To identify the hazards and define the theoretical occupational risks arising from the process of handling hazard drugs in hospital pharmacy services on the basis of expert consensus.
MethodAn expert consensus was conducted (nominal group and documentary techniques) using a mixed method of two face-to-face rounds (meeting of participants and approval of proposals) and three masked rounds (individualized review). The analysis was applied to the field of hospital pharmacy. The stages of the process were designed using the standardized graphical Business Process Model and Notation.
ResultsA specific flowchart was obtained for the management and traceability of hazardous drugs. All general process phases were characterized. A management chart included operations addressing the reception and storage, compounding, conservation, and dispensation of hazardous drugs in hospital pharmacy services. This chart provides a description of the chemical hazards and exposure routes.
ConclusionsThe hazardous drug process should be integrated in a standard management system to improve the safety of patients and healthcare professionals. Efficiency can maximized and procedural incidents minimized, thereby ensuring the quality and the safety of hazardous drugs handling in hospital pharmacy services.
Once hazards are identified, risk assessment should be implemented using a systematic and preventative methodology to minimize the risk and severity of any adverse event.
Identificar los peligros y definir los riesgos laborales teóricos derivados del proceso de manipulación de los medicamentos peligrosos en los servicios de farmacia hospitalaria mediante un consenso de expertos.
MétodoSe realizó un consenso de expertos (grupo nominal y técnicas documentales) utilizando un método mixto mediante dos rondas presenciales (reunión de los participantes y aprobación de propuestas) y tres rondas enmascaradas (revisión del material de forma individual). El análisis se aplicó al ámbito de la farmacia hospitalaria y las etapas del proceso se diseñaron mediante notación gráfica normalizada Business Process Modeling Notation.
ResultadosSe obtuvo el diagrama de flujo específico para la gestión y trazabilidad de los medicamentos peligrosos, caracterizándose cada una de las fases del proceso general, recopiladas en un cuadro de gestión de etapas y operaciones de recepción y almacenamiento, elaboración, conservación y dispensación de medicamentos peligrosos en los servicios de farmacia hospitalaria, que sirvió para la posterior descripción de riesgos químicos y vías de exposición.
ConclusionesLos medicamentos peligrosos deben integrarse en un sistema normalizado de gestión con el fin de mejorar la seguridad del paciente y de los profesionales sanitarios, a la vez que se maximizan la eficiencia de los recursos y minimizan los incidentes procesales, garantizando la calidad y la seguridad del proceso de manipulación de medicamentos peligrosos en los servicios de farmacia.
Sería deseable, una vez se han identificado los peligros, llevar a cabo una evaluación de los riesgos siguiendo una metodología sistemática y de abordaje preventivo que permita calibrar la probabilidad de ocurrencia y la gravedad de cualquier suceso adverso.
The European Agency for Safety and Health at Work (EU-OSHA) has established that the handling of hazardous drugs (HD) is one of the most relevant risk factors for the health of healthcare workers1. The evidence for this is incontrovertible, in that it has been estimated that in Europe more than 12.7 million healthcare workers handle HDs. This figure implies that occupational exposure may cause an estimated 2,220 new cases of leukaemia leading to 1,467 deaths among these workers in this continent2.
The National Institute for Occupational Safety and Health (NIOSH) defines drugs as hazardous if, in animal or human studies, they demonstrate any of the following characteristics: carcinogenicity, teratogenicity or other developmental toxicity, genotoxicity, reproductive toxicity, organ toxicity at low doses, and drugs with a structure or toxicity profile similar to that of other hazardous drugs3.
Numerous studies have shown that HDs carry chemical risks for the workers who handle them4-13.
Thus, risk assessment is one of the key points in the HD handling and control process, given that its results support all the measures adopted to guarantee the safety of the process14. The scientific literature has described several risk assessment models15. Although each one has its particular characteristics, all of them include the identification of process-associated hazards as an essential first step in their handling.
However, although risk assessment is a legal requirement in Spain16, and the need for it is recognised, there are few studies on HD risk analysis. In 2009, a French team of hospital pharmacists applied the “hazard analysis and critical control points” methodology to the preparation of anti-cancer drugs in pharmacy services17. To the best of our knowledge, the American Hazardous Drug Consensus Group is the only group that has proposed a specific methodology for HD risk analysis18.
Given the foregoing, and on the basis of expert consensus, the objective of this study was to identify the hazards and define the theoretical occupational risks arising from the process of handling HDs in hospital pharmacy services.
MethodsDesignAn expert consensus (nominal group and documentary techniques) was conducted using a mixed method of two face-to-face rounds (group meeting and approval of proposals) and three blinded rounds (individual review of the material). The study was conducted between November 2018 and May 2019.
Expert groupThe following objective criteria were used to select the expert group:
- •
Previous knowledge and experience: more than five years of professional experience in posts in which HDs are handled or risk assessment is performed.
- •
Setting: primary care, hospital care, home care, or public healthcare.
Consensus was developed in seven phases:
- •
Phase 1 (prior to the expert consensus): Bibliographic review of the antecedents and protocols related to monitoring hazards (controlled feedback), through the identification, collection, and analysis of documents related to the issue or setting under study. This review was published in 201819.
- •
Phase 2 (Nov-Dec 2018): Preparation of the initial documentation and construction of the first flowchart and the HD management chart.
- •
Phase 3 (masked) (Jan 2019): Review of this material and corrections.
- •
Phase 4 (face-to-face) (February 2019): Sharing the contributions and document correction.
- •
Phase 5 (masked) (March 2019): New document revision and new contributions if needed.
- •
Phase 6 (face-to-face) (April 2019): Acceptance of the latest revisions and production of corrected material.
- •
Phase 7 (masked) (May 2019): Final unanimous approval of the material: flowchart and HD management chart.
The analysis was applied to the setting of hospital pharmacy.
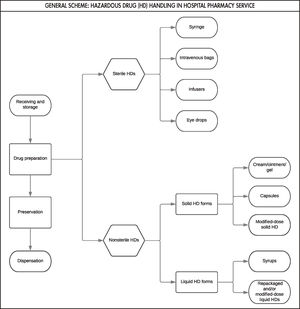
Stages of the processThe Business Process Model and Notation (BPMN) was used to design the flowchart of the general scheme of the operational management of the HD logistics chain in hospital pharmacy services. Based on this flowchart, we developed total traceability management for each of the stages, created their BPMN diagrams20, and developed the corresponding HD management chart. This model was previously implemented by Bernabeu Soria et al.21. It was used to analyse and characterize each of the steps within the process, thereby facilitating the analysis of each step and the determination of potential hazards. This technique was successfully implemented and verified by Cervera Peris et al.22. If needed, this technique allows processes to be easily scaled (i.e. extended), thereby maintaining efficiency and effectiveness when there is any change or new requirement. The steps to be managed were obtained from the systematic review conducted by Bernabeu et al.19: reception and bulk storage, preparation, drug storage, and dispensing.
On-site checkBased on the documents developed, we identified each of the stages, operations, and possible control points. Subsequently, we verified the correspondence between the documents developed (i.e. flowchart and HD management chart) and the stages comprising the HD manipulation process in the places where the operations are conducted.
NomenclatureThe following lexicon was accepted and used in the creation of the documents:
- •
Process: a set of interrelated activities that are conducted in a systematic manner by a group of agents to achieve a predefined end.
- •
Stage: each subprocess in the final flowchart.
- •
Operation: each of the activities or steps that make up a stage.
- •
Hazard: an agent with the intrinsic potential to injure the health of the healthcare worker15. These agents are classified according to their characteristics:
- –
Physical hazard: objects or fragments of objects that may injure the worker.
- –
Biohazard: any type of microorganism from a patient that, either by direct or indirect contact with tissues or fluids, can cause an infection in healthcare workers.
- –
Chemical hazard: a chemical agent (HD) that due to its intrinsic toxicity can injure the personnel handling it.
- –
- •
Risk: the possibility of a worker experiencing a specific injury due to exposure to a hazard15. These risks are categorized as follows:
- –
Physical risk: cuts from glass and other materials, puncture wounds from sharp objects, etc.
- –
Biological risk: parenteral exposure to infectious agents (i.e. via puncture wounds after administration of a HD to an infected patient).
- –
Chemical risk: exposure to a HD by inhalation, through the skin or mucous membranes, by contact with eyes, ingestion, and via parenteral routes.
- –
Documentary techniques were used to analyse any stages over which there was disagreement until a 100% consensus level was reached.
ResultsThe experts had an average of 22 ± 3.17 years of experience (median: 25.50 years; range: 8-28 years). In all cases, the degree of consensus on the objective criteria of choice was 100%. Table 1 shows the characteristics of the experts who comprised the group.
Characteristics of the Members of the Expert Group (n = 6)
| Characteristics | Participants | |
|---|---|---|
| n | % | |
| Sex | ||
| Men | 3 | 50.0 |
| Women | 3 | 50.0 |
| Knowledge and experience | ||
| Hazardous drug handling | 4 | 66.7 |
| Risk assessment | 2 | 33.3 |
| Both | 0 | 0.0 |
| Profession | ||
| Physician | 1 | 16.7 |
| Pharmacist | 5 | 83.3 |
| Work setting | ||
| Hospital | 4 | 66.7 |
| Primary care | 1 | 16.7 |
| Public health | 1 | 16.7 |
| Area of work | ||
| Home hospitalisation unit | 1 | 16.7 |
| Hospital pharmacist | 4 | 66.7 |
| Public health pharmacist | 1 | 16.7 |
| Autonomous community | ||
| The Valencian Community | 6 | 100.0 |
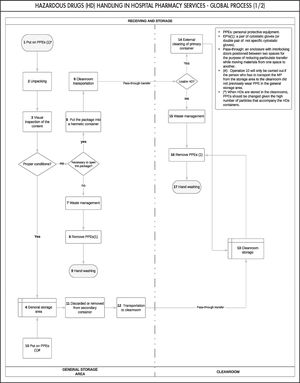
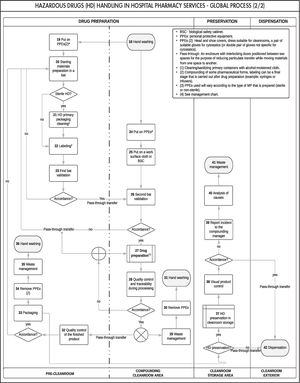
Based on the expert consensus, we first designed the general outline of the process (Figure 1), which facilitated the development of the global process (Figure 2). Figure 2 shows all the stages that comprise the HD traceability management procedure in hospital pharmacy services, which would allow its monitoring and reproducibility.
Each stage was represented in a table (Table 2) to systematize and facilitate the understanding of the results. The following variables were coded for each stage of the process: stage, operation and operation number (i.e. the number that appears in each of the operations shown in the flowchart and facilitates their identification in the different documents), potential hazard (i.e. yes/no), type of hazard identified (i.e. chemical and physical), and HD exposure routes (skin and mucous membranes, ingestion, ocular, and injection).
Risk management chart by stage
| STAGES AND OPERATIONS OF THE STERILE AND NON-STERILE HAZARDOUS DRUG HANDLING PROCESS | ||||||
|---|---|---|---|---|---|---|
| Stage | Number† | Operation | Hazard | Physical | Chemical | Chemical Risk (exposure routes) |
| 1 | Putting on PPEs* (see flowchart) | NO | − | − | − | |
| 2 | Unpacking | YES | YES (if there is a broken container, cuts from glass) | YES (exposure if loss of primary container integrity) |
| |
| 3 | Visual inspection of contents | NO | − | − | − | |
| 4 | Bulk storage in general store | YES | YES (cuts from broken ampules or glass vials) | YES (exposure if loss of packaging integrity, spills, or splashes) |
| |
| 5 | Putting the package in airtight container (if packaging conditions are inadequate) | YES | YES (if there is a broken container, cuts from glass) | YES (exposure if spills or loss of primary container integrity) | ||
| 6 | Transportation to clean rooms | YES | YES (breakage of the airtight container containing the HD) | YES (exposure if spills or loss of container integrity) | ||
| 7 | Waste management | YES | YES (cuts if glass containers) | YES (exposure if loss of packaging integrity, spills) | ||
| Reception and bulk storage | 8 | Remove PPEs(1) | YES | NO | YES (exposure by contact with HD residues if removal inadequate) |
|
| 9 | Hand washing | NO | − | − | − | |
| 10 | Putting on PPEs(1) | NO | − | − | − | |
| 11 | Discarded (removed from secondary container) | YES | YES (cuts if accidental breakage of the primary glass container) | YES (exposure if loss of packaging integrity, spills, or splashes) | Skin or mucous | |
| 12 | Transportation to clean rooms | YES | YES (breakage of the container containing HD and cuts if glass containers) | YES (exposure if spills or loss of container integrity) |
| |
| 13 | Storage in clean room | YES | YES (cuts if accidental breakage of the primary glass container) | YES (exposure if loss of packaging integrity, spills, or splashes) | □ Inhalation | |
| 14 | External cleaning of primary packaging of usable HDs | YES | NO | YES (exposure by contact with contaminated HD container) |
| |
| 15 | Waste management | YES | NO | YES (exposure by contact with HD residues) | Same as operation 7 | |
| 16 | Remove PPEs(1) | YES | NO | YES (exposure by contact with HD residues if removal inadequate) | Same as operation 8 | |
| 17 | Hand washing(2) | NO | − | − | − | |
| 18 | Hand washing | NO | − | − | − | |
| 19 | Putting on PPEs(2) (see flowchart) | NO | − | − | − | |
| 20 | Preparation of primary ingredients in tray | YES | YES (cuts if breakage of glass HD container) | YES (exposure if loss of packaging integrity, spills, or splashes) |
| |
| Preparation (previous stages in common) | 21 | Cleaning of primary HD containers (if processing sterile HD) | YES | YES (cuts if breakage of glass HD container) | YES (exposure if loss of packaging integrity, spills, or splashes) | |
| 22 | Labelling | NO | − | − | − | |
| 23 | First validation of the tray | NO | − | − | − | |
| 24 | Putting on PPEs(3) | NO | − | − | − | |
| 25 | Placing cloth on work surface | NO | − | − | − | |
| 26 | Second validation of the tray | NO | − | − | − | |
| 27.1. Preparation of syringes for parenteral administration (SC, IM, IT, bolus IV) | YES | YES (cuts if breakage of HD glass containers or ampoules, puncture wounds if using needles(4)) | YES (exposure if spills, splashes, aerosol generation, or accidental injection) | |||
| 27.2. Preparation of bags for intravenous infusion | YES | YES (cuts if breakage of HD glass containers or ampoules, puncture wounds if using needles(4)) | YES (exposure if spills, splashes, aerosol generation, or accidental injection) | |||
| 27.3. Preparation of infuser pumps | YES | YES (cuts if breakage of HD glass containers or ampoules, puncture wounds if using needles(4)) | YES (exposure if spills, splashes, aerosol generation, or accidental injection) |
| ||
| 27.4. Preparation of eyedrops | YES | YES (cuts if breakage of HD glass containers or ampoules, puncture wounds if using needles (4)) | YES (exposure if spills, splashes, aerosol generation, or accidental injection) | |||
| Preparation | ††27 | 27.5. Preparation of topical forms (creams, ointments, gels) | YES | YES (cuts if breakage of HD glass containers or ampoules, punctures if using needles(4), blunt trauma while crushing, cuts when splitting tablets) | YES (exposure if spills, splashes, aerosol generation, dust inhalation) | |
| 27.6. Preparation of capsules | YES | YES (blunt trauma if crushing starting HD) | YES (dust inhalation, exposure by contact with skin and mucous membranes) |
| ||
| 27.7. Modifying solid HDs(5) | YES | YES (blunt trauma when crushing HD, cuts when splitting tablets) | YES (dust inhalation, exposure by contact with skin and mucous membranes) | |||
| 27.8. Preparation of suspensions and solutions. | YES | YES (cuts if breakage of HD glass containers or ampoules, punctures if using needles(4), blunt trauma while crushing, cuts when splitting tablets) | YES (exposure if spills, splashes, aerosol generation, dust inhalation) |
| ||
| 27.9. Modifying/repackaging of liquid HDs | YES | YES (cuts if breakage of HD glass containers or ampoules, puncture wounds if using needles(4)) | YES (exposure if spills, splashes, aerosol generation, accidental injection) |
| ||
| 28 | Quality control and traceability during processing (using computerized traceability systems, or double checking (control without using computerized systems). | NO | − | − | − | |
| 29 | Waste management | YES | YES (cuts from ampoules or glass vials, puncture wounds from needles) | YES (exposure if spills or splashes) | Same as operation 7 | |
| 30 | Removal of PPEs(6) | YES | NO | YES (exposure by contact with HD residues if removal inadequate) | Same as operation 8 | |
| Preparation (common final stages) | 31 | Hand washing12 | NO | − | − | − |
| 32 | Quality control of finished product | NO | − | − | − | |
| 33 | Packaging | YES | NO | YES (exposure if loss of packaging integrity) |
| |
| 34 | Removal of PPEs(2) | YES | NO | YES (contact with HD residue if removal inadequate) | Same as operation 8 | |
| 35 | Waste management | YES | NO | YES (exposure by contact with HD residues) | Same as operation 7 | |
| 36 | Hand washing(2) | NO | − | − | − | |
| Preservation | 37 | Preservation (if applicable) | YES | NO | YES (exposure by loss of packaging integrity) |
|
| 38 | Visual control | NO | − | − | − | |
| 39 | Report incident to the compounding manager | NO | − | − | − | |
| 40 | Analysis of causes | NO | − | − | − | |
| 41 | Waste management | NO | − | − | − | |
| Dispensation | 42 | Dispensation | YES | NO | YES (exposure by loss of packaging integrity) |
|
Number that identifies each of the operations in the flowchart and facilitates its identification in the table
Operation 10 will only be conducted if the person responsible for transporting the HDs from the general store to the clean rooms did not previously wear PPEs in the storage area
Handwashing usually consists of disinfecting them with antiseptic solution when staying in the clean rooms because the work in them has not been completed. If the clean room is vacated, handwashing is done with soap and water
The removal of PPEs is conducted gradually, while passing through rooms with different degrees of environmental quality, following the standardized work procedure established. BSC, biological safety cabinet; HD, hazardous drug; IM, intramuscular; IT, intrathecal; IV, intravenous; PPE, personal protective equipment; SC, subcutaneous.
In total, 42 operations were established corresponding to the four stages of the handling process conducted in hospital pharmacy services: reception and bulk storage (17 operations [40.5%]); preparation (19 operations [45.2%]); drug storage (5 operations [12%]); and dispensing (1 operation [2.4%]).
Determination of potential hazardsTable 2 shows that 22 operations (52.4%) were associated with some type of hazard. These hazards were distributed as follows: physical hazard (12 operations [28.6%]; and chemical hazard (22 operations [52.4%]). No biological hazard was identified (see Table 2).
Degree of consensusThe degree of final consensus among the experts was 100% for all stages, operations, and hazards identified. However, the reception and bulk storage stage was the area that caused the greatest initial differences of opinion during its development. In cases of disagreement, the available scientific evidence was reviewed until the group reached a level of agreement of 100%.
DiscussionThe scientific literature has investigated and discussed in detail the health risks of handling HDs, regarding which there is increasing concern from the point of view of occupational health23.
The objective of the present study was to identify and analyse the theoretical hazards and risks of HDs in the HD logistics chain in hospital pharmacy services as the initial phase of a risk assessment. This objective required detailed knowledge of the entire logistics chain and its stages. The development of flowcharts played a key role in achieving this objective. As Ramos-Merino et al.24 indicated, flowcharts condense a large amount of information in a small space, visually represent the flow of the activities involved, and facilitate the rapid and efficient understanding of processes.
The main differences of opinion between the experts concerned the reception and bulk storage stage. Although this stage has been less addressed in the literature, it is the one in which there is the greatest variability in care practice, mainly due to the limited human resources, materials, and facilities available in each health centre. Nevertheless, after combining the opinions of the experts with the scientific evidence, a 100% consensus level was reached
Detailed study of the general process flowchart showed that almost half of the operations were concentrated in the preparation stage. This observation is unsurprising, given the heterogeneous catalogue of HDs that are currently prepared in pharmacy services (e.g. infusion bags, syringes, infusion pumps, topical forms, solid and liquid oral forms, and eye drops). Furthermore, in recent years, these types of processes have become more complex due to the need to improve the management of critical aspects that influence the safety of health personnel, patients, and the drugs themselves. This aspect explains the multiple control and protection operations during the preparation stage (i.e. operations 19, 23, 24, 25, 26, 28, and 32 [see Table 2 and Figure 2]), as well as the use of sophisticated devices and equipment that reduce to the greatest extent possible contamination of the work area and environment, thereby ensuring worker safety.
The main risk inherent to the use of HDs is chemical risk, which is due to their intrinsic characteristics of carcinogenicity, teratogenicity, genotoxicity, reproductive or developmental toxicity, or organ toxicity at low doses. However, physical hazards are also an issue because sharp and pointed objects (e.g. glass recipients containing the HD, needles used in their preparation) may be handled (i.e. operations 2, 4-7, 11-13, 20, 21, 27, and 29 [see Table 2]). No biological hazards were identified, because there is no direct contact with patients or their fluids during the stages of the process conducted in the pharmacy services.
However, although physical injury may occur (e.g. cuts from glass bottles or glass fragments, or puncture wounds from needles), it should be noted that in practice this type of risk has been greatly minimized by the increased use of devices and equipment without needles, luer-lock connections, and the widespread use of HD containers made of polyolefin-type plastic. Currently, the use of glass is uncommon and has been relegated to specific situations in which there are incompatibilities between plastics and HDs14.
Given the magnitude of the problem, it is unsurprising that in recent years several governmental and non-governmental organizations, scientific societies, and expert panels have encouraged health organization managers to conduct assessments of the risks associated with the HD circuit16,18,23,25-27. In any case, the first step in improving occupational safety is to identify hazards.
Although this study is useful as a basis for future projects, it has several limitations. The first of these is related to the selection of the experts. It would have been desirable and enriching to have had available the opinions of other types of expert, such as occupational risk specialists, preventionists, or occupational health technicians (due to their high level of knowledge in the field), as well as pharmacy nurses or technicians (because they work directly in these areas). However, this could not be achieved due to high workloads and the absence of incentives in the development of this study. Nevertheless, it should be noted that this study represents the initial phase of the identification and description of the process: that is, it is the preliminary phase of a risk analysis which will include the opinions of a wider range of experts. A further limitation is that the description of the process and the identification of hazards was based on the healthcare practices of two hospitals and on theoretical knowledge, concerning which there is a lack of solid evidence. The latter aspect is due to both the lack of published material on adverse events that would allow the identification of hazards, as well as the great heterogenicity of internationally published guidelines19, thus reducing the external validity and applicability of this study in other settings. However, the model obtained in this study should be highly reproducible given that it was based on the high level of knowledge of the participants in HD handling and the systematic review conducted as a preliminary to the expert consensus. In any case, risk analysis is a dynamic process that has to undergo periodical reassessment based on any nonconformities obtained, such that any bias derived from the subjectivity of the participating experts can be corrected in the future.
Based on the foregoing, we suggest that HDs should be integrated within a standardized management system to improve the safety of patients and health workers, while maximizing resource efficiency and minimizing procedural incidents. Such a system would make it possible to establish a global system with fully characterized stages that would guarantee the quality and safety of the HD handling process in pharmacy services.
Once hazards have been identified, risk assessment should be implemented using a systematic and preventive methodology to estimate the risk and severity of any adverse event.
FundingThis work was supported by the Instituto de Salud Carlos III de Madrid, Spain through the Health Research Project reference number PI16/00788.
AcknowledgmentsExpert group
Carmina Wanden-Bergue Lozano: Associate physician of the Home Hospitalization Unit, Hospital General Universitario de Alicante, Spain. Extensive experience in risk assessment. She has participated in competitive projects on Hazard Analysis and Critical Control Points (HACCP) addressing parenteral nutrient mixtures (PI13/00464), which has already been widely disseminated at an international level and led to the registration of patent 09/2014/3148.
Javier Sanz Valero: Public Health Pharmacist. Official Regulatory Control Officer of Public Health of the Department of Health of the Valencian Government. He was recently Auditor of Risk Analysis Systems, with proven experience in process quality audits through the application of risk analysis.
Scientific researcher of the Escuela Nacional de Medicina del Trabajo del Instituto de Salud Carlos III.
Associate Professor at the Universidad Miguel Hernández, Departamento de Salud Pública e Historia de la Ciencia.
María Ángeles Bernabeu Martínez: Hospital Pharmacist. Primary care pharmacist, Departamento Hospital General Universitario de Alicante, Spain.
Pedro García Salom: Hospital Pharmacist. Section Head. Hospital General Universitario de Alicante, Spain.
Coordinator of the Working Group on Hazardous Drugs of the Ministry of Health.
Member of the Expert Group of the Ministry of Health, Social Services, and Equality for the development of the “Good Practice Guide for Drug Preparation in Pharmacy Services”.
Amparo Burgos San José: Associate hospital pharmacist. Hospital General Universitario de Alicante, Spain Head of the Oncohematology Pharmacy Unit. BCOPS (Board-Certified Oncology Pharmacy Specialist).
Andrés Navarro Ruiz: Hospital Pharmacist. Head of Service. Hospital General Universitario de Elche.
Associate Professor at the Universidad Miguel Hernández. Masters in Pharmacovigilance and Post-authorization Studies. Masters in Pharmaceutical Care.
Conflicts of interestNo conflict of interests.