Critically ill patients are at increased risk of drug-drug interactions but their prevalence and clinical relevance remains unclear. The prevalence of potential drug-drug interactions in an intensive care unit according to Micromedex Drug-Reax® and Lexi-Interact® databases was studied and the concordance between the two databases was assessed. In addition, drug-drug interactions detected in 2013 were compared with those identified in 2018 to determine updates between these years.
MethodBetween January and June 2013, 152 critical care patients were prospectively included. Cardiac patients were excluded. Demographic and clinical data together with the drugs administered on the first calendar day of intensive care unit admission were recorded. Potential drug-drug interactions were searched in both Drug-Reax® and Lexi-Interact® and their prevalence, level of severity and evidence were compared considering the same sample in 2013 and 2018.
ResultsIn 2013, 1,025 potential drug-drug interactions were identified, corresponding to 438 unique pairs. Lexi-Interact® identified more interactions (92.8%) than Drug-Reax® (34.0%). The percentage of agreement between databases was 274%. The number of interactions included in both databases increased after the five years but their level of evidence decreased. The most common potential drug-drug interactions involved sedatives and analgesics, intentionally prescribed concomitantly. Only two potential drug-drug interactions were classified as contraindicated by both databases. None of the potential drug-drug interactions identified had a noticeable clinical impact. Neither did they imply a prescription change.
ConclusionsThis study shows that the prevalence of potential drug-drug interactions in the intensive care unit is high, although their clinical relevance is generally low. Our data also show a lack of concordance between Drug-Reax® and Lexi-Interact®, as well as their updates.
Los pacientes críticos presentan un mayor riesgo de interacciones farmacológicas, aunque su prevalencia y relevancia clínica siguen sin estar claras. En el presente estudio se analizó la prevalencia de interacciones farmacológicas potenciales en una unidad de cuidados intensivos mediante las bases de datos Micromedex Drug-Reax® y Lexi-Interact® y se evaluó la concordancia entre ambas bases de datos. También se compararon las interacciones farmacológicas detectadas en 2013 con las identificadas en 2018 para evaluar las actualizaciones realizadas durante este periodo de tiempo.
MétodoEntre enero y junio de 2013 se incluyeron de forma prospectiva 152 pacientes críticos. Los pacientes cardiacos fueron excluidos. Se registraron los datos demográficos y clínicos junto con los fármacos administrados durante el primer día de ingreso en la unidad de cuidados intensivos. Las interacciones se buscaron tanto en Micromedex Drug-Reax® como en Lexi-Interact® y se comparó su prevalencia, el nivel de severidad y la evidencia considerando la misma muestra en 2013 y 2018.
ResultadosEn 2013 se identificaron 1.025 interacciones farmacológicas potenciales, correspondientes a 438 pares únicos. Lexi-Interact® identificó más interacciones (92,8%) que Drug-Reax® (34,0%). El porcentaje de concordancia entre las dos bases de datos fue del 27,4%. El número de interacciones incluidas en ambas bases de datos aumentó durante los cinco años, pero su nivel de evidencia disminuyó. Las interacciones farmacológicas potenciales más comunes incluyeron sedantes y analgésicos, prescritos intencionadamente de forma concomitante. Sólo dos interacciones farmacológicas potenciales fueron clasificadas como contraindicadas por ambas bases de datos. Ninguna de las interacciones identificadas tuvo un impacto clínico notable ni supuso un cambio de prescripción.
ConclusionesEste estudio muestra que la prevalencia de interacciones farmacológicas potenciales en las unidades de cuidados intensivos es alta, aunque su relevancia clínica es generalmente baja. Nuestros datos también muestran la falta de concordancia entre Drug-Reax® y Lexi-Interact®, así como sus actualizaciones.
Drug-drug interactions (DDIs) are a major cause of preventable adverse drug reactions (ADRs) in patients admitted in intensive care units (ICU)1,2. Besides possible severe toxicity and loss of an expected therapeutic effect, DDIs can prolong ICU stays and impact patient outcomes3,4. Many critically ill patients have life-threatening diseases and multiple comorbidities. Polypharmacy is highly prevalent in this population and is associated with an increased risk of DDIs4–11. Furthermore, the first 24 hours in the critical care setting are crucial for patient outcomes12,13.
The clinical relevance of DDIs can be assessed through several databases. Among these, Micromedex Drug-Reax® (DR) and Lexi-Interact® (LI) are considered the most complete and reliable compendia14–16. They contain valuable information about the severity, management and clinical effect of DDIs, as well as their grade of reliability. This information is continuously updated as new evidence appears17–19. However, discrepancies between databases are common and hinder the work of pharmacists and intensivists in their daily clinical practice. For these reasons, detection of potential DDIs (pDDIs) implies close individualized clinical assessment. Although some DDIs are desired by the clinicians, many others require close drug monitoring, dose modification, or even drug discontinuation20.
To date, the frequency and clinical relevance of pDDIs on the first calendar day of ICU admission have not been assessed. Moreover, there is little information about the drugs most frequently involved and management options4,6,7,9. DDIs in critically ill patients are therefore of concern and many questions remain unanswered.
The main objective of this study was to determine the prevalence of pDDIs on the first calendar day of admission to the ICU and to identify the drugs most frequently involved. Another aim was to assess the concordance between two highly-used drug interaction databases (DR and LI) and how the content of the two databases changed by comparing data published in 2013 with that in 2018.
MethodsSetting and study populationA cross-sectional, prospective, observational and multidisciplinary study was carried out at Hospital de la Santa Creu i Sant Pau (HSCSP, Barcelona, Spain), a tertiary care hospital with polyvalent medical-surgery ICU beds. The study population comprised adult patients (≥ 18 years) admitted to the ICU for more than 24 hours. They were recruited between January and June 2013. We did not include cardiac or cardiothoracic patients. This research project was approved by the Institutional Ethics Committee at HSCSP. Written informed consent was not needed because patients in the study had not been applied any additional procedure.
Data collection and evaluation of potential drug-drug interactionsA multidisciplinary team composed of five intensivists and four pharmacists collected the demographic data (age and gender), clinical history data (main diagnosis, simplified acute physiology [SAP II] score, mechanical ventilation, renal replacement therapy, length of stay and main comorbidities), and medication profiles of eligible patients through chart review. The drugs administered on the first complete natural day of admission in the ICU were recorded. This was agreed with the medical team considering that these first hours in the critical care setting are determinant for patient outcomes. Additionally, this approached helped to collect data homogeneously.
All drugs prescribed to each patient were analysed in pairs to check pDDIs with both DR and LI databases, aiming to determine the prevalence, severity and level of evidence of each pDDI detected. According to both databases, severity grades were classified as contraindicated, major, moderate, or minor. The level of evidence for each DDI according to the literature was considered excellent, good, fair, or poor (only LI included “poor” category). In addition, the drugs most commonly involved were identified and the degree of agreement between the two sources used was assessed. In order to compare database updates in the period 2013-2018, data collected in 2013 was analysed at that year and was reevaluated in 2018 with the same databases. The study was designed considering that a five-year period would be suitable to detect variations.
Aiming to optimize prescription and reduce possible ADRs, every detected pDDI that required an intervention was assessed as part of usual practice in the pharmacotherapeutic follow-up.
StatisticsAll results were analyzed using descriptive statistics. Prevalence of pDDIs was expressed as the proportion of patients with at least one pDDI. Concordance between the two databases was evaluated using McNemar tests. Associations between polymedication (> 10 drugs) and presence of pDDIs were assessed using chi-square tests. These analyses were performed for each database and year of analysis. P values of less than 0.05 were regarded as statistically significant. Statistical analyses were carried out using GraphPad Prism software (version 8.3.0).
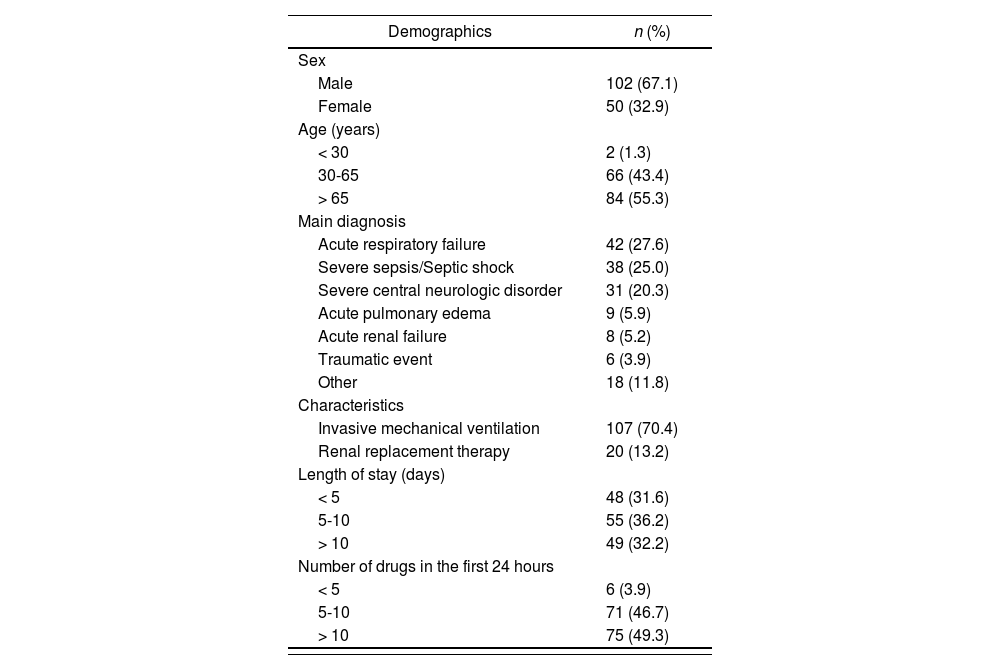
ResultsPatient populationOne-hundred and fifty-two patients were consecutively included in the study (between January and June 2013). The mean age was 64 ± 15 years and mean SAP II score was 45.5 ± 16.3 points. Most patients stayed between five and ten days in the ICU, with a mean stay of 7 days. The median (range) number of drugs prescribed on the first complete natural day of admission was 10 (3-24) drugs. Demographic data and patient clinical characteristics are shown in table 1.
Baseline patient characteristics
| Demographics | n (%) |
|---|---|
| Sex | |
| Male | 102 (67.1) |
| Female | 50 (32.9) |
| Age (years) | |
| < 30 | 2 (1.3) |
| 30-65 | 66 (43.4) |
| > 65 | 84 (55.3) |
| Main diagnosis | |
| Acute respiratory failure | 42 (27.6) |
| Severe sepsis/Septic shock | 38 (25.0) |
| Severe central neurologic disorder | 31 (20.3) |
| Acute pulmonary edema | 9 (5.9) |
| Acute renal failure | 8 (5.2) |
| Traumatic event | 6 (3.9) |
| Other | 18 (11.8) |
| Characteristics | |
| Invasive mechanical ventilation | 107 (70.4) |
| Renal replacement therapy | 20 (13.2) |
| Length of stay (days) | |
| < 5 | 48 (31.6) |
| 5-10 | 55 (36.2) |
| > 10 | 49 (32.2) |
| Number of drugs in the first 24 hours | |
| < 5 | 6 (3.9) |
| 5-10 | 71 (46.7) |
| > 10 | 75 (49.3) |
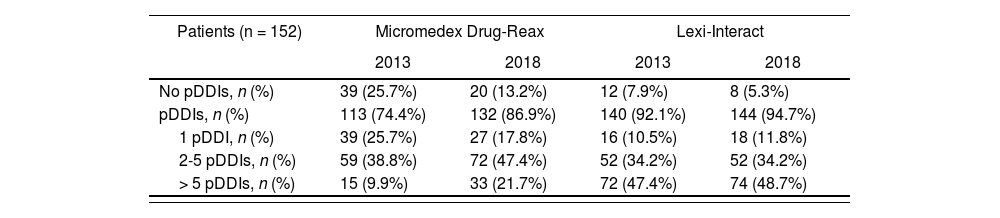
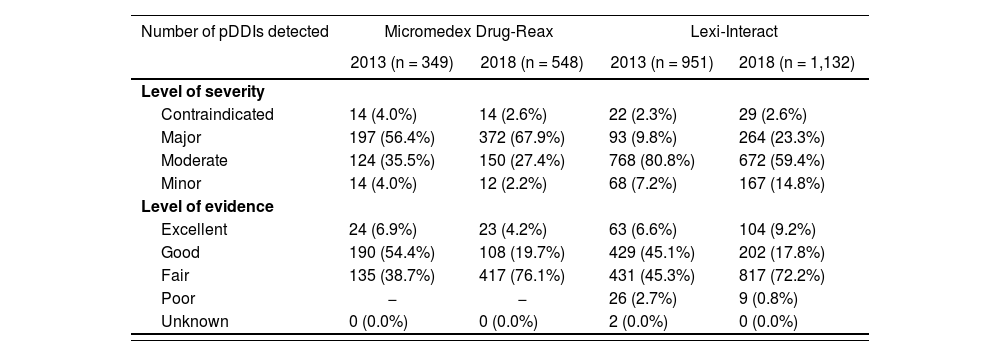
One hundred thirteen patients (according to DR) and 140 patients (according to LI) presented at least one pDDI (p < 0.0001) (Table 2). They accounted for 74.4 and 92.1% of the patients, respectively. Considering the DR database, a total of 349 potential interactions were detected, most of which were major (56.4%). With regards to the LI database, it identified 951 potential interactions, classified mainly as moderate (80.8%). A total of 1,025 pDDIs were detected when both databases were considered. Consequently, LI identified more interactions (951/1,025, 92.8%) than DR (349/1,025, 34.0%). These data are detailed in table 3.
Prevalence of potential drug-drug interactions detected with the databases
| Patients (n = 152) | Micromedex Drug-Reax | Lexi-Interact | ||
|---|---|---|---|---|
| 2013 | 2018 | 2013 | 2018 | |
| No pDDIs, n (%) | 39 (25.7%) | 20 (13.2%) | 12 (7.9%) | 8 (5.3%) |
| pDDIs, n (%) | 113 (74.4%) | 132 (86.9%) | 140 (92.1%) | 144 (94.7%) |
| 1 pDDI, n (%) | 39 (25.7%) | 27 (17.8%) | 16 (10.5%) | 18 (11.8%) |
| 2-5 pDDIs, n (%) | 59 (38.8%) | 72 (47.4%) | 52 (34.2%) | 52 (34.2%) |
| > 5 pDDIs, n (%) | 15 (9.9%) | 33 (21.7%) | 72 (47.4%) | 74 (48.7%) |
pDDI: potential drug-drug interaction.
Level of severity and evidence of potential drug-drug interaction detected with the databases
| Number of pDDIs detected | Micromedex Drug-Reax | Lexi-Interact | ||
|---|---|---|---|---|
| 2013 (n = 349) | 2018 (n = 548) | 2013 (n = 951) | 2018 (n = 1,132) | |
| Level of severity | ||||
| Contraindicated | 14 (4.0%) | 14 (2.6%) | 22 (2.3%) | 29 (2.6%) |
| Major | 197 (56.4%) | 372 (67.9%) | 93 (9.8%) | 264 (23.3%) |
| Moderate | 124 (35.5%) | 150 (27.4%) | 768 (80.8%) | 672 (59.4%) |
| Minor | 14 (4.0%) | 12 (2.2%) | 68 (7.2%) | 167 (14.8%) |
| Level of evidence | ||||
| Excellent | 24 (6.9%) | 23 (4.2%) | 63 (6.6%) | 104 (9.2%) |
| Good | 190 (54.4%) | 108 (19.7%) | 429 (45.1%) | 202 (17.8%) |
| Fair | 135 (38.7%) | 417 (76.1%) | 431 (45.3%) | 817 (72.2%) |
| Poor | − | − | 26 (2.7%) | 9 (0.8%) |
| Unknown | 0 (0.0%) | 0 (0.0%) | 2 (0.0%) | 0 (0.0%) |
pDDI, potential drug-drug interaction.
The ten most common pDDIs identified by at least one of the databases accounted for 37.8% (132/349) and 26.9% (256/951) of the interactions, for DR and LI respectively. Seventy-one patients (46.7%) presented the interaction of midazolam plus morphine, considered a major interaction by DR and moderate by LI. The drugs most frequently involved in pDDIs were those acting on central nervous system targets, such as midazolam-morphine (n = 71), morphine-propofol (n = 36), morphine-acetaminophen (n = 23), and midazolam-propofol (n = 22). Of note, none of these pDDIs required a modification of the prescription during the study period.
Year 2018: Reanalysis of the patient data collected in 1203In 2018, the number of patients with at least one pDDI increased for both databases (132 for DR and 144 for LI), and the difference remained statistically significant (p = 0.003). The percentage of patients with at least one pDDI was 86.9 and 94.7%, with DR and LI respectively (Table 2).
The number of interactions included in each databases increased in 2018 and, consequently, so did the total of pDDIs detected considering the combination of the two databases, which was 1,203. Considering the DR database, 548 potential interactions (45.6%, 548/1,203) were detected, most of which were major (67.9%) (Table 3). As for LI, 1,132 pDDIs were found (94.1%, 1,132/1,203), most of which were moderate (59.4%). Compared to the 2013 assessment, in 2018 a higher number of major pDDIs to the detriment of the moderate pDDIs was found. In relation to the level of evidence, a higher percentage of fair evidence in 2018 was found, which consequently caused a decrease in the percentage of good evidence. In 2013, up to 54% of the contraindicated and major pDDIs identified by DR had an excellent or good level of evidence versus 34.3% in 2018. Regarding LI, the percentages were 31.3% and 23.1%, in 2013 and 2018 respectively.
Patients receiving more than ten drugs showed a higher probability of presenting pDDIs regardless of the year of assessment (2013 or 2018) or the database used (DR or LI). In all cases, statistically significant differences between the two both groups of patients were found (p < 0.005).
Analysis of drug-drug unique pairsThe number of drug-drug unique pairs (when duplicates are removed) was 438 out of 1,025 in 2013 and increased to 459 out of 1,203 in 2018. In 2013, DR and LI agreed in 27.4% (120/438) of the pDDIs identified. As for the level of severity, the percentage of agreement between the two databases was 47.5% (57/120). The maximum degree of concordance (72.9%) was found for moderate pDDIs. In contrast, the percentage of concordance for the remaining categories was found to be ≤ 40%. Considering the analysis in 2018, the percentage of agreement in the detection of pDDIs was higher (38.1%, 175/459), while the agreement for the level of severity was slightly lower (40.6%, 71/175). In keeping with the results obtained in 2013, the percentage of concordance (61%) was highest for those pDDIs considered moderate by both DR and LI.
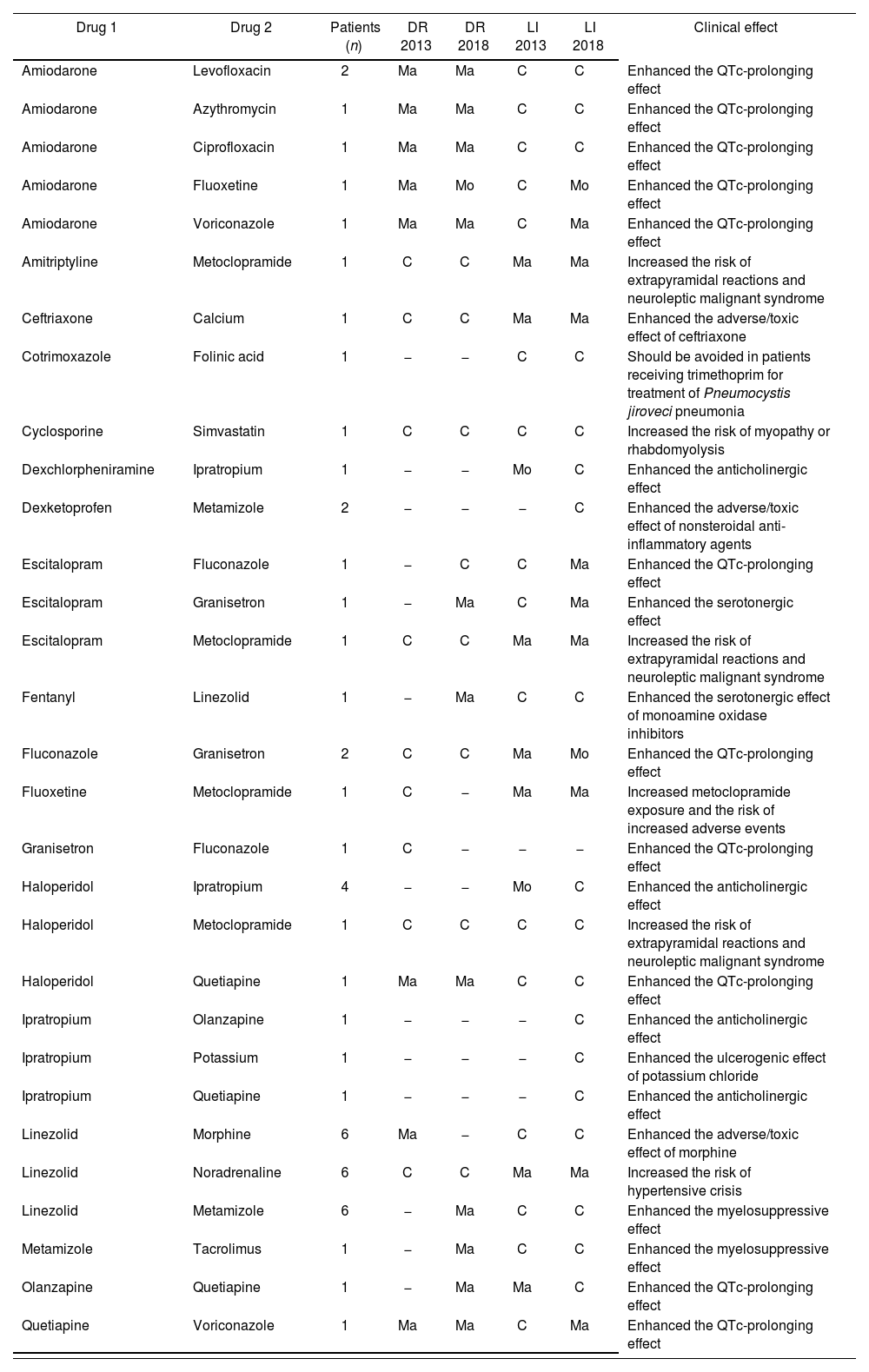
Assessment of contraindicated potential drug-drug interactionsThe percentage of contraindicated interactions was similar in both databases when analysing drug-drug unique pairs (with a percentage of around 4-5%). Only two of the 30 interactions identified by one or both databases in any year of analysis (cyclosporine-simvastatin and haloperidol-metoclopramide) were classified as contraindicated by both databases (Table 4). Each of these pDDIs was observed in only one patient of the study. All pDDIs were monitored, but none led to any changes in prescription.
Contraindicated potential drug-drug interactions in at least one database/year
| Drug 1 | Drug 2 | Patients (n) | DR 2013 | DR 2018 | LI 2013 | LI 2018 | Clinical effect |
|---|---|---|---|---|---|---|---|
| Amiodarone | Levofloxacin | 2 | Ma | Ma | C | C | Enhanced the QTc-prolonging effect |
| Amiodarone | Azythromycin | 1 | Ma | Ma | C | C | Enhanced the QTc-prolonging effect |
| Amiodarone | Ciprofloxacin | 1 | Ma | Ma | C | C | Enhanced the QTc-prolonging effect |
| Amiodarone | Fluoxetine | 1 | Ma | Mo | C | Mo | Enhanced the QTc-prolonging effect |
| Amiodarone | Voriconazole | 1 | Ma | Ma | C | Ma | Enhanced the QTc-prolonging effect |
| Amitriptyline | Metoclopramide | 1 | C | C | Ma | Ma | Increased the risk of extrapyramidal reactions and neuroleptic malignant syndrome |
| Ceftriaxone | Calcium | 1 | C | C | Ma | Ma | Enhanced the adverse/toxic effect of ceftriaxone |
| Cotrimoxazole | Folinic acid | 1 | − | − | C | C | Should be avoided in patients receiving trimethoprim for treatment of Pneumocystis jiroveci pneumonia |
| Cyclosporine | Simvastatin | 1 | C | C | C | C | Increased the risk of myopathy or rhabdomyolysis |
| Dexchlorpheniramine | Ipratropium | 1 | − | − | Mo | C | Enhanced the anticholinergic effect |
| Dexketoprofen | Metamizole | 2 | − | − | − | C | Enhanced the adverse/toxic effect of nonsteroidal anti-inflammatory agents |
| Escitalopram | Fluconazole | 1 | − | C | C | Ma | Enhanced the QTc-prolonging effect |
| Escitalopram | Granisetron | 1 | − | Ma | C | Ma | Enhanced the serotonergic effect |
| Escitalopram | Metoclopramide | 1 | C | C | Ma | Ma | Increased the risk of extrapyramidal reactions and neuroleptic malignant syndrome |
| Fentanyl | Linezolid | 1 | − | Ma | C | C | Enhanced the serotonergic effect of monoamine oxidase inhibitors |
| Fluconazole | Granisetron | 2 | C | C | Ma | Mo | Enhanced the QTc-prolonging effect |
| Fluoxetine | Metoclopramide | 1 | C | − | Ma | Ma | Increased metoclopramide exposure and the risk of increased adverse events |
| Granisetron | Fluconazole | 1 | C | − | − | − | Enhanced the QTc-prolonging effect |
| Haloperidol | Ipratropium | 4 | − | − | Mo | C | Enhanced the anticholinergic effect |
| Haloperidol | Metoclopramide | 1 | C | C | C | C | Increased the risk of extrapyramidal reactions and neuroleptic malignant syndrome |
| Haloperidol | Quetiapine | 1 | Ma | Ma | C | C | Enhanced the QTc-prolonging effect |
| Ipratropium | Olanzapine | 1 | − | − | − | C | Enhanced the anticholinergic effect |
| Ipratropium | Potassium | 1 | − | − | − | C | Enhanced the ulcerogenic effect of potassium chloride |
| Ipratropium | Quetiapine | 1 | − | − | − | C | Enhanced the anticholinergic effect |
| Linezolid | Morphine | 6 | Ma | − | C | C | Enhanced the adverse/toxic effect of morphine |
| Linezolid | Noradrenaline | 6 | C | C | Ma | Ma | Increased the risk of hypertensive crisis |
| Linezolid | Metamizole | 6 | − | Ma | C | C | Enhanced the myelosuppressive effect |
| Metamizole | Tacrolimus | 1 | − | Ma | C | C | Enhanced the myelosuppressive effect |
| Olanzapine | Quetiapine | 1 | − | Ma | Ma | C | Enhanced the QTc-prolonging effect |
| Quetiapine | Voriconazole | 1 | Ma | Ma | C | Ma | Enhanced the QTc-prolonging effect |
C: contraindicated; DR: Micromedex Drug-Reax®; LI: Lexi-interact®; Ma: major; Mi: minor; Mo: moderate.
This study assessed the prevalence of pDDIs in a Spanish ICU. Clinical data were collected and analysed in 2013, and assessed again in 2018 using the same databases (DR and LI) in order to find differences on pDDIs’ detection. Our results show that both compendia are continuously updating and incorporating new interactions. However, their concordance in terms of severity and level of evidence is low.
Our findings confirm a high frequency of pDDIs among critically ill patients, although their clinical relevance is normally low or even desired by the clinicians. The analysis performed in 2013 showed that 74.4%-92.1% of the patients presented at least one pDDI, depending on the database, whereas the rate was considerably higher in 2018 (86.9-94.7%). This increase in pDDI identification could be due to several factors, such as the publication of new scientific studies, the potentiation of postmarketing pharmacovigilance programs, and the introduction of data mining to discover novel and potentially harmful DDIs21–23. The pDDI rates found in our study are higher than the 54-79% reported earlier by other authors2,4,5,7–10,24. Additionally, the number of drugs prescribed per patient on the first calendar day of admission in our ICU is generally higher than that reported in previous studies. In fact, about half of the patients received more than ten drugs, whereas in the study of Vanham et al. this subgroup represented only 16% of patients9. As polypharmacy is associated with a greater likelihood of presenting pDDIs, the high number of drugs prescribed to our patients could explain our findings.
Concerning the ability of the databases used to detect pDDIs, we noticed that LI identified more pDDIs than DR (92.8% vs 34.0% in 2013; 94.1% vs 45.6% in 2018), in keeping with data previously reported by Hasan et al., who reported that 88% of pDDIs were identified by LI in comparison with the 33% detected by DR10. Additionally, we note that Vanham et al. detected only 13% of potential DDIs with the three compendia they used (Epocrates, Stockley and Micromedex)9. Likewise, our study also shows a poor percentage of concordance between DR and LI (27.4% in 2013; 38.1% in 2018). The higher percentage of concordance detected in our study could be due to the fact that we only used two compendia.
Regarding the level of evidence, we found that this was poorer in 2018 than in 2013, possibly attributable to the publication of new studies showing controversial results and the extrapolation of known data from similar drugs. Our results also show that, in 2013, DR identified fewer pDDIs than LI, but the level of evidence was higher. Conversely, in 2018, the percentage of pDDIs with excellent/good level of evidence was higher in LI. The aforementioned differences could be explained by differences between databases regarding inclusion criteria. Considering our findings, and given the lack of concordance between both databases, we would recommend using more than one database when performing the clinical assessment.
We focused specifically on the 10 most common pDDIs detected by DR and LI. These accounted for more than 25% of the total. None of them were classified as contraindicated, either by DR or by LI. In fact, some of them involved drugs used to regulate analgosedation (morphine-midazolam, morphine-propofol, midazolam-propofol), the clinical effect that clinicians aimed to achieve. It is worth noting that opioid analgesics, particularly morphine, were the main group of drugs involved in pDDIs, similarly to the findings described by Hasan et al.10. It should be borne in mind that we did not include either cardiac or cardiothoracic care unit patients. As expected therefore, the rate of pDDIs due to antiplatelet and anticoagulant drugs was low in comparison with other studies7,18. As previously mentioned, we found that the 10 most common pDDIs accounted for 26.9% (DR) and 37.8% (LI) of the total. Comparing our rates with the 17.5% reported by Smithburger et al., our percentage is markedly higher25. This difference could be due to the greater rate of drug-drug pairs/pDDIs detected in Smithburger's study. They found a rate of 65.0% (297/457), whereas the rate observed in our study was 42.7% (438/1,025) in 2013.
It should be emphasized that in 2013, LI identified 16 contraindicated pDDIs while DR detected only 9. Strikingly, only two of these of these pDDIs were typified equally in both databases (cyclosporine-simvastatin and haloperidol-metoclopramide). The concomitant use of cyclosporine and simvastatin increases the risk of myopathy or rhabdomyolysis, whereas the interaction between haloperidol and metoclopramide is associated with an enhanced risk of extrapyramidal reactions and neuroleptic malignant syndrome. If the cyclosporine-simvastatin pDDI is detected, it is advised to switch to a less sensitive statin to this interaction, such as pravastatin or fluvastatin, or to an alternative type of LDL-lowering medication. However, the patient taking simvastatin and cyclosporine concomitantly was a hearttransplanted patient who received both drugs as chronic medication. Consequently, despite the interaction detected, simvastatin was not changed. With regard to the interaction between haloperidol and metoclopramide, it should be borne in mind that haloperidol is frequently administered as a single-dose, so this interaction is normally harmless and no interventions are needed. It should be noted that although no modifications in the treatment were made, patients were continuously and prospectively followed and monitored by clinical pharmacists along with the medical team.
The major strength of our study is that we evaluated the detection of pDDIs by two databases in two different years. In 2013, we prospectively collected the medication profiles of the patients included, which were analysed to identify pDDIs in that year and reassessed in 2018. Thereby, we were able to assess how databases were updated. The study also has limitations that should be taken into account. First, some drugs, such as urapidil, did not appear in either of the two databases tested. Others were included in only one of them (such as dexketoprofen, which was only included in LI) or only in one of the years of analysis (metamizole, for example, was only found in DR in 2018). Second, we did not use all the databases available, although we selected those considered most reliable and complete. Third, we did not include cardiac or cardiothoracic patients given that they are not assigned to intensive care clinicians in our hospital. Therefore, some drugs with significant potential interactions may have been excluded. Finally, we tested only the pDDIs caused by the drugs given during the first complete calendar day of admission in the ICU. Consequently, drugs prescribed afterwards were not evaluated. However, we consider that in the first day of admission pharmacotherapy is complex enough to highlight the incidence of pDDIs. Our results are not therefore comparable to those reported from studies carried out for other intervals of time9.
In conclusion, our results show that the prevalence of pDDIs in the critical care setting is high, although their clinical relevance is generally harmless and manageable by clinicians. Our data also show a lack of concordance between DR and LI, as well as their continuous updating. Interpreting the overwhelming amount of information provided by the two compendia is a clinical challenge in daily clinical practice. To tackle this issue, it is mandatory to perform an individualized assessment of the pDDIs identified, taking into account the information given by the databases along with the clinical situation of the patient.
FundingNo funding.
AcknowledgementsWe thank Carolyn Newey for her linguistic revision of the paper.
Conflict of interestsNo conflict of interests.
Contribution to the scientific literatureThis study provides an analysis of the information found by using two databases about drug-drug interactions in critically ill patients.
The results show the importance of using several databases for interaction analyses.
Early Access date (08/26/2022).