Patients included in MAINRITSAN2 trial received either an individually tailored or a fixed-schedule therapy with rituximab as maintenance treatment of antineutrophil cytoplasm antibody associated vasculitides. The aim of this study was to compare the real-world costs of both arms.
MethodWe performed a cost-minimization analysis over an 18-month time period, estimating direct costs –drug acquisition, preparation, administration and monitoring costs- from the health system perspective. We conducted a number of additional sensitivity analyses with different assumptions for unit costs, with further scenarios including the interquartile range of the tailored-infusion group results, different number of monitoring visits for fixed-schedule regimen and different number of reported severe adverse events. A cost-effectiveness analysis was conducted as a sensitivity analysis using the absolute difference in the relapse rate and its confidence interval.
ResultsThe individually tailored maintenance therapy with rituximab was shown to be a cost-saving treatment compared to the fixed-schedule therapy (6,049 euros vs. 7,850 euros). Savings resulted primarily from lower drug acquisition costs (2,861 vs. 4,768 euros) and lower preparation and administration costs (892 vs. 1,486 euros), due to the lower number of infusions per patient in the tailored-infusion regimen. The tailored-infusion regimen presented higher monitoring costs (2,296 vs. 1,596 euros). This result was replicated in all assumptions considered in the sensitivity analysis of cost-minimization approach.
ConclusionsFrom the perspective of the health system, the tailored-therapy regimen seems to be the preferable option in terms of direct costs. Further studies assessing all the effects and costs associated to vasculitides maintenance treatment with rituximab are needed to support clinical management and healthcare planning.
Los pacientes incluidos en el ensayo MAINRITSAN2 recibieron una pauta individualizada o un esquema fijo de rituximab como tratamiento de mantenimiento para la vasculitis asociada con anticuerpos contra el citoplasma de los neutrófilos. El objetivo de este estudio es comparar los costes reales de ambos esquemas de tratamiento.
MétodoSe llevó a cabo un análisis de minimización de costes sobre un periodo de 18 meses, estimando los costes directos —adquisición del fármaco, preparación, administración y costes de monitorización— desde la perspectiva del sistema de salud. Se realizaron varios análisis de sensibilidad con diferentes supuestos para los costes unitarios, añadiendo escenarios que incluían el rango intercuartílico de los resultados en el grupo de la pauta individualizada, diferente número de visitas de control para el grupo que seguía el esquema fijo y distinto número de eventos adversos registrados. Se realizó un análisis de coste-efectividad como parte del análisis de sensibilidad usando la diferencia absoluta en la tasa de recaída y su intervalo de confianza.
ResultadosEl esquema de tratamiento con la pauta individualizada demostró una reducción del coste en comparación con el esquema de dosis fijas (6.049 versus 7.850 euros). El ahorro se debió principalmente a un menor coste en la adquisición del fármaco (2.861 versus 4.768 euros) y a menos costes de preparación y administración (892 versus 1.486 euros), debido al menor número de infusiones por paciente en el brazo del esquema individualizado. Este esquema individualizado presentó mayores costes de monitorización (2.296 versus 1.596 euros). Este resultado se repitió en todos los supuestos considerados en el análisis de sensibilidad desde el enfoque de minimización de costes.
ConclusionesDesde la perspectiva del sistema de salud, la pauta individualizada parece ser la opción preferible en términos de costes directos. No obstante, son necesarios más estudios que evalúen todos los efectos y costes asociados al tratamiento de mantenimiento con rituximab de la vasculitis por anticuerpo anticitoplasma de neutrófilo para respaldar el manejo clínico y la asistencia sanitaria.
The use of rituximab as maintenance treatment of antineutrophil cytoplasm antibody (ANCA) associated vasculitides (AAVs) was supported by MAINRITSAN trial that demonstrated rituximab superiority to azathioprine1. Moreover, a recent publication concluded that maintenance treatment by rituximab instead of azathioprine could be cost-effective for preventing relapses in patients with AAVs2.
MAINRITSAN2 was a randomised, open-label, multicenter, phase III trial conducted in France which evaluated the difference between an individually tailored and a fixed-schedule maintenance therapy with rituximab3. The trial included 162 patients (81 per group) with newly diagnosed or relapsing granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA) in complete remission after induction therapy and those general characteristics were comparable. ANCA and circulating CD19+ B cells were the biological parameters monitored to re-infuse rituximab in tailored regimen. The conclusion was that AAVs relapse rates did not differ significantly between the two groups over a period of 28 months (18 months of treatment plus 10 months of follow-up). At month 28, 21 patients had suffered 22 relapses: 14/81 (17.3%) in 13 tailored-infusion recipients and 8/81 (9.9%) in 8 fixed-schedule patients (p = 0.22). Furthermore, individually tailored-arm patients received fewer rituximab infusions, 248 vs 381 infusions, with medians (interquartile range [IQR]) of 3 (2-4) vs 5 (5-5) administrations, respectively. An extension of MAINRITSAN2 is currently underway (MAINRITSAN3) to assess a long-term maintenance therapy with rituximab4.
The fact that fewer rituximab infusions were required with the individually tailored regimen is important in terms of cost. Although, cost-minimisation analysis can not be conducted unless two alternative treatments are demonstrated to be equivalent, in clinical practice, this type of economic evaluation is frequently applied when a clinical relevant difference has not been demonstrated between two alternative options. Based on this, the present study used a cost-minimization analysis as the base approach to examine the real-world costs of an individually tailored-therapy compared to a fixed-schedule therapy with rituximab for remission maintenance of AAVs to facilitate decision making in clinical practice. A cost-effectiveness analysis has been considered in the sensitivity analysis.
MethodsModel structure & key assumptionsIn MAINRITSAN2, tailored-infusion-arm patients received 500 mg of rituximab at randomization and another 500 mg when CD19+ B cell count and/or ANCA changes were documented in trimestral testing. The fixed-schedule therapy patients received 500 mg rituximab infusion on days 0 and 14 post randomization and at months 6, 12, 18 after the first infusion. There were 81 patients in each arm who accumulated 248 vs 381 infusions, with medians (IQR) of 3 (2-4) vs 5 (5-5) administrations per patient, respectively3.
We conducted a cost-minimization analysis (CMA) using Excel® 2016. The model we developed adopted the perspective of the health system, and evaluated direct costs incurred by treating patients. Total costs included drug acquisition, intravenous drug preparation, administration and monitoring costs.
The model time horizon was 18 months in line with the treatment phase of MAINRITSAN2. Costs were calculated in 2018 euros. We could not apply any cost discounting despite costs being measured over a period of more than 1 year because we were unable to determine exact rituximab administration dates in the tailored-infusion group from the trial paper.
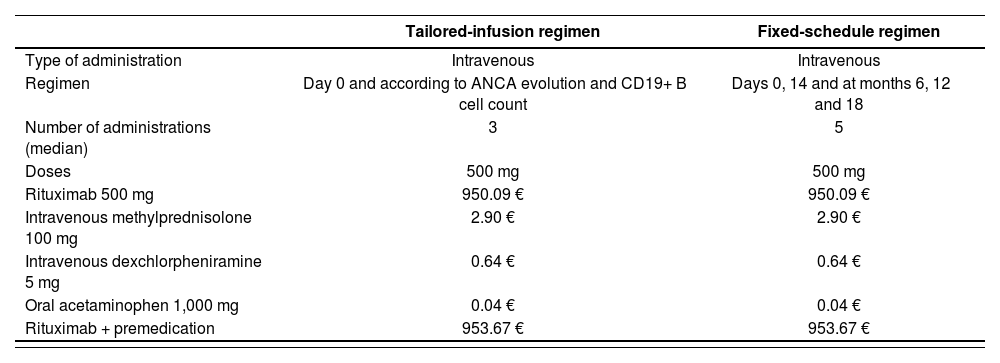
Drug acquisitionDrug acquisition costs (Table 1) included cost of rituximab and premedication: intravenous methylprednisolone (100 mg), intravenous dexchlorpheniramine (5 mg) and oral acetaminophen (1,000 mg) as reported by the MAINRITSAN2 clinical trial3.
Drug unit costs
| Tailored-infusion regimen | Fixed-schedule regimen | |
|---|---|---|
| Type of administration | Intravenous | Intravenous |
| Regimen | Day 0 and according to ANCA evolution and CD19+ B cell count | Days 0, 14 and at months 6, 12 and 18 |
| Number of administrations (median) | 3 | 5 |
| Doses | 500 mg | 500 mg |
| Rituximab 500 mg | 950.09 € | 950.09 € |
| Intravenous methylprednisolone 100 mg | 2.90 € | 2.90 € |
| Intravenous dexchlorpheniramine 5 mg | 0.64 € | 0.64 € |
| Oral acetaminophen 1,000 mg | 0.04 € | 0.04 € |
| Rituximab + premedication | 953.67 € | 953.67 € |
ANCA: antineutrophil cytoplasm antibody.
For base case analysis, we adopted our hospital's drug prices including 4% value added tax (VAT) and 7.5% discount for rituximab biosimilar (Truxima® 500 mg5, available on the European market since 2017 and indicated in GPA and MPA treatment), as indicated in Royal Decree of Law 8/20106.
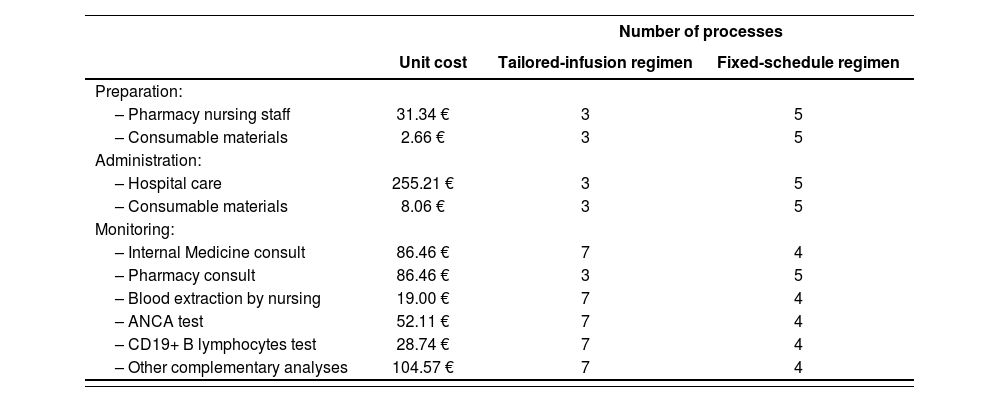
Preparation, administration and monitoringWe based the decision on which resources to use in preparation, administration and monitoring (Table 2) on the opinion of clinical experts. The unit costs associated with these resources were an average obtained from the health costs database eSalud7.
Preparation, administration and monitoring costs
| Number of processes | |||
|---|---|---|---|
| Unit cost | Tailored-infusion regimen | Fixed-schedule regimen | |
| Preparation: | |||
| – Pharmacy nursing staff | 31.34 € | 3 | 5 |
| – Consumable materials | 2.66 € | 3 | 5 |
| Administration: | |||
| – Hospital care | 255.21 € | 3 | 5 |
| – Consumable materials | 8.06 € | 3 | 5 |
| Monitoring: | |||
| – Internal Medicine consult | 86.46 € | 7 | 4 |
| – Pharmacy consult | 86.46 € | 3 | 5 |
| – Blood extraction by nursing | 19.00 € | 7 | 4 |
| – ANCA test | 52.11 € | 7 | 4 |
| – CD19+ B lymphocytes test | 28.74 € | 7 | 4 |
| – Other complementary analyses | 104.57 € | 7 | 4 |
ANCA: antineutrophil cytoplasm antibody.
Preparation costs consisted of consumable materials (500 mL saline solution for dilution, 50 mL syringe, vented spike, alcohol, gauze, gown, latex gloves, disposable surgical cap and mask, and plastic bag for transport) and rituximab reconstitution and dilution in aseptic conditions by pharmacy nursing staff (31.34 euros per preparation). Equipment costs such as maintenance contracts of electromedical equipment (laminar flow cabinet) were considered negligible.
Costs for administration process included the patient's stay in the treatment room receiving specialized care provided by nurses (252.24 euros per stay) and auxiliary nurses during the first 30 minutes (5.94 euros per hour). Intravenous sets and solutions for premedication dilutions (50 mL saline solutions) were included as consumable materials.
In the MAINRITSAN2 trial, therapy monitoring consisted of extraction of blood samples and ANCA and CD19+ B lymphocytes tests. In addition, costs for Internal Medicine consult, Pharmacy consult before rituximab administration and other complementary analyses were included in keeping with routine clinical practice. Internal Medicine consults, extraction of blood samples and blood tests were trimestral in the tailored-infusion group and before infusion in the other arm, except for the first two administrations (days 0 and 14) that were scheduled in the first visit. Other complementary analyses involved complete blood count, kidney function test, liver function test, reactive C protein, globular sedimentation rate, serum immunoglobulins and urine test. Costs from routine complementary tests before the beginning of treatment were excluded from the model.
Sensitivity analysisTo explore the robustness of the results, we performed several one-way sensitivity analyses (S) to test the impact of using different assumptions for unit costs. To perform S2 and S3 analysis, we consulted different European drug price databases8-10.
- •
S1: price of rituximab MabThera® 500 mg in our hospital including VAT (4%) and 15% discount as indicated in Royal Decree of Law 8/20106.
- •
S2: the highest price for rituximab in Europe without VAT.
- •
S3: the lowest price for rituximab in Europe without VAT.
- •
S4: considering maximum unit costs for preparation, administration and monitoring7.
- •
S5: considering minimum unit costs for preparation, administration and monitoring7.
The analysis also considered two scenarios (S6 and S7) including the interquartile range of number of cycles in the tailored-infusion group3, two monitoring visits instead of one before infusions of days 0 and 14 in the fixed-schedule regimen (S8) and serious adverse effect costs (S9).
- •
S6: four administrations per patient in the tailored-infusion group.
- •
S7: two administrations per patient in the tailored-infusion group.
- •
S8: five Internal Medicine consults, five blood extractions and five blood tests for monitoring of fixed-schedule regimen.
- •
S9: 26 of 81 tailored-infusion recipients vs 31 of 81 patients in the fixed-scheduled group reported at least one severe adverse event (SAE), with no statistically significant differences between the groups and 37 vs 53 SAEs per group3. A unit cost for each adverse effect of 294 euros was used11.
Finally, a cost-effectiveness analysis was conducted as sensitivity analysis using the absolute difference in the relapse rate between groups and its 95% confidence interval 7.4% (95%CI -3.1%; 17.9%), although it was not statistically significant.
- •
S10: 3.1% less relapses with tailored-infusion regimen.
- •
S11: 7.4% more relapses with tailored-infusion regimen.
- •
S12: 17.9% more relapses with tailored-infusion regimen.
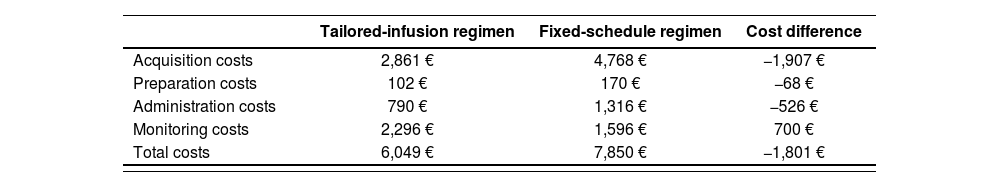
In the base case, the per-patient total cost for the tailored-infusion regimen with rituximab for remission maintenance of AAVs was 6,049 euros vs 7,850 euros for the fixed-schedule regimen. This represents a saving of 1,801 euros (23%) per patient in direct costs for the health system (Table 3).
Results of base case cost-minimization analysis
| Tailored-infusion regimen | Fixed-schedule regimen | Cost difference | |
|---|---|---|---|
| Acquisition costs | 2,861 € | 4,768 € | −1,907 € |
| Preparation costs | 102 € | 170 € | −68 € |
| Administration costs | 790 € | 1,316 € | −526 € |
| Monitoring costs | 2,296 € | 1,596 € | 700 € |
| Total costs | 6,049 € | 7,850 € | −1,801 € |
Savings were primarily due to lower drug acquisition costs for the tailored-infusion regimen (2,861 euros vs 4,768 euros) in addition to lower preparation and administration costs (892 euros vs 1,486 euros) due to the lower number of infusions per patient in the tailored-infusion regimen. The tailored-infusion regimen presented higher monitoring costs (2,296 euros vs 1,596 euros).
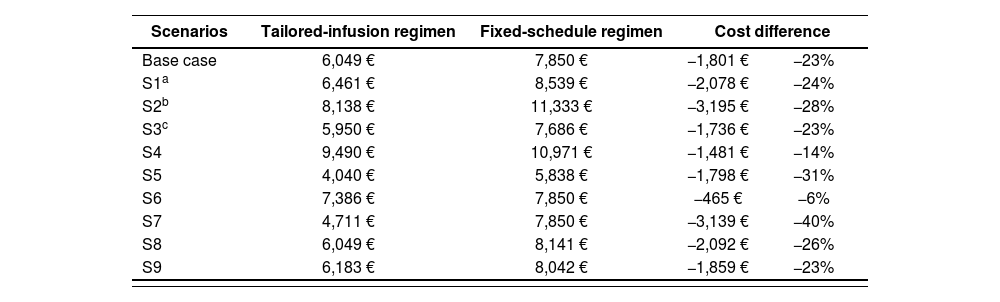
The results of the sensitivity analysis S1 to S9 are shown in Table 4.
Sensitivity analysis of base case
| Scenarios | Tailored-infusion regimen | Fixed-schedule regimen | Cost difference | |
|---|---|---|---|---|
| Base case | 6,049 € | 7,850 € | −1,801 € | −23% |
| S1a | 6,461 € | 8,539 € | −2,078 € | −24% |
| S2b | 8,138 € | 11,333 € | −3,195 € | −28% |
| S3c | 5,950 € | 7,686 € | −1,736 € | −23% |
| S4 | 9,490 € | 10,971 € | −1,481 € | −14% |
| S5 | 4,040 € | 5,838 € | −1,798 € | −31% |
| S6 | 7,386 € | 7,850 € | −465 € | −6% |
| S7 | 4,711 € | 7,850 € | −3,139 € | −40% |
| S8 | 6,049 € | 8,141 € | −2,092 € | −26% |
| S9 | 6,183 € | 8,042 € | −1,859 € | −23% |
Considering the uncertainty about the difference in relapse rate, in the S10 scenario the tailored-infusion regimen would be a dominant strategy. It would be associated with lower rate of relapses, 3% lower, and lower cost, 1,801 euros less per patient. In the S11 and S12 scenarios, the fixed-schedule regimen would be an alternative more costly and more effective, with an incremental cost effectiveness ratio (ICER) of 24,338 euros per avoided relapse in S11. This means that if the fixed schedule is used instead of the tailored-infusion regimen, it would cost 24,338 euros per avoided relapse. Similarly, in the S12 scenario, the ICER would be 10,061 euros per avoided relapse.
DiscussionThe tailored-therapy had higher monitoring costs than the fixed-schedule maintenance therapy, which had higher drug acquisition, preparation and administration costs owing to patients undergoing this regimen receiving more cycles of rituximab. Overall, tailored-therapy costs less than the fixed regimen. This result was replicated in all assumptions considered in the sensitivity analysis of cost-minimization approach, emphasizing its robustness.
Regardless of the global reduction of drug acquisition price due to biosimilar commercialization, drug acquisition cost will still represent the main cost in the overall attention of these patients. Therefore, regimens able to reach the same effectiveness with fewer drug infusions will be less onerous. Furthermore, the reduction in the number of infusions in the tailored-infusion arm decreased the theoretical probability of the occurrence of adverse events and severe adverse events (SAEs). However, the clinical trial found no statistically significant differences between the two groups regarding safety3.
We found a large number of studies evaluating rituximab as a maintenance treatment in AAVs, but only a few looked at economic considerations. Richard A. Watts et al. carried out a study to quantify the global burden of AAVs. They declared that the introduction of biological therapies increased drug costs, but some of those increases might be offset by better disease control12. Turner-Stokes T. et al. studied the induction treatment of AAVs with a single dose of rituximab instead of several doses. They questioned the need to repeat dosing and adopted a standard single-dose protocol to treat active AAVs13. Pentek M. et al. evaluated the impact of biosimilars on chronic immune-mediated inflammatory diseases. Their results suggested that, given the lower price of biosimilars, formerly established biological treatment sequence practices and the eligibility criteria for biological treatment should be reconsidered. They concluded that biosimilars may contribute to better patient-access and provide savings to governments14.
The MAINRITSAN2 study3 has shown that either of the two treatment modalities could be implemented in clinical practice with similar efficacy. The main strength of our study is that it provides added information for decision-making in clinical practice over the choice of the maintenance treatment of ANCA associated vasculitides.
The main limitation of the present analysis is that the economic evaluation is based on the results of a single study, the MAINRITSAN2, with a limited number of patients, and, therefore, limited statistical power to detect as significant some clinically relevant differences between groups in relapse rate or in the incidence of serious adverse effects. However, this study provides the best evidence available to choose in clinical practice between a tailored or fixed rituximab regimen, and this economic evaluation adds costs information for decision making. In the base-case scenario, similar efficacy and safety was assumed and therefore a cost-minimization analysis was performed, and in the sensitivity analysis, a cost-effectiveness analysis was performed to consider the uncertainty in effectiveness. The latter has also the limitation of not including survival or quality of life data; however, this information is not currently available. In addition, cost of relapses were not included. In the cost-effectiveness analysis, the fixed dose strategy goes from being dominated by the tailored strategy to being more effective and more costly. To know whether it is an efficient strategy is difficult as there is uncertainty regarding long term or final outcome consequences of relapses and serious adverse effects. When this information will be available the best strategy will have to be reconsidered, until then this economic evaluation can guide us as the best alternative to select.
A discount rate could not be applied because of the absence of information about when the successive treatments occurred in the tailored therapy arm. However, this should not have been significant because the total period of the study was only 18 months. We were also unable to find data on the cost of patient hospital care depending on the duration of the treatment administration; we had to use the cost of a full visit with an average duration calculated among many other types of treatments and medical procedures. This fact would not seem to have influenced results because of the small contribution of this cost to the total cost. Our study was affected by the lack of transparency regarding official prices in the western world and, above all, by the opacity of the real purchase prices of public health systems and large health maintenance organizations or insurers. We have applied the prices available in the databases8-10, although some of them may not be completely updated. In any case, this CMA is easily reproducible for those who wish to apply it in other scenarios.
In this analysis, we have established a point of view of the health system without considering patients’ preferences, or indirect and intangible costs (although these are very difficult to measure). The tailored-infusion option requires more medical visits and blood extractions. It also generates uncertainty in patients since their therapeutic plan is not defined from the outset. For these reasons, in the future we need to establish which treatment option generates less expense for patients, less discomfort and less interference in their daily life.
This economic evaluation based on available data shows the cost-saving option between a tailored-therapy and a fixed-schedule regimen with rituximab for the maintenance treatment of AAVs. From the perspective of the health system, the tailored-therapy regimen seems to be the preferable option in terms of direct costs. Further studies assessing all the effects and costs associated to AAVs maintenance treatment with rituximab are needed to support clinical management and healthcare planning.
FundingNo funding.
Conflict of interestsNo conflict of interest.
Presentation in CongressesEuropean Congress of Hospital Pharmacy. European Association of Hospital Pharmacists. Barcelona: March 27, 2019
Contribution to scientific literature
This cost-minimization analysis serves as comparison between costs associated to, either tailored or fixed-schedule maintenance therapy with rituximab, of antineutrophil cytoplasm antibody associated vasculitides. Numerous studies have been published evaluating rituximab as maintenance treatment for this type of vasculitis. However, few of them have considered its economical perspective. Thus, this study provides additional information for decision-making in clinical practice over the choice of maintenance treatment of antineutrophil cytoplasm antibody associated vasculitides.
According to this analysis, tailored rituximab regimen would be the most economic therapeutic option against fixed rituximab regimen. Drug acquisition cost will continue being the main expense of the patients’ general care. Thus, all regimens that are able to reach the same effectiveness with less infusions will result in being more affordable.