There are many medicinal products that, although having shown efficacy and safety in different ophthalmological indications, they are not authorized or commercially available for ophthalmic administration. This implies, on one hand, that they must be used according to legislation that regulates the availability of medicines in special situations and, on the other hand, that they must be prepared in the pharmacy services for ophthalmic administration, according to quality criteria to ensure its effectiveness, stability and sterility. This document gathers the consensus between the Spanish Society of Ophthalmology and the Spanish Society of Hospital Pharmacy about these selected preparations which have shown enough evidence in their efficacy and safety for their ophthalmic use (off label) and ophthalmic administration. This document includes recommendations about its use according to the current legislation. In addition, with the aim of harmonizing the preparation of intraocular injections in the hospital pharmacy services, general recommendations are set in this document to ensure the compliance with standards established in the Spanish Guideline for GoodPreparation Practices of Medicinal Products in Hospital Pharmacies. These recommendations include sections such as the area of preparation, material, technique, packaging, stability, quality control, prescription and traceability of intraocular preparations.
Son muchos los medicamentos que, aun habiendo demostrado eficacia y seguridad en diferentes indicaciones oftalmológicas, no están autorizados ni disponibles comercialmente en una forma adecuada para esta vía de administración. Esto implica, por un lado, que se deban utilizar según la legislación que regula la disponibilidad de medicamentos en situaciones especiales y, por otro, que se deban preparar en los Servicios de Farmacia para su administración por vía oftálmica, conforme a unos criterios de calidad que aseguren su efectividad, estabilidad y esterilidad. Este documento recoge un consenso entre la Sociedad Española de Oftalmología y la Sociedad Española de Farmacia Hospitalaria sobre aquellas preparaciones con suficiente evidencia respecto a su eficacia y seguridad para su uso no autorizado en indicaciones y vía de administración oftálmicas. Se incluyen recomendaciones para su utilización de acuerdo con la legislación vigente. Además, con el ánimo de armonizar la preparación de inyecciones intraoculares en los Servicios de Farmacia Hospitalaria, se establecen unas recomendaciones generales para su elaboración siguiendo los estándares establecidos en la Guía de Buenas Prácticas de Preparación de Medicamentos en los Servicios de Farmacia Hospitalaria. En estas recomendaciones se incluyen apartados como el lugar de preparación, el material, la técnica, el envasado, el periodo de validez, el control de calidad, la prescripción y la trazabilidad de las preparaciones intraoculares.
Currently, the pharmaceutical industry does not offer forms that cover all the needs of ophthalmological treatment. Thus, this therapeutic gap should be addressed by the centralised preparation of such forms in hospital Pharmacy Services (PS) using authorized medications for other indications or administration routes.
The use of medicines under conditions other than those described in the summary of product characteristics is regulated by the Spanish Royal Decree (RD) 1015/20091, which defines this approach as “The use of medicines under conditions other than those authorized” (Spanish acronym: UMCDA). Chapter III, article 13 of the RD establishes the requirements for such use and includes the following statement:
- •
The doctor must provide a sound justification in the clinical history of the need to use the medication.
- •
The responsible doctor must obtain the consent of the patient according to Spanish Law 41/2002 on patient autonomy.
- •
Suspected adverse reactions must be notified according to the provisions of RD 1344/2007 regulating the Pharmacovigilance of Medicinal Products for Human Use.
- •
Restrictions that have been established related to the prescription and dispensation of the medication and the therapeutic protocol of the centre will be respected.
The fact that the UMCDA may be linked to a protocol prepared by the health centre serving the patient involves the need to carefully assess the available evidence on each medication for each of the indications for which it is intended.
Furthermore, in 2011, in resolution CM/ResAP (2011)12, the Council of Europe aimed to standardise the quality of medicinal products, and recommended the development of practical guidelines on drug preparation to avoid differences in quality and safety between medications prepared in pharmacies and those manufactured on an industrial scale. Thus, in 2012, in its adaptation to Spanish regulations, RD Law 16/2012 was published on urgent measures to guarantee the sustainability of the National Health System and improve the quality and safety of its services3. RD Law 16/2012, article 7 establishes that the PSs in which these operations are conducted must guarantee adherence to the good practice technical guidelines. Therefore, the preparation of medicines must meet the quality criteria outlined in the Good Practice Guidelines on Pharmaceutical Preparation (Spanish acronym: GBPP)4 in order for them to be dispensed in a form ready to be administered under the required conditions after risk assessment and assignment of the necessary quality criteria. The preparation of pre-filled syringes ready to use is included as one of the operations of the manipulation and adaptation of preparations3. Specifically, the division of very high-cost therapeutic substances from a commercial single-use vial into multiple individual doses is frequently performed by hospital PSs in the attempt to minimize the high economic impact of these therapies.
Based on the risk assessment, preparations for intraocular administration should be prepared centrally in PSs in a laminar flow cabinet (LFC) with a controlled environment given the possibility of patients experiencing adverse effects caused by the contamination of these types of preparations4. However, although there are many general recommendations for the preparation of these types of products, to date there is no unanimous consensus on specific techniques to maximize benefits, while obtaining the largest number of individual syringes possible and ensuring the sterility, stability, and effectiveness of the doses prepared.
The objectives of this document are: (1) to establish which drugs and under what conditions there is sufficient pharmaceutical and clinical evidence to support their use other than that described in the summary of product characteristics in order to cover the most common therapeutic gaps in ophthalmological treatment, and thus facilitate the development of care protocols in health centres; and (2) to establish a set of general recommendations for the preparation of intraocular injections that are useful for the health personnel involved in their preparation and that increase patient safety.
MethodsThis document was developed by a working group comprising members of the Spanish Group of Pharmaceutical Compounding of the Spanish Society of Hospital Pharmacy (Spanish acronym: SEFH) and members appointed by the board of the Spanish Society of Ophthalmology (Spanish acronym: SEO).
The document was developed in several phases:
- •
The members of the Spanish group of Hospital Pharmacy Compounding shared information on the ophthalmological medications they most frequently prepared in their own health centres, their indications, and the galenic and clinical evidence on which they were based.
- •
After drawing up an initial list, it was reviewed by the SEO assessment group for possible corrections or the inclusion of new preparations.
- •
The list reviewed by the SEO underwent a final literature search of the galenic evidence related to the selected preparations using PubMed. Thus, the following keywords were used: ophthalmic solutions, drug stability, keratitis, endophthalmitis therapy, intravitreal injections, intraocular injections, and drug compounding.
- •
Members of the Spanish Group of Hospital Pharmacy Compounding used the GBPP4 as a reference to establish recommendations on the preparation of intraocular injections. This group also used PubMed and other electronic literature sources to conduct a literature search using the following keywords: intraocular injections, intravitreal injections, pharmaceutical preparations, drug compounding, drug stability. In this way, a draft recommendation was created on the characteristics of the environment, material required, preparation technique, shelf-life, and quality control of the intraocular preparations. This draft was shared among the members for its review and for further contributions taking into account the expertise of each professional in each of the sections mentioned. In this phase, it was decided to include aspects related to syringe packaging, prescription, and traceability of the samples.
- •
Finally, the document was reviewed and endorsed by the SEO assessment group within the framework of the SEO-SEFH collaboration agreement. The document was signed by the board of the SEO on November 16, 2017.
As mentioned, the use of medications under conditions other than those authorized involves respecting the established restrictions on the prescription and dispensation of the medications and following the health care protocol of the centre. This protocol (or protocols if it has been decided to design one for each active ingredient) must be agreed upon by the services involved in the use of the medication and approved by the Pharmacy and Therapeutics Commission and by the management of the health centre.
Table 1 shows a proposed protocol for the use of ophthalmic drugs under conditions other than those authorized.
Proposed protocol for the use of ophthalmic drugs under conditions other than those authorized
| Objective: Define the treatment and indication. |
|
| Related documentation and references. |
|
| Responsibilities. |
|
| Management of the implementation of the procedure: Management/Commission/Working group to sponsor the protocol, those who have prepared it, date of coming into force, dissemination, date of review, indicators for the assessment of the objectives, and those responsible for their measurement. |
|
In each application the prescribing physician provides a sound justification in the clinical history of the need to use the medication, inform the patient of the potential benefits and risks, and obtain their informed consent.
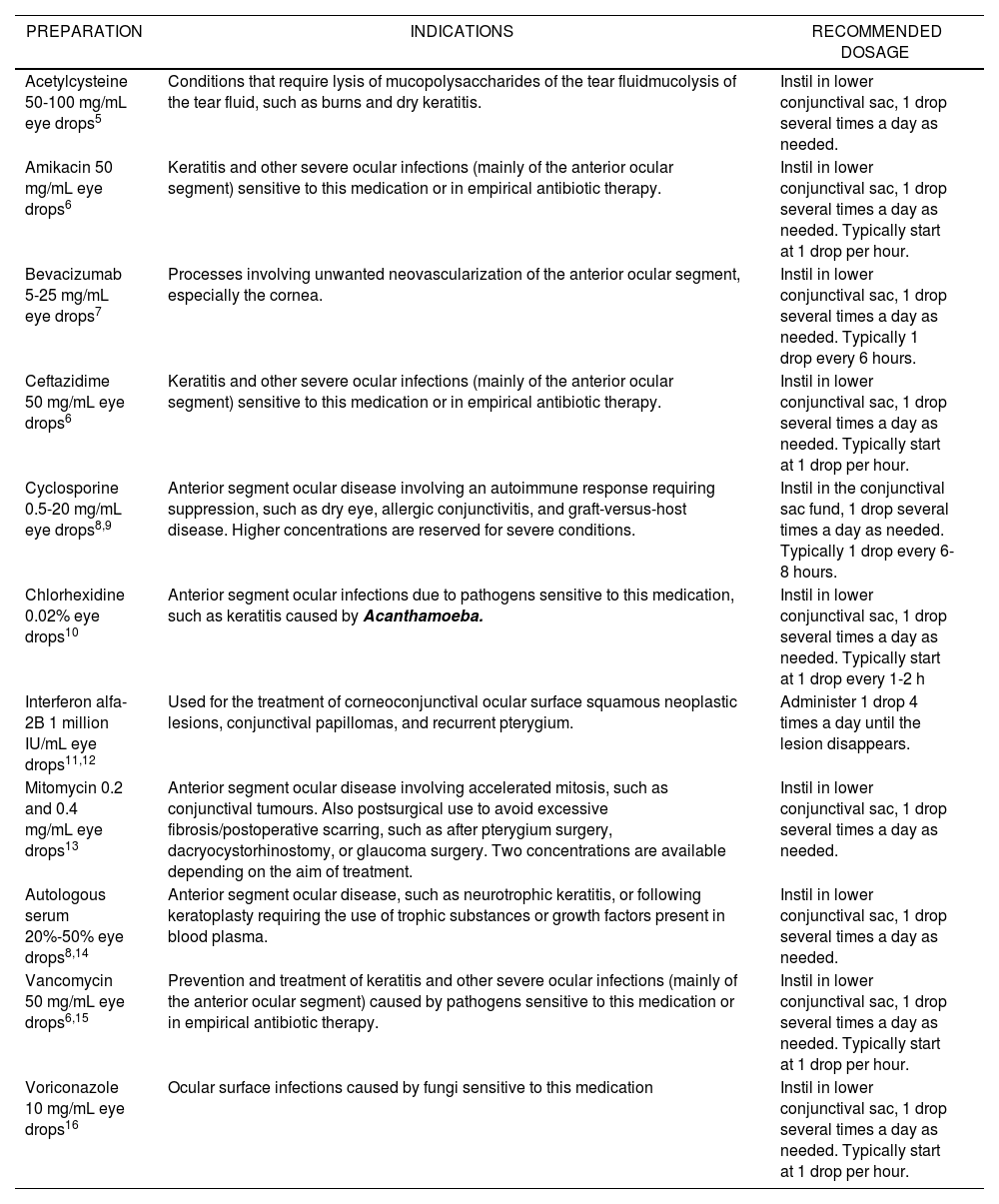
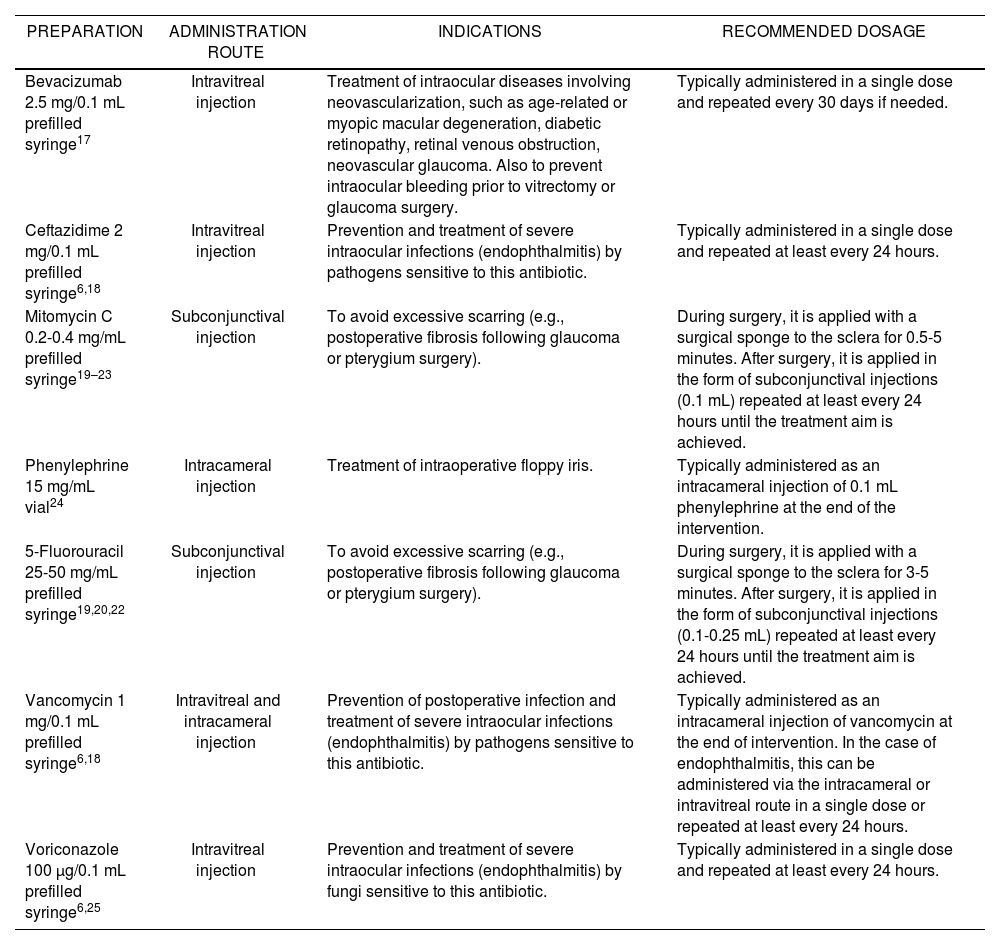
Tables 2 and 3 provide examples of eye drops and intraocular injections, respectively, their indications (unauthorized), and the most common posology.
Preparations of Eye Drops, Indications and Posology, and Recommended Dosage
| PREPARATION | INDICATIONS | RECOMMENDED DOSAGE |
|---|---|---|
| Acetylcysteine 50-100 mg/mL eye drops5 | Conditions that require lysis of mucopolysaccharides of the tear fluidmucolysis of the tear fluid, such as burns and dry keratitis. | Instil in lower conjunctival sac, 1 drop several times a day as needed. |
| Amikacin 50 mg/mL eye drops6 | Keratitis and other severe ocular infections (mainly of the anterior ocular segment) sensitive to this medication or in empirical antibiotic therapy. | Instil in lower conjunctival sac, 1 drop several times a day as needed. Typically start at 1 drop per hour. |
| Bevacizumab 5-25 mg/mL eye drops7 | Processes involving unwanted neovascularization of the anterior ocular segment, especially the cornea. | Instil in lower conjunctival sac, 1 drop several times a day as needed. Typically 1 drop every 6 hours. |
| Ceftazidime 50 mg/mL eye drops6 | Keratitis and other severe ocular infections (mainly of the anterior ocular segment) sensitive to this medication or in empirical antibiotic therapy. | Instil in lower conjunctival sac, 1 drop several times a day as needed. Typically start at 1 drop per hour. |
| Cyclosporine 0.5-20 mg/mL eye drops8,9 | Anterior segment ocular disease involving an autoimmune response requiring suppression, such as dry eye, allergic conjunctivitis, and graft-versus-host disease. Higher concentrations are reserved for severe conditions. | Instil in the conjunctival sac fund, 1 drop several times a day as needed. Typically 1 drop every 6-8 hours. |
| Chlorhexidine 0.02% eye drops10 | Anterior segment ocular infections due to pathogens sensitive to this medication, such as keratitis caused by Acanthamoeba. | Instil in lower conjunctival sac, 1 drop several times a day as needed. Typically start at 1 drop every 1-2 h |
| Interferon alfa-2B 1 million IU/mL eye drops11,12 | Used for the treatment of corneoconjunctival ocular surface squamous neoplastic lesions, conjunctival papillomas, and recurrent pterygium. | Administer 1 drop 4 times a day until the lesion disappears. |
| Mitomycin 0.2 and 0.4 mg/mL eye drops13 | Anterior segment ocular disease involving accelerated mitosis, such as conjunctival tumours. Also postsurgical use to avoid excessive fibrosis/postoperative scarring, such as after pterygium surgery, dacryocystorhinostomy, or glaucoma surgery. Two concentrations are available depending on the aim of treatment. | Instil in lower conjunctival sac, 1 drop several times a day as needed. |
| Autologous serum 20%-50% eye drops8,14 | Anterior segment ocular disease, such as neurotrophic keratitis, or following keratoplasty requiring the use of trophic substances or growth factors present in blood plasma. | Instil in lower conjunctival sac, 1 drop several times a day as needed. |
| Vancomycin 50 mg/mL eye drops6,15 | Prevention and treatment of keratitis and other severe ocular infections (mainly of the anterior ocular segment) caused by pathogens sensitive to this medication or in empirical antibiotic therapy. | Instil in lower conjunctival sac, 1 drop several times a day as needed. Typically start at 1 drop per hour. |
| Voriconazole 10 mg/mL eye drops16 | Ocular surface infections caused by fungi sensitive to this medication | Instil in lower conjunctival sac, 1 drop several times a day as needed. Typically start at 1 drop per hour. |
Preparations, Indications, and Recommended Dosage
| PREPARATION | ADMINISTRATION ROUTE | INDICATIONS | RECOMMENDED DOSAGE |
|---|---|---|---|
| Bevacizumab 2.5 mg/0.1 mL prefilled syringe17 | Intravitreal injection | Treatment of intraocular diseases involving neovascularization, such as age-related or myopic macular degeneration, diabetic retinopathy, retinal venous obstruction, neovascular glaucoma. Also to prevent intraocular bleeding prior to vitrectomy or glaucoma surgery. | Typically administered in a single dose and repeated every 30 days if needed. |
| Ceftazidime 2 mg/0.1 mL prefilled syringe6,18 | Intravitreal injection | Prevention and treatment of severe intraocular infections (endophthalmitis) by pathogens sensitive to this antibiotic. | Typically administered in a single dose and repeated at least every 24 hours. |
| Mitomycin C 0.2-0.4 mg/mL prefilled syringe19–23 | Subconjunctival injection | To avoid excessive scarring (e.g., postoperative fibrosis following glaucoma or pterygium surgery). | During surgery, it is applied with a surgical sponge to the sclera for 0.5-5 minutes. After surgery, it is applied in the form of subconjunctival injections (0.1 mL) repeated at least every 24 hours until the treatment aim is achieved. |
| Phenylephrine 15 mg/mL vial24 | Intracameral injection | Treatment of intraoperative floppy iris. | Typically administered as an intracameral injection of 0.1 mL phenylephrine at the end of the intervention. |
| 5-Fluorouracil 25-50 mg/mL prefilled syringe19,20,22 | Subconjunctival injection | To avoid excessive scarring (e.g., postoperative fibrosis following glaucoma or pterygium surgery). | During surgery, it is applied with a surgical sponge to the sclera for 3-5 minutes. After surgery, it is applied in the form of subconjunctival injections (0.1-0.25 mL) repeated at least every 24 hours until the treatment aim is achieved. |
| Vancomycin 1 mg/0.1 mL prefilled syringe6,18 | Intravitreal and intracameral injection | Prevention of postoperative infection and treatment of severe intraocular infections (endophthalmitis) by pathogens sensitive to this antibiotic. | Typically administered as an intracameral injection of vancomycin at the end of intervention. In the case of endophthalmitis, this can be administered via the intracameral or intravitreal route in a single dose or repeated at least every 24 hours. |
| Voriconazole 100 μg/0.1 mL prefilled syringe6,25 | Intravitreal injection | Prevention and treatment of severe intraocular infections (endophthalmitis) by fungi sensitive to this antibiotic. | Typically administered in a single dose and repeated at least every 24 hours. |
Annex 1 shows the recommendations for the preparation of intraocular preparations. This annex provides information on the following aspects: preparation area, characteristics of the material, preparation technique, packaging, shelf-life, quality control, prescription, and traceability.
Depending on the type of preparation, special requirements will be taken into account for the preparation of terminal sterilization products or for aseptic preparation as outlined in the GBPP4.
ConclusionsThere is ample clinical evidence supporting the use of the drugs selected in this study for ophthalmic use, although the health authorities do not recognize such use in the majority of cases.
From a legal point of view, these preparations would be included in what is known as the use of medicines under conditions other than those authorized. Their use should be incorporated in the health care protocols agreed by the ophthalmology services and approved by the Pharmacy and Therapeutics Commission and the centre's management body.
There is also galenic evidence supporting the preparation of these medications such that they can be administered by the ophthalmic route in the form of eye drops or intraocular injections.
Ophthalmic medications must be prepared in accordance with the guidelines established in the GBPP. In addition, the specific recommendations on intraocular preparations established in this document will help to standardize and facilitate the preparation of these medications in hospital PSs, thereby making these types of preparations equally accessible to all patients.
Annex 1Recommendations for the Compounding of Intraocular Preparations| 1. PREPARATION AREA |
|
| 2. MATERIAL |
|
| 3. PREPARATION TECHNIQUE |
|
|
| 4. PACKAGING |
|
| 5. SHELF-LIFE |
|
| 6. QUALITY CONTROL |
|
| 7. PRESCRIPTION |
|
| 8. TRACEABILITY |
|
| 9. OTHER OBSERVATIONS |
|
No funding
AcknowledgmentsThe authors of this document wish to thank Dr. José Luis Encinas Martín (president of the SEO), Dr. Miguel Ángel Calleja Hernández (president of the SEFH), Dr. Ana Lozano Blázquez (Vice-President of the SEFH), and Dr. Montserrat Pérez Encinas (Secretary of the SEFH) for supporting the project and signing the agreement of collaboration between both Societies.
Conflict of interestsNo conflict of interest.