los medicamentos biológicos autoinyectables para la psoriasis (inhibidores de interleucinas y del factor de necrosis tumoral alfa). Todos los costes (euros de 2020) se consideraron desde la perspectiva del Sistema Nacional de Salud.
ResultadosConsiderando la dispensación en la farmacia hospitalaria, la frecuencia de dispensación cada 4 semanas y la telefarmacia en cada administración, el coste anual de dispensación por paciente osciló entre 194,9 € y 2.088,0 €. En los diferentes escenarios simulados, los fármacos biológicos que se asociaron a un coste inferior fueron los que se administran de forma más espaciada en el tiempo (cada 12 semanas).
ConclusionesEn la era post-COVID-19, los nuevos modelos de atención farmacéutica hospitalaria que consideran cambios en la dispensación farmacológica y la telefarmacia tendrán consecuencias económicas para el Sistema Nacional de Salud que merecen consideración.
Hospital pharmacy departments have had to adapt to the unexpected health crisis unleashed by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with a view to maintaining and/or improving the quality of their services and the safety of their patients1. Telepharmacy and remote drug dispensation have been added to outpatient pharmaceutical care with the aim of giving patients themselves greater accessibility while at the same time minimizing the risk of infection with SARS-CoV-21. Remote dispensation and telepharmacy make it possible to guarantee safe and effective pharmaceutical care and also allow for personalized pharmacotherapeutic follow-up within much more flexible timeframes, thereby improving the wellbeing of patients1.
Some national and international hospitals had already experienced with telepharmacy before COVD-192,3. MAPEX (Mapa estratégico de Atención farmacéutica al Paciente EXterno), a project that is currently being developed, aims at setting up an action framework for hospital pharmacists in outpatient care. In this project, telepharmacy is one of the three elements that have been introduced across the board to respond to patient needs, also offers health professionals an opportunity for career development and is one of the engines driving the paradigm shift in models of care within the health system2.
On 25 March 2020 the Ministry of Health approved Order SND/293/2020, setting conditions for the dispensation and administration of drugs under the National Health System (NHS) in the face of the health crisis resulting from COVD-194. This exceptional order determined that each of the country's autonomous regions could establish the measures that were deemed necessary to guarantee drug dispensation without patients having to access hospitals in person. It also stipulated that no drug requiring dispensation at the hospital could be prescribed for treatment periods of more than two months4.
The results of the ENOPEX study, in which one hundred hospitals and about 9,500 patients took part in Spain during the COVD-19 pandemic, revealed that three in every four patients prefer drug dispensation at home and 95% of patients favor telepharmacy for pharmaceutical care5. A survey among pharmacists of 185 NHS hospitals showed that remote pharmaceutical care involving telepharmacy and home delivery of drugs was practically not used before the pandemic (83.2%), whereas 119,972 patients had been served and 134,142 drug deliveries had been made within the first 6 weeks of COVD-19 lockdown. Most cases involved home dispensation (87.0%) and telematic consultation (87.6%)6.
The aim of the present study is to estimate the economic consequences of replacing drug dispensation at the hospital's pharmacy department with new methods of delivery that considered the use of telepharmacy services. The analysis focused on potential post-COVID-19 dispensation scenarios in the context of biological treatment of psoriasis in Spain.
MethodsThe potential scenarios of biological drug dispensation in Spain, for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy, were modelled from an economic perspective.
The study considered self-injectable biological drugs, interleukin inhibitors (anti-IL) tildrakizumab, guselkumab, risankizumab, secukinumab, ixekizumab, kizumab, brodalumab and ustekinumab, and tumor necrosis factor alpha inhibitors (anti-TNF-a) adalimumab, etanercept and certolizumab. Infliximab was excluded since it is administered intravenously.
The parameters used to determine the economic consequences of implementing different drug dispensation systems were: dispensation site and frequency, frequency of telematic follow-up, and associated unit costs.
Four potential drug dispensation sites were modelled. First of all, the economic cost of continuing to dispense drugs at the hospital's pharmacy department, as was mostly the case prior to the COVD-19 pandemic, was estimated. The new dispensation sites that were assessed included the primary care facility, the community pharmacy and the patient's home.
To analyze different dispensation frequencies, the dosing of biological drugs in maintenance regimens and in smaller containers (fewer syringes/ pens and fewer milligrams per syringe/pen) were considered (Table 1)7 Three dispensation frequencies were assessed: every 4 weeks (1 month), every 8 weeks (2 months), and every 12 weeks (3 months) (Table 1).
Number of annual dispensations, administrations and injections for the different biological drugs
| Group | Drug | Posology during maintenance phase7 | Contents of container7 | Dispensations/year | Administrations/year | Injections/year | ||
|---|---|---|---|---|---|---|---|---|
| Every 4 weeks | Every 8 weeks 1 | Every weeks | ||||||
| Tildrakizumab | 100 mg every 12 weeks |
| 4.3 | 4.3 | 4.3 | 4.3 | 4.3 | |
| Guselkumab | 100 mg every 8 weeks |
| 6.5 | 6.5 | 4.3 | 6.5 | 6.5 | |
| Risankizumab | 150 mg every 12 weeks |
| 4.3 | 4.3 | 4.3 | 4.3 | 8.7 | |
| Anti-IL | Secukinumab | 300 mg every month* |
| 13.0 | 6.5 | 4.3 | 13.0 | 13.0 |
| Ixekizumab | 80 mg every 4 weeks |
| 13.0 | 6.5 | 4.3 | 13.0 | 13.0 | |
| Brodalumab | 210 mg every 2 weeks |
| 13.0 | 6.5 | 4.3 | 26.0 | 26.0 | |
| Ustekinumab | 45 mg every 12 weeks |
| 4.3 | 4.3 | 4.3 | 4.3 | 4.3 | |
| Adalimumab | 40 mg every 2 weeks |
| 13.0 | 6.5 | 4.3 | 26.0 | 26.0 | |
| Anti-TNF-a | Etanercept | 50 mg weekly |
| 13.0 | 6.5 | 4.3 | 52.0 | 104.0 |
| Certolizumab | 200 mg every 2 weeks |
| 13.0 | 6.5 | 4.3 | 26.0 | 26.0 | |
Anti-IL: interleukin inhibitors; anti-TNF-α: tumor necrosis factor alpha inhibitors.
In terms of estimating the frequency of follow-up using telepharmacy, telematic consultations with the patient at each drug administration or at each drug dispensation were both considered (Table 1).
The combination of all these potential dispensation sites, dispensation frequencies and telematic sessions with the patient produced a total of 18 theoretical drug dispensation scenarios.
All costs were appraised from the perspective of the NHS and updated to 2020 euros using the consumer price index8. The following unit costs were applied depending on the place of delivery of the drugs: €6.4 at the hospital's pharmacy department9, €13.8 at home 10, €4.6 at community pharmacy11, and €4.6 at primary care facility (assuming the same cost as at community pharmacy). The unit cost for telepharmacy service was €38.512.
Results are presented as the average cost, with standard deviation, of the 18 theoretical dispensation scenarios for each biological drug.
With a view to accounting for the influence of variability and uncertainty on the considered costs, several univariate sensitivity analyses were performed in which the unit costs applied in the study were reduced and increased by 50%.
ResultsResults for the potential post-COVID-19 dispensation scenarios in the treatment of psoriasis in Spain are presented in Table 2.
Annual dispensation cost per patient according to treatment, site of dispensation, dispensation frequency and telepharmacy follow-up
| Disp. site | Anti-IL | Anti-TNF-a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tildrakizumab | Guselkumab | Risankizumak | Secukinumab | Ixekizumab | Brodalumab | Ustekinumab | Adalimumab | Etanercept | Certolizumab | ||
| Telepharmacy follow-up at each administration | |||||||||||
| HP | €194.9 | €292.4 | €194.9 | €584.7 | €584.7 | €1,085.8 | €194.9 | €1,085.8 | €2,088.0 | €1,085.8 | |
| HD | €227.0 | €340.4 | €227.0 | €680.9 | €680.9 | €1,182.0 | €227.0 | €1,182.0 | €2,184.2 | €1,182.0 | |
| Dispensation every 4 weeks | PC/CP | €187.4 | €281.0 | €187.4 | €562.1 | €562.1 | €1,063.2 | €187.4 | €1,063.2 | €2,065.4 | €1,063.2 |
| Telepharmacy follow-up at each dispensation | |||||||||||
| HP | €194.9 | €292.4 | €194.9 | €584.7 | €584.7 | €584.7 | €194.9 | €584.7 | €584.7 | €584.7 | |
| HD | €227.0 | €340.4 | €227.0 | €680.9 | €680.9 | €680.9 | €227.0 | €680.9 | €680.9 | €680.9 | |
| PC/CP | €187.4 | €281.0 | €187.4 | €562.1 | €562.1 | €562.1 | €187.4 | €562.1 | €562.1 | €562.1 | |
| Telepharmacy follow-up at each administration | |||||||||||
| HP | €194.9 | €292.4 | €194.9 | €542.9 | €542.9 | €1,044.0 | €194.9 | €1,044.0 | €2,046.2 | €1,044.0 | |
| HD | €227.0 | €340.4 | €227.0 | €591.0 | €591.0 | €1,092.1 | €227.0 | €1,092.1 | €2,094.3 | €1,092.1 | |
| Dispensation every 8 weeks | PC/CP | €187.4 | €281.0 | €187.4 | €531.6 | €531.6 | €1,032.7 | €187.4 | €1,032.7 | €2,034.9 | €1,032.7 |
| Telepharmacy follow-up at each dispensation | |||||||||||
| HP | €194.9 | €292.4 | €194.9 | €292.4 | €292.4 | €292.4 | €194.9 | €292.4 | €292.4 | €292.4 | |
| HD | €227.0 | €340.4 | €227.0 | €340.4 | €340.4 | €340.4 | €227.0 | €340.4 | €340.4 | €340.4 | |
| PC/CP | €187.4 | €281.0 | €187.4 | €281.0 | €281.0 | €281.0 | €187.4 | €281.0 | €281.0 | €281.0 | |
| Telepharmacy follow-up at each administration | |||||||||||
| HP | €194.9 | €278.4 | €194.9 | €529.0 | €529.0 | €1,030.1 | €194.9 | €1,030.1 | €2,032.3 | €1,030.1 | |
| HD | €227.0 | €310.5 | €227.0 | €561.0 | €561.0 | €1,062.1 | €227.0 | €1,062.1 | €2,064.3 | €1,062.1 | |
| Dispensation every 12 weeks | PC/CP | €187.4 | €270.9 | €187.4 | €521.4 | €521.4 | €1,022.5 | €187.4 | €1,022.5 | €2,024.7 | €1,022.5 |
| Telepharmacy follow-up at each dispensation | |||||||||||
| HP | €194.9 | €194.9 | €194.9 | €194.9 | €194.9 | €194.9 | €194.9 | €194.9 | €194.9 | €194.9 | |
| HD | €227.0 | €227.0 | €227.0 | €227.0 | €227.0 | €227.0 | €227.0 | €227.0 | €227.0 | €227.0 | |
| PC/CP | €187.4 | €187.4 | €187.4 | €187.4 | €187.4 | €187.4 | €187.4 | €187.4 | €187.4 | €187.4 | |
Anti-IL: interleukin inhibitors; anti-TNF-α: tumor necrosis factor alpha inhibitors; CP: community pharmacy; disp.: dispensation; HD: home delivery; HP: hospital pharmacy; PC: primary care.
Considering that the dispensation of biological drugs for the treatment of psoriasis is undertaken at the hospital's pharmacy department, a dispensation frequency of every 4 weeks, added to telematic monitoring of each administration of the drug, resulted in an annual dispensation cost, per patient, of between i194.9 (for tildrakizumab, risankizumab and ustekinumab, which are administered every 12 weeks) and i2,088.0 (for etanercept, which is administered every week) (Table 2).
The costliest theoretical scenario was that of home dispensation of the drug every 4 weeks and telematic monitoring of each administration, with costs ranging from €227.0 (tildrakizumab, risankizumab and ustekinumab) to €2,184.2 (etanercept) (Table 2).
The least costly potential scenario was dispensation at the primary care facility or community pharmacy every 12 weeks and telematic consultation at the time of each dispensation, at a cost of €187.4 for all biological drugs (Table 2).
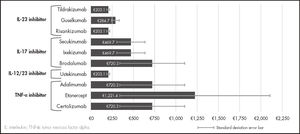
Figure 1 shows that the biological drugs associated with a lower average cost were those that are administered at longer intervals in time. The average annual cost of dispensation per patient can thus be up to 6 times lower for biological drugs like tildrakizumab, risankizumab and ustekinumab, which are administered every 12 weeks, than for etanercept, which is administered weekly.
In sensitivity analyses, the cost that caused a greater variation in results was that of telematic consultations, regardless of the biological drug under consideration (Figure 2). When halving or doubling such expense, the average annual cost of dispensation per patient varied from 41% to 47% depending on the type of treatment.
DiscussionIt has been apparent for some years that outpatient pharmaceutical care at hospitals is evolving and exploring potential changes, such as distance drug dispensation and telepharmacy2. However, the COVID-19 pandemic has accelerated and definitely moved forward these new forms of care within health services1,4.
There are discrepancies and methodological difficulties associated with estimating the economic consequences of changing conventional forms of hospital drug dispensation13. There are also few Spanish studies assessing the unit costs associated with different dispensation sites9-11. The present analysis is the first Spanish study to assess the economic impact resulting from the potential introduction of different hospital drug dispensation systems in the NHS within the context of psoriasis. The only similar publication we have identified was a recent cost-minimization analysis comparing drug delivery at health centers, through outsourced courier services and at pharmacies14. Although the dispensation costs for each of these three scenarios were similar to those of our study, total costs differ, mainly due to the fact that in our study we also added the cost of telematic consultation at the time of drug administration or dispensation, which increases the economic impact considerably.
The present analysis shows that differences in the dosing regimens of biological drugs for the treatment of psoriasis have a direct impact on dispensation costs. Thus, drugs that are administered every 12 weeks (tildrakizumab, risankizumab and ustekinumab) are associated with lower dispensation costs than more frequently administered agents. Regarding drugs with lower dispensation costs, it should be pointed out that risankizumab requires twice as many injections as tildrakizumab and ustekinumab, which can lead to increased reactions at the injection site15 and worse therapeutic compliance, in addition to having a greater negative impact on patient quality of life16. In fact, patients with psoriasis prefer treatment regimens requiring a lower frequency of injections17.
The study also reveals that the differences in dispensation costs disappear if dispensation and telematic consultation take place every 12 weeks for all drugs equally. However, it is important to remember that patients that are dispensed several syringes at once, for subsequent self-injection at different points in time, may forget doses or make mistakes as a result of misinterpreting instructions for the conservation and/or administration of their medication. Although the optimal time for telematic consultation has not been clearly established, in real-life practice it would seem advisable to schedule consultations shortly before each administration in order to avoid the above-mentioned risks.
An added advantage of drugs associated with lower number of injections is that they generate less device waste and are therefore more environmentally friendly.
Since direct comparisons of the efficacy and safety of the different biological drugs that inhibit IL-23 are not available, it is important to bear in mind other factors that determine which drug may be the most efficient18. In addition to direct dispensation costs, the pharmacological costs must be considered. Such costs have not been considered in the present study, given the differences that exist between listed prices and invoiced prices, and the fact that manufacturers sometimes offer added discounts, making it impossible to accurately determine the actual price of the drugs. At any rate, high pharmacological costs can be offset with lower dispensation expenses in the case of drugs with long dosing intervals.
Another aspect which should be borne in mind during the decision-making process involves the drug's conservation requirements. The only syringes that may be stored for 1 month at room temperature are those used with tildrakizumab, ustekinumab, adalimumab and etanercept; the remainder must be kept under refrigeration (guselkumab and risankizumab) and/ or can only be stored at room temperature for up to 14 days (secukinumab, ixekizumab, brodalumab and certolizumab)7.
This study has some limitations. On the one hand, due to the lack of data on dispensation unit costs, the economic impact we have determined might not exactly reflect the actual cost of the potential dispensation scenarios. We must also point out that estimates have been determined for maintenance regimens, and for one kind of drug container only (the smallest in size). Furthermore, the economic impact of replacing the standard system of hospital drug dispensation with new forms of dispensation has only been studied in one disease, psoriasis. Further studies looking at the economic consequences of these changes in the setting of other diseases are therefore required.
In conclusion, adapting the dispensation system of biological drugs for the treatment of psoriasis in the post-COVID-19 era will have economic implications for the NHS that must be borne in mind when making planning and management decisions in the context of pharmaceutical care. These implications will vary especially depending on the frequency of telematic care and drug dosing regimens. Drugs that are administered every 12 weeks (tildrakizumab, risankizumab and ustekinumab) are associated with the lowest dispensation costs.
FundingThis study has been sponsored by Almirall.
Conflict of interestIgnacio Martí-Ragué and Mónica Casañas-Domingo are staff members at Almirall, the company that sponsored this study. Miguel Ángel Calleja-Hernández and Pere Ventayol-Bosch participated in the study in their capacity as hospital pharmacy experts. Jaume Costa-Samarra and Diana Nieves-Calatrava are staff members at Oblikue Consulting S.L., an independent consultancy company hired for the preparation of the study.
Presentation at congressesPreliminary results of this article were presented at the following European congress: The International Society for Pharmacoeconomics and Outcomes Research (ISPOR). Online. Date: 16-19 November 2020.
Contribution to the scientific literature
The COVID-19 pandemic has accelerated remote dispensation of medicines and the use of telepharmacy, making it possible to render outpatients the hospital pharmacy services they need. This is, to the best of our knowledge, the first study to gage the economic impact of these changes on the dispensation of hospital-based medications to patients with psoriasis treated in the Spanish National Health System.
The results obtained show that the cost associated to the dispensation of biological treatments depends mainly on the dosing regimen and the frequency of pharmaceutical care with telepharmacy. For that reason, these new dispensation models are particularly interesting from the point of view of healthcare management and healthcare policy.
Early Access date (10/22/2021).