Daptomycin is a cyclic lipopeptide antibiotic with bactericidal activity that was discovered in 1986 from Streptomyces roseosporus1. It has been shown to be effective in the treatment of right-sided infective endocarditis and skin infections caused by gram-positive pathogens, including methicillinresistant Staphylococcus aureus (MRSA)2,3.
Tolerance to daptomycin is usually acceptable and the most frequent adverse effects are elevated creatine phosphokinase (CPK), myalgia, rhabdomyolysis, and gastrointestinal symptoms3. However, evidence is scarce on daptomycin-associated neurotoxicity.
We describe the case of a male patient who presented with daptomycinassociated resting myoclonus.
Case descriptionAn 80-year-old man with a history of hypertension, insulin-dependent type-2 diabetes mellitus with multiple target organ damage (ischemic heart disease, chronic kidney failure, and ophthalmoplegia due to third right cranial pair palsy), atrial fibrillation, prostate adenocarcinoma controlled with hormone therapy, and permanent VVI pacemaker due to sinus node disease. He had no history of medication allergies or intolerances. His usual medication consisted of pantoprazole, rivaroxaban, cilostazol, thioctic acid, amlodipine, melatonin, ezetimibe, fenofibrate, tamsulosin, and insulin detemir.
He was admitted to our hospital for severe coronavirus pneumonia (COVID-19) requiring management in the intensive care unit without the need for mechanical ventilation. Subsequently, he developed prolonged febrile syndrome with no isolated germs in the microbiological studies. This condition was interpreted as inhospital pneumonia, so he was given treatment with vancomycin and meropenem for 7 days. The febrile syndrome persisted after the end of the antibiotic regimen. Having ruled out other causes of hyperthermia, on the 41st day after admission a transesophageal echocardiogram was performed, which showed the presence of a 3-mm vegetation on the aortic valve. Given the diagnosis of infective endocarditis of the native aortic valve with no isolated germs, empirical treatment was started with cefepime 2 g/8 h and vancomycin 1 g/12 h. Due to the need for prolonged intravenous antibiotic treatment with empirical coverage for MRSA in a patient with chronic kidney disease, we decided to replace vancomycin with daptomycin 10 mg/kg/24 h (dose = 900 mg/24 h). On the sixth day after administration, the patient presented with myoclonus at rest in the upper limbs. In view of this picture, metabolic causes were ruled out through laboratory studies (Table 1). Likewise, an analysis of concomitant drugs was performed in the search for another drug that could explain the phenomenon under study, with negative results (Table 2). In view of the suspicion of daptomycin-associated neurotoxicity, this antibiotic was changed back to vancomycin, with remission of the tremor. The patient evolved with progressive deterioration of kidney function and pharmacodermia in the upper and lower limbs and dorsum in the setting of supratherapeutic plasma levels of vancomycin (26.3 µg/mL). For this reason, vancomycin was once again discontinued and after 5 days, we decided to restart daptomycin at 8 mg/kg/24 h (dose = 720 mg/24 h). After the first infusion, the patient again presented with myoclonus in the upper and lower limbs, which later became generalized. On physical examination he was awake and oriented in the three spheres. His osteotendinous reflexes, cranial nerves, and strength were preserved. Involuntary movements persisted at night, making sleep difficult. Serial electroencephalograms were performed and showed no significant alterations, and so the movements were interpreted as asterixis secondary to daptomycin. Daptomycin was discontinued, with gradual improvement in the picture during the following week. During the periods in which the patient received daptomycin, CPK values were monitored, which always remained within the normal range. After this, the patient completed 4 weeks of intravenous antibiotic treatment with linezolid and cefepime. Given the patient's fragility and preferences, we decided not to replace the pacemaker and to continue with chronic suppressive antibiotic therapy with ciprofloxacin 500 mg/12 h plus oral minocycline 100 mg/12 h. We conducted a causality analysis using the algorithm developed by Naranjo et al.4, which yielded a value of 8 for the reaction to daptomycin and 7 for the reaction to vancomycin. These values correspond to probable causality between exposure to and the respective reactions observed for each drug. Both adverse effects were reported to the National Pharmacovigilance System.
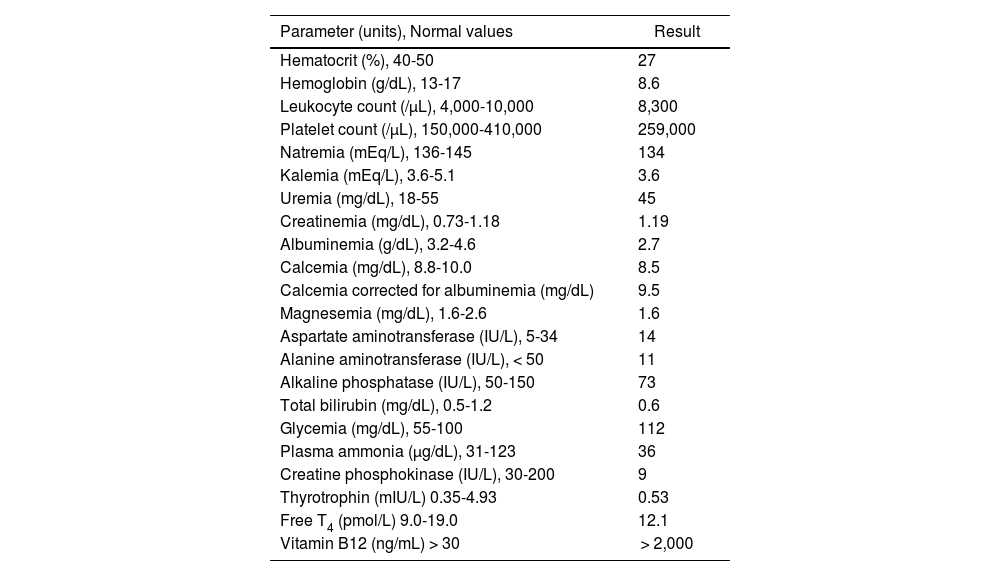
Laboratory studies performed after possible daptomycin-associated myoclonus
| Parameter (units), Normal values | Result |
|---|---|
| Hematocrit (%), 40-50 | 27 |
| Hemoglobin (g/dL), 13-17 | 8.6 |
| Leukocyte count (/μL), 4,000-10,000 | 8,300 |
| Platelet count (/μL), 150,000-410,000 | 259,000 |
| Natremia (mEq/L), 136-145 | 134 |
| Kalemia (mEq/L), 3.6-5.1 | 3.6 |
| Uremia (mg/dL), 18-55 | 45 |
| Creatinemia (mg/dL), 0.73-1.18 | 1.19 |
| Albuminemia (g/dL), 3.2-4.6 | 2.7 |
| Calcemia (mg/dL), 8.8-10.0 | 8.5 |
| Calcemia corrected for albuminemia (mg/dL) | 9.5 |
| Magnesemia (mg/dL), 1.6-2.6 | 1.6 |
| Aspartate aminotransferase (IU/L), 5-34 | 14 |
| Alanine aminotransferase (IU/L), < 50 | 11 |
| Alkaline phosphatase (IU/L), 50-150 | 73 |
| Total bilirubin (mg/dL), 0.5-1.2 | 0.6 |
| Glycemia (mg/dL), 55-100 | 112 |
| Plasma ammonia (μg/dL), 31-123 | 36 |
| Creatine phosphokinase (IU/L), 30-200 | 9 |
| Thyrotrophin (mIU/L) 0.35-4.93 | 0.53 |
| Free T4 (pmol/L) 9.0-19.0 | 12.1 |
| Vitamin B12 (ng/mL) > 30 | > 2,000 |
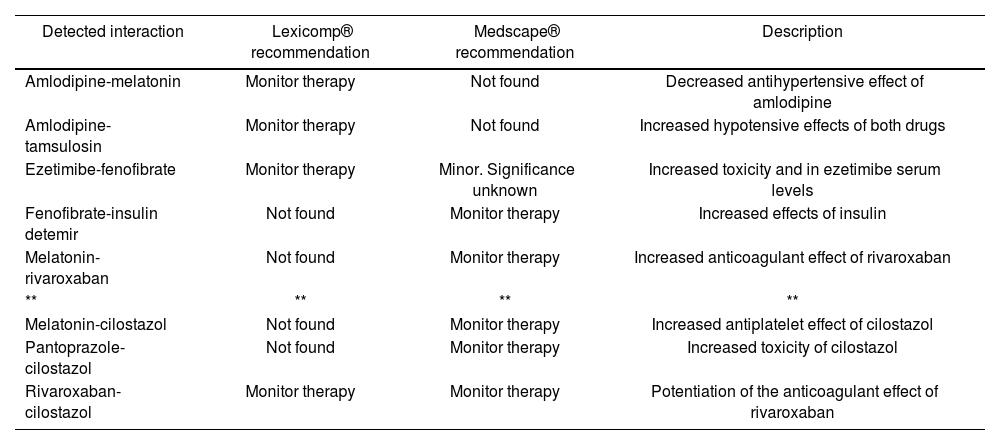
Description of the profile of pharmacological interactions extracted from the patient's medical prescription according to two online platforms (Lexicomp® and Medscape®). Note that none of the interactions detected would explain the observed myoclonus. Drugs assessed: daptomycin, cefepime, pantoprazole, rivaroxaban, cilostazol, thioctic acid, amlodipine, melatonin, ezetimibe, fenofibrate, tamsulosin, and insulin detemir
| Detected interaction | Lexicomp® recommendation | Medscape® recommendation | Description |
|---|---|---|---|
| Amlodipine-melatonin | Monitor therapy | Not found | Decreased antihypertensive effect of amlodipine |
| Amlodipine-tamsulosin | Monitor therapy | Not found | Increased hypotensive effects of both drugs |
| Ezetimibe-fenofibrate | Monitor therapy | Minor. Significance unknown | Increased toxicity and in ezetimibe serum levels |
| Fenofibrate-insulin detemir | Not found | Monitor therapy | Increased effects of insulin |
| Melatonin-rivaroxaban | Not found | Monitor therapy | Increased anticoagulant effect of rivaroxaban |
| ** | ** | ** | ** |
| Melatonin-cilostazol | Not found | Monitor therapy | Increased antiplatelet effect of cilostazol |
| Pantoprazole-cilostazol | Not found | Monitor therapy | Increased toxicity of cilostazol |
| Rivaroxaban-cilostazol | Monitor therapy | Monitor therapy | Potentiation of the anticoagulant effect of rivaroxaban |
Prior to the completion of this manuscript, written informed consent was requested from the patient.
DiscussionDaptomycin is an effective antibiotic for infections caused by gram-positive organisms and is generally well tolerated, even in patients with comorbidity and under prolonged treatment2,5. Its most frequent adverse effect is myotoxicity, characterized by elevated CPK values, which in some cases results in myalgia and rhabdomyolysis, requiring discontinuation of the drug5,6. Other less frequently reported adverse reactions include digestive intolerance, allergic reactions, eosinophilia, and eosinophilic pneumonia5,7. Although there have been cases of kidney failure associated with daptomycin5,7, its safety has been highlighted in patients with varying degrees of pre-existing nephropathy8.
A literature search in Medline (keywords: daptomycin, neurotoxicity, myoclonus, adverse reactions) found that the data on daptomycin neurotoxicity comprised a case of paralysis of the external popliteal sciatic nerve9 and a patient with posterior reversible encephalopathy syndrome10. As it was observed in our case, these adverse effects occurred with CPK values within the normal range. However, the World Health Organization database of adverse drug events (VigiAccess; available at: http://www.vigiaccess.org/) contains 574 registered cases of adverse effects at the level of the nervous system. Among these adverse events, at the level of the central nervous system, we highlight dizziness (8.4%), headache (8.2%), seizures (8.0%), and altered states of consciousness (8.7%).
We do not know the pathophysiological mechanism that may underlie the tremor. At onset, we ruled out possible metabolic causes that could explain it, such as hepatic encephalopathy, uremia, hypocalcemia, hypoglycemia, hyponatremia, hypomagnesemia, hyperthyroidism, and vitamin B12 deficiency. Likewise, the patient had not received other drugs that could cause this manifestation. The reproduction of the clinical picture upon reintroduction of the drug strengthened our suspicion.
Although the pre-existing nephropathy of our patient could have been a predisposing factor for what was observed in the present case, the findings of Azanza and Quetglas8 would suggest that there was a low probability of this having occurred.
Although we have not found any publications on daptomycin-associated myoclonus, we believe that our patient may not be the first to present with it. To date, there are 162 reports in VigiAccess associated with abnormal movements. This fact highlights the relevance and need to exercise pharmacovigilance to promote safety in health care and to encourage the construction of real-world safety profiles of the drugs used worldwide.
FundingNo funding.
Conflict of interestNo conflict of interests.
Early Access date (11/21/2021).