To assess critically oritavancin, a second-generation lipoglycopeptide, for the treatment of Acute Bacterial Skin and Skin Structure Infections caused by susceptible Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus.
MethodAn evaluation report of oritavancin in Acute Bacterial Skin and Skin Structure Infections was carried out according to the methodology of the Group for drug evaluation, standardization and research in drug selection of the Spanish Society of Hospital Pharmacy (SEFH)1, with the MADRE 4.0 program. A search was made in PubMed, in the web www.clinicaltrials.gov, Embase, PubMed and UptoDate. The European Medication Agency and Food and Drug Administration evaluation reports were also used.
ResultsSingle-dose oritavancin demonstrated its non-inferiority efficacy versus vancomycin in Acute Bacterial Skin and Skin Structure Infections, with a similar safety profile. Its potential advantage over other therapeutic alternatives lies in its administration in single dose and in its no need for plasma levels monitoring, which would allow its administration on an outpatient basis. Regarding to the other alternative possibilities of oral (linezolid, tedizolid) or IM (teicoplanin) treatment, oritavancin would improve the adherence to the treatment.
Although oritavancin could be more efficient in certain scenarios (outpatient treatment versus inpatient treatment with alternatives), there are no convincing studies in this regard so far. On the other hand, alternative drugs above-mentioned, can also allow outpatient treatment, reducing advantages of oritavancin and further increasing cost differences. Therefore, given that the efficacy is similar to the alternatives, a cost minimization analysis could be considered.
ConclusionsOritavancin is comparable in terms of efficacy and safety to the existing alternatives in Acute Bacterial Skin and Skin Structure Infections, without improvements in the cost-effectiveness ratio, because of the proposed positioning is to consider it for the treatment of vancomycin-resistant enterococcal infection in adult patients when the use of linezolid or tedizolid is contraindicated.
Evaluar críticamente la oritavancina, lipoglicopéptido de segunda generación, para el tratamiento de la infección bacteriana aguda de la piel y tejidos blandos causada por bacterias Gram-positivas susceptibles, incluyendo Staphylococcus aureus resistente a meticilina.
MétodoSe realizó un informe de evaluación según la metodología del Grupo de Evaluación de Novedades, Estandarización e Investigación en Selección de Medicamentos de la Sociedad Española de Farmacia Hospitalaria, con el programa MADRE 4.0. Se llevó a cabo una búsqueda en PubMed, en www.clinicaltrials.gov, Embase y UptoDate. También se utilizaron informes publicados de agencias de evaluación.
ResultadosLa oritavancina en dosis única demostró no ser inferior a la vancomicina en Infección bacteriana aguda de la piel y tejidos blandos, con un perfil de seguridad similar. Sus ventajas potenciales frente a otras alternativas terapéuticas radicarían en su administración en dosis única y en la no necesidad de monitorización de los niveles plasmáticos (lo que posibilitaría su administración ambulatoria), y en la mejora de la adherencia. Aunque podría ser eficiente en determinados escenarios (tratamiento ambulatorio frente al hospitalario con las alternativas), no hay estudios convincentes en este sentido. Por otra parte, los fármacos alternativos por vía oral (linezolid, tedizolid) o IM (teicoplanina) pueden permitir también el tratamiento ambulatorio, reduciendo las ventajas de la oritavancina y agrandando las diferencias de coste. Dado que su eficacia es similar a las alternativas, cabría considerar un análisis de minimización de costes.
ConclusionesLa oritavancina es de una eficacia y seguridad comparables a las alternativas existentes en Infección bacteriana aguda de la piel y tejidos blandos y no mejora la relación coste-efectividad, por lo que el posicionamiento propuesto sería el tratamiento de la infección por enterococo resistente a vancomicina en pacientes adultos cuando esté contraindicado el uso de linezolid o tedizolid.
The current concept of acute bacterial skin and soft tissue infections (SSTIs) includes, according to the Food and Drug Administration (FDA)1 all those infections with a minimum lesion surface area of approximately 75 cm2 which are included in one of the following categories: cellulitis/erysipelas, wound infection, and major cutaneous abscess. The European Medication Agency (EMA)2,3 also recommends, for the assessment of infection severity, the presence of signs or symptoms associated with an acute course of the infectious process.
Given the variable presentation of SSTIs and the frequency of recurrent episodes, it is complicated to estimate their incidence and prevalence. Different studies have been conducted in U.S.A. which show an increase during the past years4–6. In Spain, SSTIs share with gastrointestinal infections the fourth position within infections; while in a selected population, such as the elderly, they might even be the second cause of infection. According to series, SSTIs represent between 0.66% and 2.5% of the total infections7.
SSTI treatment requires a multidisciplinary approach which includes antibiotic treatment, and surgery in those cases necessary. Antimicrobial treatment, very heterogeneous and usually empirical8,9, will be conditioned by the microorganisms that colonize the skin in the affected area, the place where the infection has been acquired (hospital or community), its clinical presentation, risk factors, previous administration of antibiotics, and the local epidemiology of resistance to antimicrobial agents10.
Although there is no overall consensus about the empirical therapy for this type of infection, it seems to be acknowledged that beta-lactams are one of the most adequate treatments, in those cases where there is no suspected involvement by methicillin-resistant Staphylococcus aureus (MRSA)11,12. When it is suspected that the infection can be caused by MRSA, or there is evidence of this, the recommendation is to use any of the antimicrobial agents that have activity against this microorganism. There are publications highlighting the high rate of failures in first-line antibiotic therapies13.
Glycopeptides, such as vancomycin and teicoplanin, have been until recently the basis for the treatment of severe MRSA infections. However, the concern about the efficacy and gradual development of resistance (MRSA strains reach 22.1% in our country, above the European mean of 17.4%14) has led to focusing on the development of new active agents against Gram-positive bacteria. The agents approved for SSTI treatment are: linezolid, tedizolid, daptomycin and tigecycline.
Oritavancin has not been authorized by the Spanish Agency of Medicines and Medical Devices (AEMPS) at the time of preparing this report; but it has been approved by the EMA15 and by the FDA16 for the treatment of SSTIs in adults. Its mechanism of action is triple: on one hand, it causes the inhibition of the transglycosylation and transpeptidation stage of cell wall biosynthesis, and also causes a rupture in the integrity of the bacterial membrane17,18. This turns it active against organisms sensitive and resistant to vancomycin, as well as having a fast bactericide activity, concentration-dependent, against Gram-positive bacteria in active growth, stationary stage, and during biofilm formation18.
The GENESIS Group (Group for drug evaluation, standardization and research in drug selection) of the Spanish Society of Hospital Pharmacy (SEFH), has a program called MADRE, available for the preparation of reports on medication evaluation that must be submitted to the Pharmacy Committees in order to make decisions about the positioning of medications in treatment and their selection for the therapeutic approach of patients.
This MADRE program contains sections about the basic cornerstones recommended to make these decisions about medication positioning: efficacy, safety and efficiency. This last section includes costs, economic evaluation, and budgetary impact.
The objective of this paper is, therefore, a critical evaluation of oritavancin according to the methodology of this work group.
MethodsAn exhaustive review of oritavancin in SSTI was conducted according to the MADRE 4.0 program of the GENESIS Group (Group for drug evaluation, standardization and research in drug selection) of the Spanish Society of Hospital Pharmacy (SEFH)19.
According to this program, a search was conducted for efficacy and safety in the Clinical Queries tool of PubMed, with the following words: “oritavancin AND phase III” in the “narrow” field, and another search with “oritavancin AND trial”, in the “narrow” field. A search was conducted in the www.clinicaltrials.gov website with the word “oritavancin”.
The EPAR Report by the EMA (2015) and the CDER Report by the FDA (2014) were also used. In these, there is a description of 2 pivotal clinical trials on Phase III and a Phase II clinical trial (the CDER Report also mentions 2 pivotal clinical trials on Phase III that were described in the 2008 CDER Report).
A search was also conducted in PubMed and Embase with the descriptor “oritavancin”, which was limited to “systematic reviews” OR “meta-analysis”.
For the economic evaluation, a search was conducted in Pubmed and Embase with the descriptors “oritavancin” and “economic”. Data were analyzed according to the guidelines 20.
ResultsOn 16/12/15 there was a search in the Clinical Queries tool of PubMed, with the following words: “oritavancin AND phase III” in the “narrow” field, without any results. On 16/12/15, there was a search in the Clinical Queries tool of PubMed, with the following words: “oritavancin AND trial” in the “narrow” field, achieving 5 results: 4 clinical trials (2 Phase III, 1 Phase II, and 1 Phase I), and an opinion article.
On 15/12/15 there was a search in the www.clinicaltrials.gov website with the words “oritavancin”, and 10 clinical trials were retrieved in adults (two in Phase III known as TMC-ORI-10-01 and TMC-ORI-10-02, one in Phase II called TAR-ORI-SD001, and seven in Phase I), as well as a clinical trial on paediatric population in the recruitment stage (Phase I).
There are four published pivotal clinical trials, and another clinical trial of interest. Out of the clinical trials found, two compared the drug evaluated vs. vancomycin + cefalexin, two with vancomycin, and one with oritavancin at different doses and frequencies.
There was also a search in PubMed and Embase with the descriptor “oritavancin”, which was limited to “systematic reviews” OR “meta-analysis”. Respectively, seven and 21 results were obtained, and after eliminating duplicates and articles that did not meet the criteria required, these were limited to one single network meta-analysis.
For economic evaluation, a search was conducted in Pubmed and Embase with the descriptors “oritavancin” and “economic”. Four articles were retrieved.
1EfficacyThree clinical trials have been analyzed in order to conduct the evaluation. Other articles have not been taken into account because they had been evaluated in a previous application for marketing (which the company decided to withdraw when the conclusion by the Committee for Medicinal Products for Human Use of the EMA was that there was not enough evidence for its use), because they had used patient populations with a less strict diagnosis of STTI, or because they used doses and frequencies different from those currently studied.
In the EPAR Report by the EMA15 (2015) and the CDER16 Report by the FDA (2014), three clinical trials are mentioned:
- •
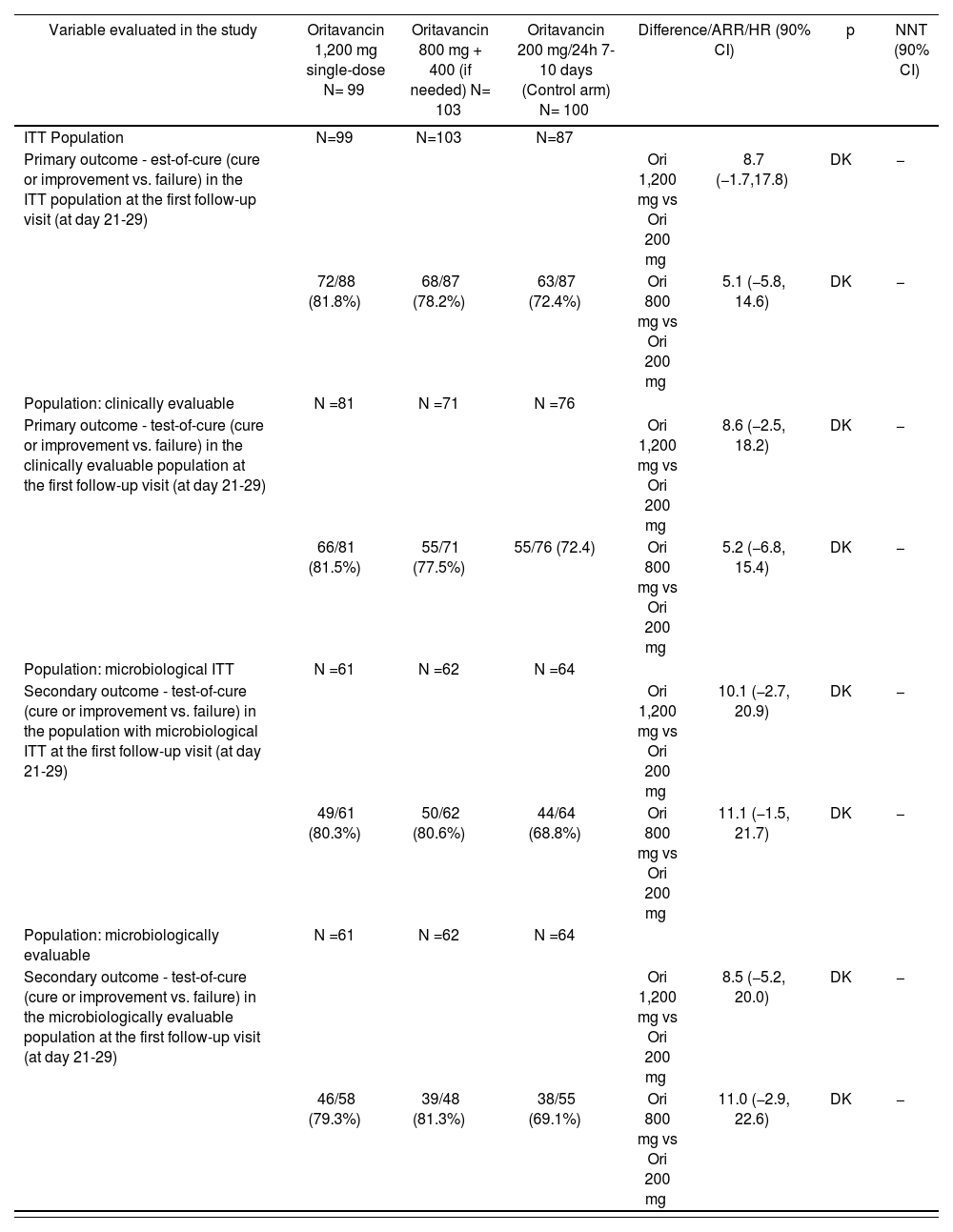
SIMPLIFI (TAR-ORI-SD001)21: A Phase II, multicenter, randomized, double-blind, non-inferiority, parallel clinical trial, with active comparator, on patients with complicated skin and soft tissue infection, assumed or confirmed to be caused by Gram-positive pathogens. In total, 311 patients were included, and 302 of them received the study medication: 100 patients in the arm with daily dose of oritavancin (control arm), 99 in the arm with 1,200 mg single dose, and 103 in the 800 mg arm (+ 400 mg optional). This is a dose-ranging clinical trial which compared 3 different doses of oritavancin. Its outcomes appear on table 1.
Table 1.TAR-ORI-SD001 (SIMPLIFI) trial outcomes20
Variable evaluated in the study Oritavancin 1,200 mg single-dose N= 99 Oritavancin 800 mg + 400 (if needed) N= 103 Oritavancin 200 mg/24h 7-10 days (Control arm) N= 100 Difference/ARR/HR (90% CI) p NNT (90% CI) ITT Population N=99 N=103 N=87 Primary outcome - est-of-cure (cure or improvement vs. failure) in the ITT population at the first follow-up visit (at day 21-29) Ori 1,200 mg vs Ori 200 mg 8.7 (−1.7,17.8) DK − 72/88 (81.8%) 68/87 (78.2%) 63/87 (72.4%) Ori 800 mg vs Ori 200 mg 5.1 (−5.8, 14.6) DK − Population: clinically evaluable N =81 N =71 N =76 Primary outcome - test-of-cure (cure or improvement vs. failure) in the clinically evaluable population at the first follow-up visit (at day 21-29) Ori 1,200 mg vs Ori 200 mg 8.6 (−2.5, 18.2) DK − 66/81 (81.5%) 55/71 (77.5%) 55/76 (72.4) Ori 800 mg vs Ori 200 mg 5.2 (−6.8, 15.4) DK − Population: microbiological ITT N =61 N =62 N =64 Secondary outcome - test-of-cure (cure or improvement vs. failure) in the population with microbiological ITT at the first follow-up visit (at day 21-29) Ori 1,200 mg vs Ori 200 mg 10.1 (−2.7, 20.9) DK − 49/61 (80.3%) 50/62 (80.6%) 44/64 (68.8%) Ori 800 mg vs Ori 200 mg 11.1 (−1.5, 21.7) DK − Population: microbiologically evaluable N =61 N =62 N =64 Secondary outcome - test-of-cure (cure or improvement vs. failure) in the microbiologically evaluable population at the first follow-up visit (at day 21-29) Ori 1,200 mg vs Ori 200 mg 8.5 (−5.2, 20.0) DK − 46/58 (79.3%) 39/48 (81.3%) 38/55 (69.1%) Ori 800 mg vs Ori 200 mg 11.0 (−2.9, 22.6) DK − ITT: Intention to treat.
- •
SOLO I (TMC-ORI-10-01) AND SOLO II (TMC-ORI-10-02) CLINICAL TRIALS: These are pivotal studies with the same design, on Phase III, multicenter, double-blind, non-inferiority, randomized, comparing a single-dose of IV oritavancin 1,200 mg vs. vancomycin 1g or 15 mg/kg/12h during 7-10 days. The inclusion criteria were: patients ≥ 18 year-old, with informed consent, diagnosed with SSTI on a minimum 75 cm2 surface or knowledge that it had been caused by a Gram-positive pathogen, requiring at least 7 days of IV therapy. Some of the exclusion criteria were: previous systemic or topical treatment with agents active against Gram-positive pathogens 14 days before randomization, associated infections with prosthetic devices, severe sepsis or refractory shock, known or suspected bacteremia at the time of screening, CD4 < 200 cells/µL in HIV patients, neutropenia with an absolute neutrophil count (ANC) < 500 cells/µL, contraindication for administering vancomycin, liver function tests ≥ 3x upper limit of normal (ULN) or total bilirubin ≥ 2x ULN; presence of hyperuricemia or gouty arthritis, or patients not willing to abstain from the chronic use of any medication with antipyretic properties.
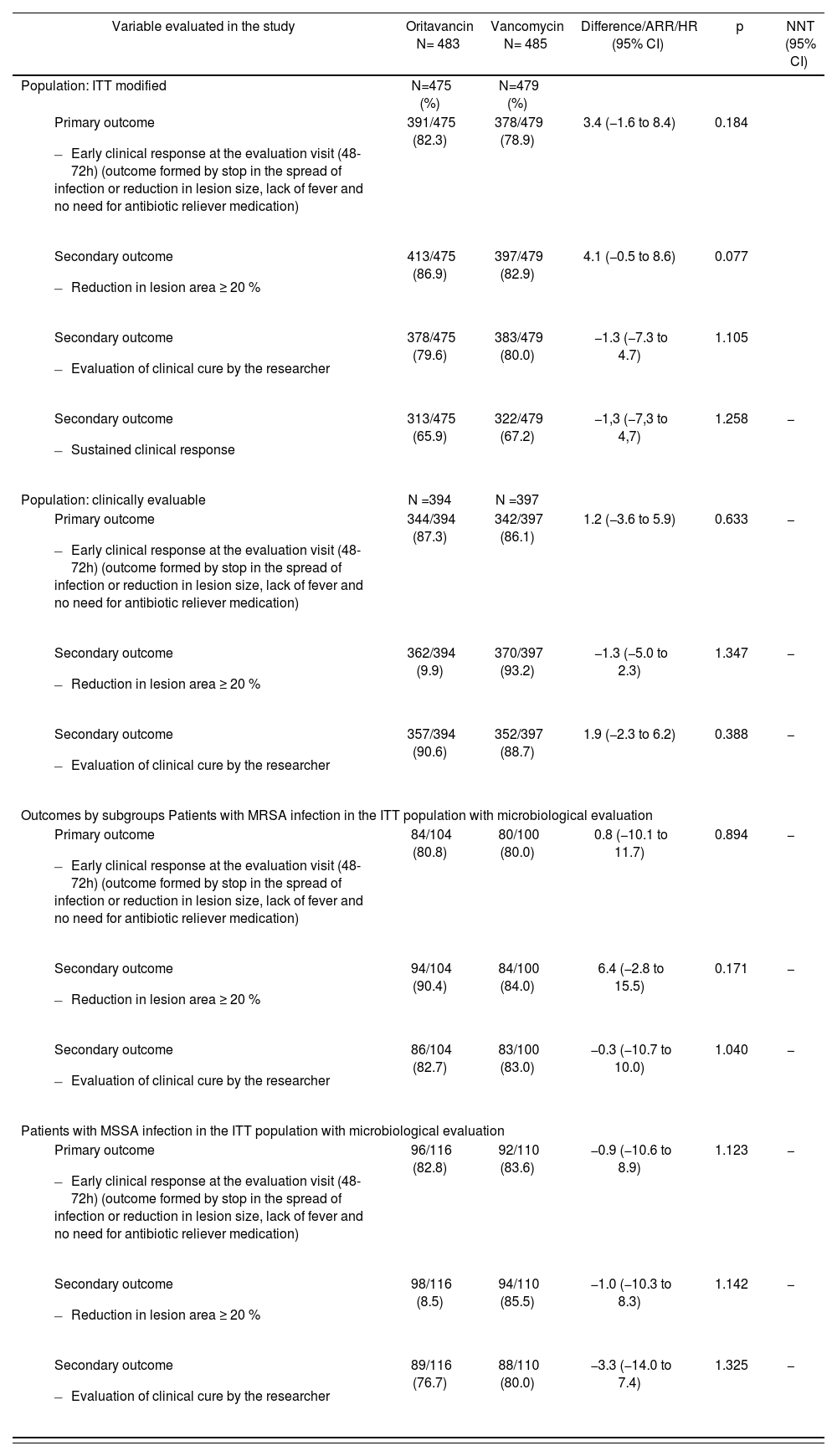
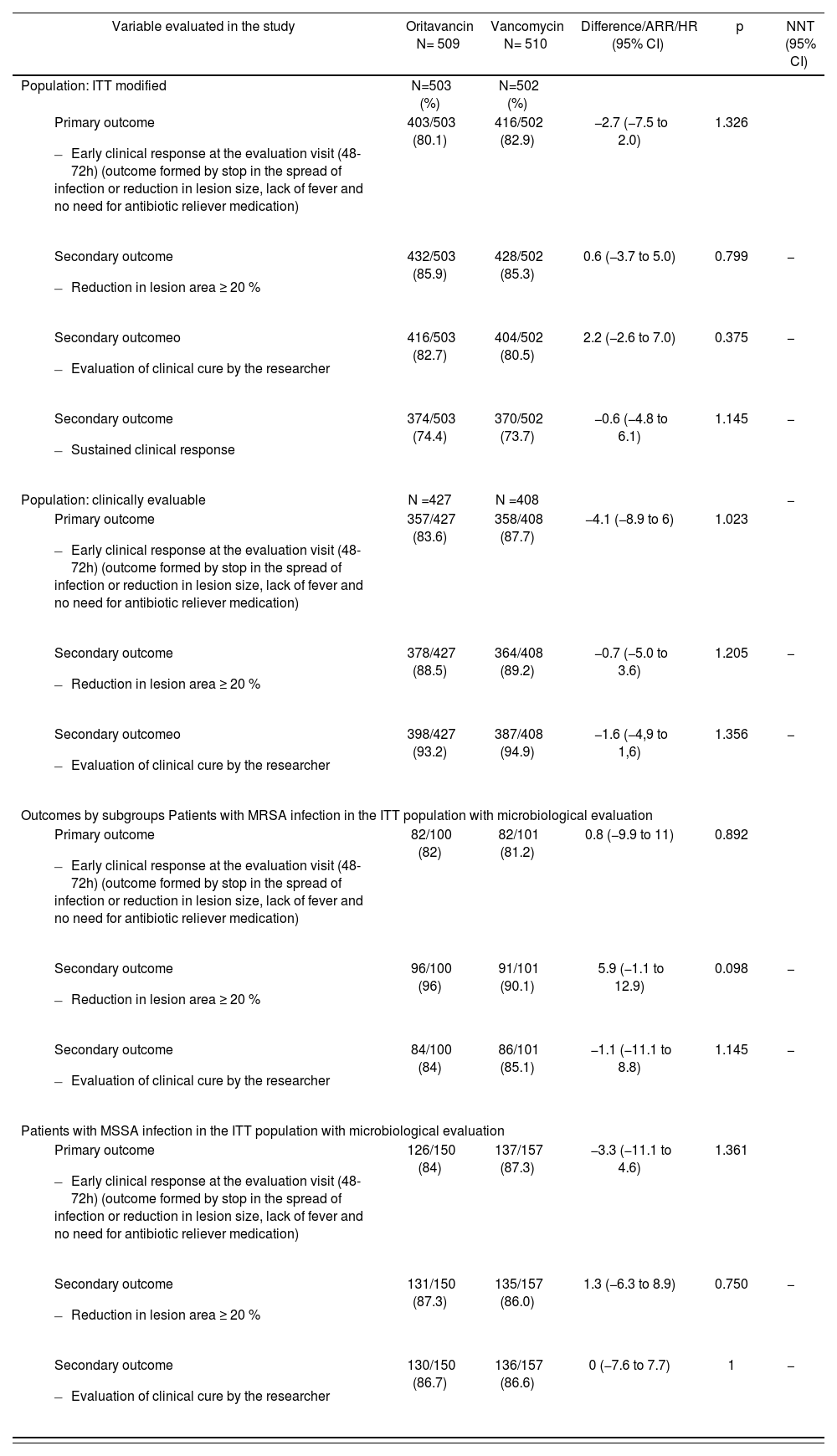
The outcomes of both studies have been published by Corey et al. in two articles22,23 and are summarized in Tables 2 and 3, respectively.
SOLO I trial outcomes21
| Variable evaluated in the study | Oritavancin N= 483 | Vancomycin N= 485 | Difference/ARR/HR (95% CI) | p | NNT (95% CI) |
|---|---|---|---|---|---|
| Population: ITT modified | N=475 (%) | N=479 (%) | |||
| 391/475 (82.3) | 378/479 (78.9) | 3.4 (−1.6 to 8.4) | 0.184 | |
| 413/475 (86.9) | 397/479 (82.9) | 4.1 (−0.5 to 8.6) | 0.077 | |
| 378/475 (79.6) | 383/479 (80.0) | −1.3 (−7.3 to 4.7) | 1.105 | |
| 313/475 (65.9) | 322/479 (67.2) | −1,3 (−7,3 to 4,7) | 1.258 | − |
| Population: clinically evaluable | N =394 | N =397 | |||
| 344/394 (87.3) | 342/397 (86.1) | 1.2 (−3.6 to 5.9) | 0.633 | − |
| 362/394 (9.9) | 370/397 (93.2) | −1.3 (−5.0 to 2.3) | 1.347 | − |
| 357/394 (90.6) | 352/397 (88.7) | 1.9 (−2.3 to 6.2) | 0.388 | − |
| Outcomes by subgroups Patients with MRSA infection in the ITT population with microbiological evaluation | |||||
| 84/104 (80.8) | 80/100 (80.0) | 0.8 (−10.1 to 11.7) | 0.894 | − |
| 94/104 (90.4) | 84/100 (84.0) | 6.4 (−2.8 to 15.5) | 0.171 | − |
| 86/104 (82.7) | 83/100 (83.0) | −0.3 (−10.7 to 10.0) | 1.040 | − |
| Patients with MSSA infection in the ITT population with microbiological evaluation | |||||
| 96/116 (82.8) | 92/110 (83.6) | −0.9 (−10.6 to 8.9) | 1.123 | − |
| 98/116 (8.5) | 94/110 (85.5) | −1.0 (−10.3 to 8.3) | 1.142 | − |
| 89/116 (76.7) | 88/110 (80.0) | −3.3 (−14.0 to 7.4) | 1.325 | − |
ITT: Intention to treat; MRSA: Methicillin-resistant Staphylococcus aureus; MSSA: Methicillin-sensitive Staphylococcus aureus.
SOLO II trial outcomes22
| Variable evaluated in the study | Oritavancin N= 509 | Vancomycin N= 510 | Difference/ARR/HR (95% CI) | p | NNT (95% CI) |
|---|---|---|---|---|---|
| Population: ITT modified | N=503 (%) | N=502 (%) | |||
| 403/503 (80.1) | 416/502 (82.9) | −2.7 (−7.5 to 2.0) | 1.326 | |
| 432/503 (85.9) | 428/502 (85.3) | 0.6 (−3.7 to 5.0) | 0.799 | − |
| 416/503 (82.7) | 404/502 (80.5) | 2.2 (−2.6 to 7.0) | 0.375 | − |
| 374/503 (74.4) | 370/502 (73.7) | −0.6 (−4.8 to 6.1) | 1.145 | − |
| Population: clinically evaluable | N =427 | N =408 | − | ||
| 357/427 (83.6) | 358/408 (87.7) | −4.1 (−8.9 to 6) | 1.023 | |
| 378/427 (88.5) | 364/408 (89.2) | −0.7 (−5.0 to 3.6) | 1.205 | − |
| 398/427 (93.2) | 387/408 (94.9) | −1.6 (−4,9 to 1,6) | 1.356 | − |
| Outcomes by subgroups Patients with MRSA infection in the ITT population with microbiological evaluation | |||||
| 82/100 (82) | 82/101 (81.2) | 0.8 (−9.9 to 11) | 0.892 | |
| 96/100 (96) | 91/101 (90.1) | 5.9 (−1.1 to 12.9) | 0.098 | − |
| 84/100 (84) | 86/101 (85.1) | −1.1 (−11.1 to 8.8) | 1.145 | − |
| Patients with MSSA infection in the ITT population with microbiological evaluation | |||||
| 126/150 (84) | 137/157 (87.3) | −3.3 (−11.1 to 4.6) | 1.361 | |
| 131/150 (87.3) | 135/157 (86.0) | 1.3 (−6.3 to 8.9) | 0.750 | − |
| 130/150 (86.7) | 136/157 (86.6) | 0 (−7.6 to 7.7) | 1 | − |
ITT: Intention to treat; MRSA: Methicillin-resistant Staphylococcus aureus; MSSA: Methicillin-sensitive Staphylococcus aureus.
Regarding the validity of clinical trials, the risk of bias has only been analyzed in the pivotal clinical trials, SOLO I and SOLO II, because SIMPLIFI is a dose escalation and determination study.
Besides, there is an indirect comparison available that has been published, where the combined “Test-of-Cure” (cure at 1-2 weeks after completing treatment) for the population clinically assessable included 14 treatments in 20 studies. Only the SOLO I and SOLO II studies were included in order to evaluate the early clinical response in the clinically assessable population. The combined “Test-of-Cure” for the population by Intent to Treat according to FDA standards included five treatments in four studies; while for the clinically assessable population it included eight treatments in seven studies. The conclusions of this network meta-analysis were that oritavancin 1200 mg was considered equivalent to vancomycin. Indirect evidence also suggests that oritavancin 1200 mg has demonstrated equivalence with linezolid (OR 1.55; CrI 95% 0.91-2.57), teicoplanin (OR 0.72; CrI 95% 0.61-1.26), tedizolid (1.51; CrI 95°% 0.82-2.73) and daptomycin (OR = 2.18; 95°% CrI = 0.90-5.42)24.
2SafetyOverall, oritavancin is a well-tolerated drug, with manageable toxicity. In studies conducted until its marketing, the most common adverse effects were nausea, headache and vomiting; and the most severe, cellulitis and osteomyelitis22,23.
The safety database consisted of 3,017 patients treated with oritavancin, from 22 clinical trials, including four Phase III studies, four Phase II studies, and 14 Phase I studies. The adverse effects of interest in the SOLO trials that appeared to a higher extent in the oritavancin arm than in the vancomycin arm included essentially infection and infestation. There were 40 cases (4%) vs. 31 (3%) respectively. These cases included 4 patients in the oritavancin arm who developed osteomyelitis (in the subsequent review, it was put forward that this could be due to the lack of efficacy of oritavancin in osteomyelitis, or failure to diagnose osteomyelitis at screening). There were a slightly higher number of cases of subcutaneous abscesses in the oritavancin arm, which represents a failure in efficacy, because the infection appeared in the site of the index infection. Cellulitis cases were balanced in both arms, which could indicate lack of efficacy, lack of the adequate incision and drainage for infection control, or recurring infection due to underlying comorbidities.
For those subjects randomized to any of the arms, the highest incidence of drug-related adverse reactions (DRAE) which led to treatment interruption was infections and infestations (1.6% vs. 1.9% respectively). Twenty-one (21) patients (2.2%) in the oritavancin arm and 19 (1.9%) in the vancomycin arm suffered a severe adverse event (AE) which led to treatment interruption. The most common DRAEs in both the oritavancin and the vancomycin arms were nausea (17.7% and 18.3%), headache (12.6% and 11.7%), vomiting (8.2% and 8.2%), diarrhoea (6.6% and 5.7%), cellulitis (6.8% and 5.7%), constipation (6% and 6.7%), and extravasation in the infusion site (6% and 5.9%). The incidence in ALT and AST elevation, cellulitis, abscesses, subcutaneous abscesses, physical integrity of abscesses, and infection on infections and infestation, tachycardia and myalgia, were slightly higher than in patients treated with oritavancin. There were 24 subjects (4.4%) in the oritavancin arm and 11 subjects (1.9%) in the vancomycin arm in the set of SOLO trials (SOLO pool) with the adverse event of tachycardia. No specific conclusions can be drawn from this analysis. There were 27 (2.8%) and 16 (1.6%) patients with elevated ALT in the oritavancin and vancomycin arms, respectively. There were 18 (1.8%) and 16 (1.6%) patients with elevated AST in the oritavancin and vancomycin arms, respectively. Even though the history of hepatitis or liver disease (9 subjects) or the use of intravenous drugs (12 subjects) could predispose subjects to transaminase elevation, there were subjects without this past history where anomalies appeared in their liver function tests. These cases don't seem to be the result of severe sepsis or septic shock. None of the subjects met Hy's Law criteria.25. There was a slightly higher incidence of severe DRAEs in diabetic subjects, with 23/138 (16.7%) in the oritavancin arm versus 18/141 (12.8%) in the vancomycin arm. However, the total number of subjects with >1 DRAE was similar in both arms. In those subjects with creatinine clearance of 30-60ml/min, 12/70 in the oritavancin arm vs. 3/54 in the vancomycin arm presented one severe adverse effect.
Within the set of patients in the SOLO I and II trials, 5 patients died (2 in the oritavancin arm and 3 in the vancomycin arm). There were 5/302 (1.7%) deaths in the SIMPLIFI study (3 in the arm with oritavancin daily dose, 2 in the arm with infrequent dosing, and none in the single-dose arm). None of the deaths seemed to be related to the research medication.
Oritavancin does not require dose adjustment in patients with mild or moderate renal or liver impairment, and it has not been researched in paediatric patients.
3Economic areaThere are four published pharmacoeconomic studies available; of these, three are budgetary impact studies and one is a cost-minimization study.
- •
The study by Wu26 analyzed a theoretical model on the economic impact represented by the inclusion of oritavancin in a U.S.A. hospital for SSTI treatment. An analytical decision making model was designed, based on current clinical practice guidelines, limiting the use of oritavancin to patients with moderate-severe SSTI (Eron Classes II and III) at risk of MRSA. The model simulated a cohort of 1,000 patients with SSTI. The base case shows the mean national use of antibiotics active against MRSA (vancomycin 92%, linezolid 2%, daptomycin 6 %, oritavancin 0%). In the hypothetical case, it was assumed that oritavancin will be used for 25.75% of patients (5% in hospitalized patients, 15% in ER/outpatient unit, and 80% in observation units), replacing vancomycin but not the rest of antibiotics. As a result of this change, fewer patients were treated as hospitalized, and there was an increase in the use of observation units. Direct costs were taken into account: medication, administration, monitoring, hospital stay and others. According to this model, there would be savings of 2,752 $ per patient; most of it would be caused by a reduction in the number of hospitalizations and the use of observation units, cheaper than traditional hospitalization units.
- •
The study by Jensen27 analyzed a theoretical model on the economic impact represented by the inclusion of oritavancin in a U.S.A. hospital for SSTI treatment, identically to the previous study 43, but it evaluated two scenarios: hospital with outpatient services and without them. The results were that the use of oritavancin in 26% of patients instead of vancomycin would represent total savings of 13% from the hospital perspective, or approximately 1,235 $ per patient. In the model of economic impact on a hospital without outpatient services, the use of oritavancin in 26% of patients would also represent savings, though lower (9%, or approximately 634 $ per patient).
- •
Another study by Wu28 repeated the model of the two previous studies, but applied to a hospital in the United Kingdom. In this case, it was assumed that oritavancin would be used in 3.6% of patients; and the conclusion was that its use would represent total savings by 0.63% from the hospital perspective, or 29.23£ per patient.
- •
The study by Lodise29 developed a cost-minimization model in order to compare the costs of patients on treatment with vancomycin while hospitalized vs. those with oritavancin administered as outpatient regimen, in patients with SSTI and few or no comorbidities (Charlson Comorbidity Index [CCI] 0 or 1). The costs associated with the use of oritavancin in the Emergency Unit (3,409.46 $) and in the observation unit (4,220.27 $) were lower to those for vancomycin in hospitalized patients (5,972.73-9,885.33 $). To switch a hospitalized patient on vancomycin to outpatient treatment with oritavancin could save 1,752.46-6,475.87 $ depending on the CCI, presence of systemic symptoms, and the use of the observation unit. If all patients hospitalized on vancomycin were treated with oritavancin at the Emergency Unit, savings per patient could be of 3,102.43 $. Assuming that some patients could be admitted to hospital after receiving treatment with oritavancin in the Emergency Unit, it is expected that savings with oritavancin in the observation unit vs. treatment hospitalized with vancomycin will be 2,291.62 $.
The limitations of these studies lie essentially in: a) the difficulty to extrapolate data from models based on U.S.A. data to our country, and even to Europe; b) the lower prevalence of MRSA in Europe; c) the lower impact in Spain of hospitalization and drug administration costs than in other countries; d) the risky assumption that all patients treated with oritavancin can be treated as outpatients, and those patients treated with alternative options (vancomycin, linezolid, teicoplanin, etc.) must be hospitalized.
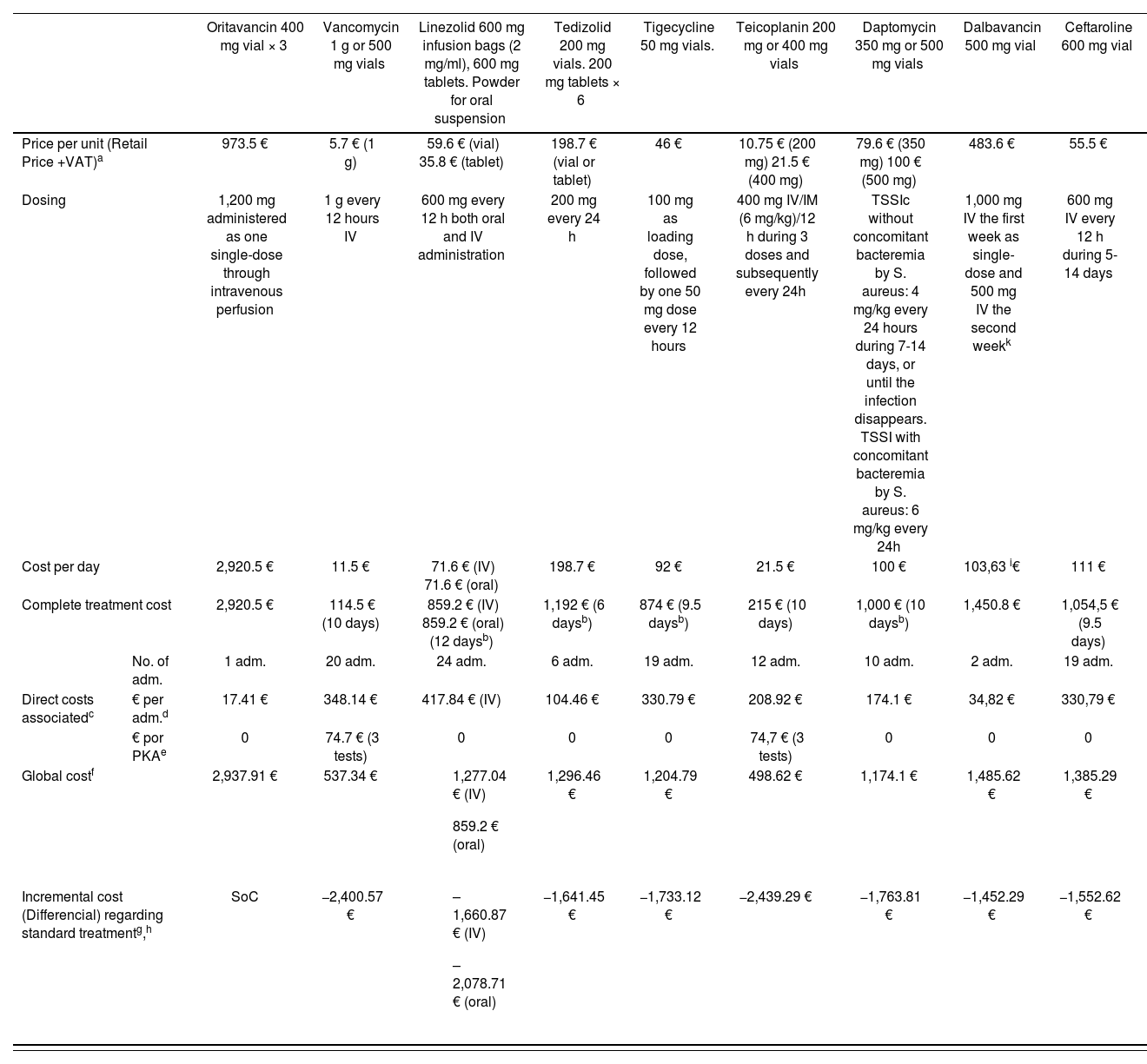
We have conducted our own comparison of the cost of the treatment evaluated vs. those alternative options currently available in Spain (Table 4). Given that these are equivalent treatments, an incremental cost-efficacy analysis is not adequate, and a cost-minimization analysis should be conducted, considering oritavancin as a therapeutic alternative vs. the rest of drugs considered for the indication under study.
Cost comparison between the treatment evaluated vs. other alternative options
| Oritavancin 400 mg vial × 3 | Vancomycin 1 g or 500 mg vials | Linezolid 600 mg infusion bags (2 mg/ml), 600 mg tablets. Powder for oral suspension | Tedizolid 200 mg vials. 200 mg tablets × 6 | Tigecycline 50 mg vials. | Teicoplanin 200 mg or 400 mg vials | Daptomycin 350 mg or 500 mg vials | Dalbavancin 500 mg vial | Ceftaroline 600 mg vial | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Price per unit (Retail Price +VAT)a | 973.5 € | 5.7 € (1 g) | 59.6 € (vial) 35.8 € (tablet) | 198.7 € (vial or tablet) | 46 € | 10.75 € (200 mg) 21.5 € (400 mg) | 79.6 € (350 mg) 100 € (500 mg) | 483.6 € | 55.5 € | |
| Dosing | 1,200 mg administered as one single-dose through intravenous perfusion | 1 g every 12 hours IV | 600 mg every 12 h both oral and IV administration | 200 mg every 24 h | 100 mg as loading dose, followed by one 50 mg dose every 12 hours | 400 mg IV/IM (6 mg/kg)/12 h during 3 doses and subsequently every 24h | TSSIc without concomitant bacteremia by S. aureus: 4 mg/kg every 24 hours during 7-14 days, or until the infection disappears. TSSI with concomitant bacteremia by S. aureus: 6 mg/kg every 24h | 1,000 mg IV the first week as single-dose and 500 mg IV the second weekk | 600 mg IV every 12 h during 5-14 days | |
| Cost per day | 2,920.5 € | 11.5 € | 71.6 € (IV) 71.6 € (oral) | 198.7 € | 92 € | 21.5 € | 100 € | 103,63 i€ | 111 € | |
| Complete treatment cost | 2,920.5 € | 114.5 € (10 days) | 859.2 € (IV) 859.2 € (oral) (12 daysb) | 1,192 € (6 daysb) | 874 € (9.5 daysb) | 215 € (10 days) | 1,000 € (10 daysb) | 1,450.8 € | 1,054,5 € (9.5 days) | |
| No. of adm. | 1 adm. | 20 adm. | 24 adm. | 6 adm. | 19 adm. | 12 adm. | 10 adm. | 2 adm. | 19 adm. | |
| Direct costs associatedc | € per adm.d | 17.41 € | 348.14 € | 417.84 € (IV) | 104.46 € | 330.79 € | 208.92 € | 174.1 € | 34,82 € | 330,79 € |
| € por PKAe | 0 | 74.7 € (3 tests) | 0 | 0 | 0 | 74,7 € (3 tests) | 0 | 0 | 0 | |
| Global costf | 2,937.91 € | 537.34 € |
| 1,296.46 € | 1,204.79 € | 498.62 € | 1,174.1 € | 1,485.62 € | 1,385.29 € | |
| Incremental cost (Differencial) regarding standard treatmentg,h | SoC | −2,400.57 € |
| −1,641.45 € | −1,733.12 € | −2,439.29 € | −1,763.81 € | −1,452.29 € | −1,552.62 € | |
IM: intramuscular; IV: intravenous; adm: administrations; TSSIc: Complicated skin and soft tissue infections.
Direct costs associated: These are costs that we can consider besides the cost of the medication studied. For example, other additional medications required, monitoring and lab tests, screening tests (pharmacogenetics, biomarkers), infusion materials or management of complications. To be considered whenever relevant.
Cost for the administration of the IV doses indicated (according to the public rates in the Community of Valencia, DOCV 5166 of 30.12.2005 updated at 24-5-2013, applying a 3% per year discount rate for its 2016 update.)
PKA = pharmacokinetic analysis. Cost for plasma level monitoring (according to the public rates in the Community of Valencia, DOCV 5166 of 30.12.2005 updated at 24-5-2013, applying a 3% per year discount rate for its 2016 = 24,9 € per test).
The cost of hospitalization has not been considered because it is assumed to be equivalent for all treatments.
A recent clinical trial (Dunne MW et al. Clin Infect Dis. (2015)doi: 10.1093/cid/civ982First published online: November 26, 2015) has demonstrated non-inferiority between the usual dalbavancin regimen (1000 mg IV + 500 mg IV the following week) and the 1,500 mg single dose. Given that this latter dosing regimen is not collected in the product specifications, it has not been considered in the table, but the difference in costs would be reduced by one administration, that is to say, 17.41 € less.
On September, 1st, 2016, oritavancin had not been yet approved by the AEMPS; therefore, its price in U.S.A. has been used for its economic evaluation: 1 vial 400 mg = 1,035 $ = 973.5 €.
For the estimation of the overall economic impact at national level, there are no data available about the prevalence of SSTI in Spain. It is known that in U.S.A. there are 500 episodes per 10,000 persons and per year30. According to the January, 2015 census by the National Statistics Institute, there were 46.449.565 inhabitants in Spain; applying the American prevalence, this would represent a figure of 2.322.478 episodes per year. If we take the proportion of patients who required hospitalization for treatment in the SOLO I trial (19%)22, we would have 441.270 patients. For an oritavancin introduction rate of 2.5% per year (11,032 patients), on the first year we would have costs of 32,411,023€.
DiscussionOritavancin is a semi-synthetic derivate of chloroeremomycin, a glycopeptide antibiotic that has been approved by the FDA and the EMA for the treatment of SSTI caused by susceptible Gram-positive bacteria. This new 2nd generation lypoglycopeptide antibiotic has activity against a broad spectrum of Gram-positive bacteria, including MRSA. Its mechanism of action through three different mechanisms turns it particularly immune to microbial resistances, at least in theory. However, in vitro data indicate that very few Staphylococci that have intermediate susceptibility or are resistant to glycopeptides could be treated with oritavancin, and that there are no clinical data for the use of oritavancin when MIC > 1mg/l. It seems unlikely that it could be used to treat Intermediate Vancomycin-resistance Staphylococcus aureus or Vancomycin-resistance Staphylococcus aureus, and there are few conclusive data about its utility in hetero-VISA. Oritavancin still presents limited data regarding the development of resistance, but in vitro resistance has been observed in vancomycin-resistant Staphylococcus aureus. No cross-resistance is known between oritavancin and the non-glycopeptide antibiotic classes, and it presents reduced in vitro activity against certain Gram-positive organisms of the Lactobacillus, Leuconostoc and Pediococcus classes, which are intrinsically resistant to glycopeptides17.
From a clinical point of view, oritavancin as single-dose has demonstrated its non-inferiority in controlled studies vs. vancomycin in skin and soft tissue infection, with a safety profile similar to the comparator. Due to its lower development, there is limited experience in clinical trials with patients with bacteremia, peripheral vascular disease, those under immunosuppression, >65-year-old, and in infections caused by S. pyogenes. Its safety has not been established in pregnant women or the paediatric population.
Oritavancin is incorporated into a well-provided therapeutic class, where it is difficult to find any gaps. Its potential advantage over other treatment alternative options available would be based on its single administration and lack of monitoring required for plasma levels, which at least in theory makes its outpatient administration possible, reducing direct treatment costs, shortening the duration of hospital stay, and indirectly minimizing the risk of nosocomial complications. Regarding the alternative options for oral treatment (linezolid, tedizolid), it would eliminate the likelihood of lack of treatment compliance.
However, its longer duration of action could represent a safety problem in case of reactions due to lack of tolerability or hypersensitivity. The long elimination half-life also causes concern about the development of resistance, particularly when the drug concentration falls below the MIC for the pathogen causing the infection. On the other hand, it is expected that the multiple mechanisms of action of oritavancin will protect against the development of resistance during treatment.
Finally, given its prolonged half-life, its “off-label” use must be foreseen in specific situations such as, for example, completing the osteomyelitis treatment and other osteoarticular infections.
In terms of economic evaluation, even though so far there is no official price available for oritavancin in Spain, we know its price in U.S.A. (973.5 €). This cost is overall much higher than the one for the rest of antibiotics it is compared with (vancomycin, linezolid, teicoplanin, tedizolid, etc.), and it has the advantage over them of its single-dose. This aspect could lead to higher efficiency in specific scenarios (outpatient treatment with oritavancin vs. hospital treatment with the alternative options), but so far there are no compelling or sufficiently detailed studies in this sense. On the other hand, some alternative drugs (linezolid, tedizolid or teicoplanin) can also allow outpatient treatment (oral or IM), at some point in the clinical process; this would reduce the advantages of oritavancin and would increase even more the differences in cost.
Therapeutic positioning and conditions of useGiven that, in the indications evaluated, the medication shows efficacy and safety comparable to the alternative options available, and its efficiency profile does not offer improvements in the cost-effectiveness ratio, the proposed positioning is to consider it within the Category D-1: Included in the Formulary with specific recommendations: treatment of infection by vancomycin-resistant enterococcus in adult patients, when there is contraindication to the use of linezolid or tedizolid.
FundingNo funding
AcknowledgmentsThe authors would like to thank Dr. Javier Cobo Reinoso, of the Infectious Diseases Service of the Hospital Ramón y Cajal (Madrid, Spain) and member of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), for his valuable contributions, which have contributed to the improvement of the document.
Conflict of interestsNo conflict of interest.
Este artículo es un resumen del informe de evaluación de la oritavancina realizado por GENESIS-SEFH (Grupo de Evaluación de Novedades, Estandarización e Investigación en Selección de Medicamentos de la Sociedad Española de Farmacia Hospitalaria), que puede obtenerse de forma completa desde la página web de GENESIS (http://gruposdetrabajo.sefh.es/genesis/). Esta evaluación se ha realizado con la ayuda de la aplicación MADRE 4.01