To compare the persistence, retention rate and prescription pattern of original infliximab and infliximab CT-P13 in biologic-naive patients with ulcerative colitis.
MethodThis was an ambispective study of biologic-naive patients with ulcerative colitis who received non-simultaneous first-line treatment with Remicade® (infliximab) and Remsima® (infliximab CT-P13) over a 10-year study period (2012-2021). Data on their age, weight, persistence, retention rate and on whether they required intensification or deintensification throughout the study period was collected. The real patient/year cost of Remicade® and Remsima® was determined individually based on the amounts administered during the study period.
Results27 biologic-naive patients were treated with Remicade® and 53 with Remsima®. Neither patient group presented with differences in terms of weight and age. Persistence (median ± interquartile range) with Remicade® was 42.49 ± 57.48 months, as compared to 27.50 ± 58.50 months for Remsima®, without significant differences (p = 0.455). The retention rate at 6, 12, and 24 months was 81%, 63%, and 33%, respectively, for the Remicade® group and 71%, 47%, and 37%, respectively, for the Remsima® group. Nine subjects in the Remicade® group vs 11 patients in the Remsima® group were intensified. Regarding deintensification, five patients treated with Remicade® were deintensified, as compared with 7 patients on Remsima®. Savings obtained with the use of Remsima® amounted to 203,649 €, which would allow treating an additional 118 patients with biosimilar infliximab for one year.
ConclusionsThere are no significant differences in persistence, retention, and number of intensifications or deintensifications between biologicna'tve patients treated with Remicade® and those treated with Remsima®, the latter being an effective, safe and economical alternative for the treatment of ulcerative colitis.
Comparar la persistencia, tasa de retención y pauta de prescripción de infliximab original e infliximab CT-P13 en pacientes naive a biológicos con colitis ulcerosa.
MétodoEstudio ambispectivo de pacientes naive a biológicos en colitis ulcerosa que recibieron tratamiento en primera línea con Remicade® (infliximab) y Remsima® (infliximab CT-P13) de forma no simultánea durante un periodo de estudio de 10 años (2012-2021). Se tomaron datos de su edad, peso, persistencia, tasa de retención y si precisó de intensificación o desintensificación a lo largo del periodo de estudio. Se determinó el coste paciente/año real de Remicade® y Remsima® de forma individualizada en función de las administraciones durante el periodo del estudio. Resultados: Un total de 27 pacientes naive a biológicos fueron tratados con Remicade® y 53 con Remsima®. Ambos grupos de pacientes no presentaron diferencias en cuanto al peso y edad. La persistencia (mediana ± rango intercuartílico) con Remicade® fue de 42,49 ± 57,48 meses frente a 27,50 ± 58,50 meses para Remsima®, sin demostrar diferencias significativas (p = 0,455). La tasa de retención a los 6, 12 y 24 meses fue del 81%, 63% y 33%, respectivamente, para el grupo de Remicade®, y del 71%, 47% y 37%, respectivamente, para el grupo de Remsima®. En el grupo de pacientes tratados con Remicade®, 9 pacientes fueron intensificados frente a 11 pacientes en el grupo de Remsima®. En cuanto a las desintensificaciones, 5 pacientes que recibieron tratamiento con Remicade® fueron desintensificados frente a 7 pacientes en tratamiento con Remsima®. El ahorro obtenido con el uso de Remsima® fue de 203.649 €, que equivaldría a tratar a 118 pacientes adicionales con infliximab biosimilar durante un año.
ConclusionesNo existen diferencias significativas en la persistencia, tasa de retención y número de intensificaciones y desintensificaciones entre los pacientes naive que fueron tratados con Remicade® y aquellos tratados con Remsima®, siendo una alternativa eficaz, segura y económica en el tratamiento biológico de la colitis ulcerosa.
Infliximab (IFX) is a chimeric IgG1 monoclonal antibody that targets tumor necrosis factor alpha (TNFα). It is a proinflammatory cytokine that plays an important role in the context of ulcerative colitis and Crohn's disease1. TNF-inhibitors such as infliximab, used either as monotherapy or in combination with immunosuppressants, have allowed a more effective treatment of inflammatory bowel disease (IBD)2. Initially approved in 1998, infliximab has been used to treat over 2.6 million patients and has a wellestablished long-term safety profile.3,4. Nevertheless, the high cost of these drugs makes it necessary for healthcare providers to do their utmost to use them correctly, maximize their efficacy, minimize their toxicity and avoid unnecessary costs5. The European patent for original infliximab (Remicade®) expired in 2015, and in February of that same year biosimilar infliximab CT-P13 (Remsima®) was licensed for use in Spain. The advent of the biosimilar has resulted in a significant cost reduction and improved access to this treatment.
Adherence to medications has been defined as the process by which patients take their medication as prescribed6. Lack of adherence affects the effectiveness of treatment, which an ensuing increase in medicationassociated risks and costs7. Given that no agreed-upon yardstick exists to measure adherence, a variety of different methods are usually used for this purpose such as questionnaires, dispensing registers, dispensing records and electronic devices, no single one of them —however— adequate sensitivity8. For that reason, the term persistence has come to be used as an adjunct to the concept of adherence. Persistence, an easily measured indicator of the long-term therapeutic benefit of a drug, has been defined as “the duration of time from initiation to discontinuation of therapy”9. Against this background, it is essential to confirm clinical results in everyday clinical practice, where some authors have hailed persistence as an appropriate indicator for evaluating the effectiveness and safety of a treatment and the patients’ satisfaction with it10.
The purpose of this study is to compare the persistence, retention rate, dosing changes (intensification and deintensification) and economic impact of infliximab CT-P13 as compared with original infliximab in two cohorts of patients with ulcerative colitis who were naïve to biologics in a third-level general hospital.
MethodsA non-randomized ambispective observational study was carried out from January 2012 to November 2021 including adult patients diagnosed with biologic-naive ulcerative colitis. Patients were randomized to receive either first line infliximab (Remicade®) or first line infliximab CT-P13 (Remsima®). The study included all adult patients with moderate to severe ulcerative colitis where treatment with glucocorticoids, immunosuppressants or both had failed and who completed the induction process to Remicade® or Remsima®. The variables analyzed were sex, age, diagnosis, initiation date, number of administrations of the drug, dosing regimen and number of intensifications and deintensifications. The number of patients who had discontinued treatment was calculated as well as persistence and the retention rates for Remicade® and Remsima® at 6, 12 and 24 months. The result was analyzed using Kaplan-Meier curves and the Log Rank statistical test.
Persistence on Remicade® and Remsima® was calculated based on the dates of initiation and end of treatment. The end-of-treatment date was considered to be the date at which the attending physician decided to discontinue the treatment as reflected in the patient's medical record. If the patient was still on the treatment, persistence was calculated based on the date when the follow-up ended (1 November 2021). Losses to follow-up, understood as failure by patients to visit their digestive doctor or the pharmacist over the course of one year, were considered to be errors in the persistence analysis9. Data was obtained from the Pharmacy Department's IV therapy preparation and validation software (Oncofarm® IMF) and the patients’ electronic medical record (Integrador® and Abucasis®). The yearly cost per patient of Remicade® and Remsima® was calculated individually depending on the number of administrations during the study period; real acquisition costs were obtained from a public procurement database (https://www. acobur.es. Last access: 2 February 2022).
The statistical analysis was conducted using the SPSS Statistics® v23 software. The results of categorical variables were described by means of frequencies (%) and compared through Pearson's chi squared test. The results of the quantitative variables were described using means and standard deviation (SD) in cases where they followed a normal distribution, which was previously determined by Shapiro-Wilk's normality test, and by means and interquartile ranges (IQRs) in cases where the distribution was not normal.
The study was approved by the hospital's Clinical Research Ethics Committee in compliance with the guidelines set by the Helsinki Declaration.
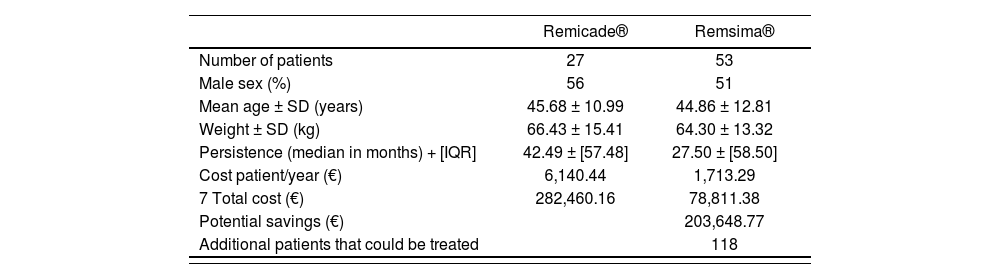
ResultsA total of 80 adult patients were included in the study. All of them had been diagnosed with biologic-naïve ulcerative colitis and were receiving first line treatment with either original infliximab (27 patients) or biosimilar infliximab (53 patients). Table 1 shows the subjects’ demographic data (sex, age and weight), with no significant differences being observed between the two groups. At the end of the study (November 2021), 3 patients on Remicade® and 32 on Remsima® were still being treated.
Patient demographic characteristics and persistence and cost assessment of Remicade® and Remsima®
| Remicade® | Remsima® | |
|---|---|---|
| Number of patients | 27 | 53 |
| Male sex (%) | 56 | 51 |
| Mean age ± SD (years) | 45.68 ± 10.99 | 44.86 ± 12.81 |
| Weight ± SD (kg) | 66.43 ± 15.41 | 64.30 ± 13.32 |
| Persistence (median in months) + [IQR] | 42.49 ± [57.48] | 27.50 ± [58.50] |
| Cost patient/year (€) | 6,140.44 | 1,713.29 |
| 7 Total cost (€) | 282,460.16 | 78,811.38 |
| Potential savings (€) | 203,648.77 | |
| Additional patients that could be treated | 118 |
IQR: inter-quartile range; SD: standard deviation.
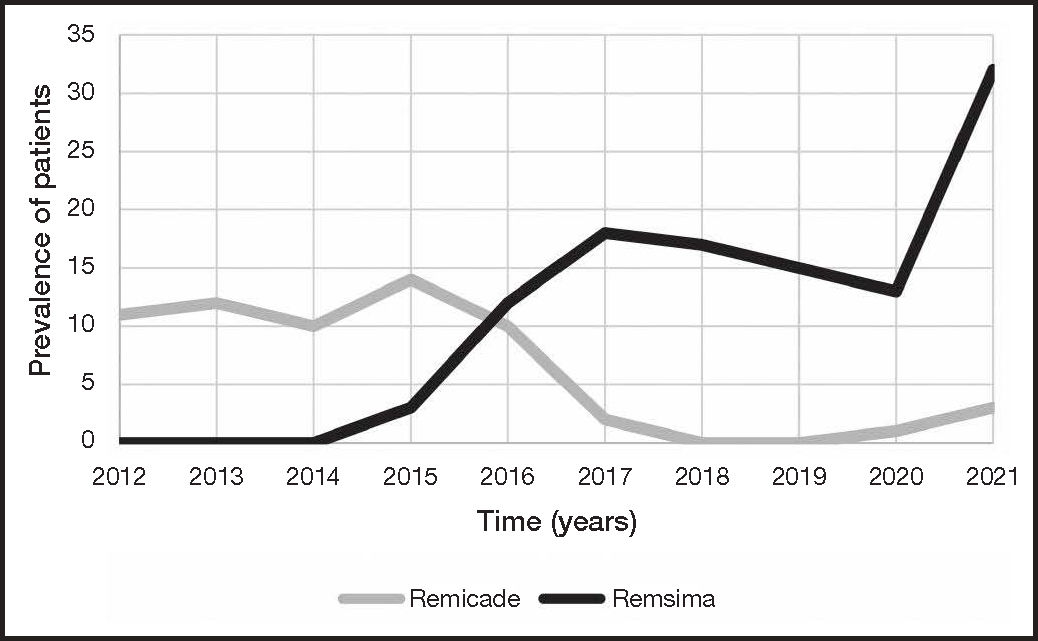
Figure 1 shows the number of active patients on Remicade® and Remsima® for each year (2012-2021). The number of patients on Remicade® remained stable until 2015. That year, after inclusion of Remsima® in the hospital's formulary and the positioning of the drug as a treatment of choice in patients with biologic infliximab naïve ulcerative colitis, a gradual increase was observed in the number of patients taking this medication, which reaching its peak in 2021 with 32 patients. The annual treatment initiation rate was 6.75 for Remicade® and 8.83 for Remsima®.
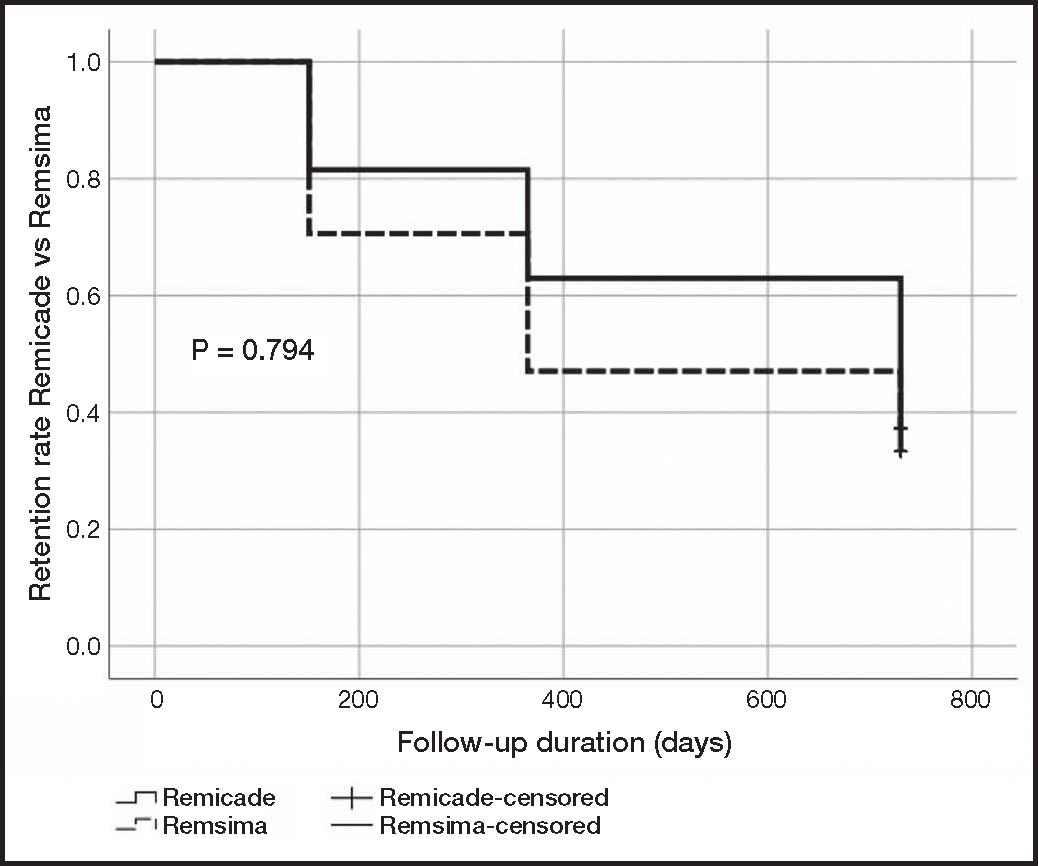
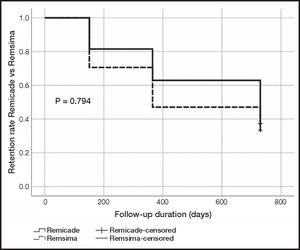
Persistence on treatment was similar for the original and the biosimilar infliximab. Remicade® exhibited a median (± IQR) of 42.49 ± 57.48 months (6-63 months) while median persistence on Remsima® was 27.50 ± 58.50 months (5-59 months). The statistical analysis showed an absence of statistically significant differences between both treatments (p = 0.455) (Table 1). The retention rate was expressed as the percentage of patients that remained on treatment at 6, 12 and 24 months. The retention rate at 6 months was 81% (22/27) for Remicade® as compared with 71% (36/51) for Remsima®; 63% (17/27) for Remicade® as compared with 47% (24/51) for Remsima® at 12 months; and 33% (9/27) for Remicade® as compared with a 37% (19/51) for Remsima® at 24 months. The Kaplan-Meier curve analysis of the retention rate did not show statistically significant differences between both treatments (p = 0.794) (Figure 2).
Treatment intensifications based on shortening dosing intervals were as follows: 9 intensifications for the group of patients on Remicade® (1 patient with a 4-week interval and 8 patients with a 6-week interval) as compared with 11 intensifications (4 patients with a 4-week interval and 7 patients with a 6-week interval) for patients treated with Remsima®. Intensifications based on increasing the infliximab dose (> 5 mg/kg) were as follows: 1 for the Remicade® group as compared with 5 for the Remsima® group. Treatment deintensifications based on lengthening dosing intervals were as follows: 5 deintensifications for the Remicade® group (4 patients with a 10-week interval and 1 patient with a 12-week interval) as compared with 7 deintensifications (5 patients with a 10-week interval and 2 patients with a 12-week interval) for patients treated with Remsima®. Deintensifications based on reducing the infliximab dose (< 5 mg/kg) were as follows: 2 deintensifications for the Remicade® group and 5 for the Remsima® group.
The mean annual cost per patient was €6,140.44 € for patients treated with Remicade® as compared with 1,713.29 € for those treated with Remsima®. The savings that the hospital would have made if the 27 patients treated with Remicade® had been treated with Remsima® would have amounted to 203,648.77 € equivalent to the cost of treating 118 new patients for one year (Table 1).
DiscussionThe results of this study showed that the cost-driven inclusion of biosimilar infliximab (CT-P13) in the hospital's formulary in 2015 facilitated access of patients with biologic-naive ulcerative colitis to treatment as a result of the drug's lower cost. This also resulted in a higher annual treatment initiation rate for Remsima® (Figure 1).
It should be mentioned that age, sex and weight were not significantly different in the two cohorts studied. In addition, the two cohorts were treated by the same specialists, who followed the same clinical management protocols (specific to patients with biologic-naïve ulcerative colitis). None of these factors can therefore be considered to induce a bias in the comparison of the persistence values, retention rates and modifications of the prescribed dosing regimen presented in this study. Persistence on infliximab treatment was similar for original and biosimilar infliximab. Indeed, although the mean value obtained for the Remicade® group was higher, the analysis of data showed an absence of statistically significant differences between both treatments. A possible reason for these absolute differences could be that given that no patients had been started on Remicade® since mid-2015, this persistence value could be considered to be close to its maturity (only three patients were still on the drug in November 2021). Contrary to this, 32 patients were still receiving Remsima® in November 2021. This means that an analysis of persistence carried out in the future would probably yield substantially different values. The persistence values presented in this study are in line with those of other real-world studies analyzing both Remicade®11 and Remsima®12. In a retrospective multicenter study evaluating persistence on Remicade® and Remsima® in patients with biologic-naïve ulcerative colitis, Martínez-Lozano et al.13 showed similar mean persistence values for both treatment groups analyzed. At weeks 14 and 54, both groups reached a similar clinical outcome with comparable response and remission rates13.
As regards retention, the retention rate for Remsima® at 6 and 12 months was somewhat lower than that for Remicade®, yet the difference was not statistically significant. Moreover, the 24-month retention rates for both groups reached equal values (33% for Remicade® and 37% for Remsima®), showing a very similar profile in the Kaplan-Meier curve analysis (Figure 2). Other authors13, however, obtained retention rates of 47.5% in patients on Remicade® at 59 months and of 76.1% in patients on Remsima® at 33 months. Avouac et al.14 switched a series of patients diagnosed with chronic inflammatory conditions from original to biosimilar infliximab. The retention rate reached 85% at the third infusion, without any objective clinical differences being observed following the change of treatment. Kin et al.15 obtained a retention rate of 69.7%, 46.0% and 26.7% at 1, 3 and 5 years, respectively, in biosimilar infliximab-naïve patients. Additionally, patients who were switched from original to biosimilar infliximab achieved a retention rate of 73.9%, 42.5% and 42.5% at 1, 3 and 5 years respectively. No statistically significant differences were found between patients started on biologic therapy with a biosimilar infliximab and those who were switched.
Therapy intensifications, both those based on a shortening of the dosing interval and those where the standard dose was increased, exhibited a prevalence of 37% in patients in the Remicade® group and of 30% in those on Remsima®. These results are similar to those obtained by Long et al.16 who found a prevalence of intensifications of 34.8%. Martínez-Lozano et al.13 recently published that 40% of patients treated with Remicade® and 32.6% of those treated with Remsima® required an intensified dosing regimen. Deintensifications exhibited a prevalence of 30% in patients on Remicade® and of 22% in those on Remsima®. In this case, differences may be attributable to the fact that over 30% of patients in the Remsima® group had been on the drug for less than one year. Moreover, it must be taken into consideration that deintensification requires the patient to have been in clinical remission for at least 6 months.17.
As regards the pharmacoeconomic aspect, biosimilar treatment entails a significant potential benefit as infliximab CTP-13 has shown the same level of efficacy as original infliximab at a lower economic cost13. The mean annual cost per naive patient was €6,140.44 for patients treated with Remicade® as compared with €1,713.29 for those on Remsima®. The advent of biosimilars has allowed a reduction in the cost of biologic treatments, which in our study resulted in savings of €203,648.77. This amount is equivalent to the cost of treating up to 118 new patients for one year, i.e., a larger number of patients can avail themselves of the treatment for the same cost. In addition, given that biologic treatment of ulcerative colitis results in significant savings, this strategy could be applied to other clinical areas, thus increasing the efficiency of the health system18.
This study presents with a series of limitations. First of all, as it was a real-world study, treatments were not assigned through a randomization process. Additionally, efficacy, safety, adherence and patient satisfaction were not measured. No pharmacokinetic monitoring was carried out in the analyzed cohorts to adjust the Remicade® or the Remsima® dose; only neutralizing antibodies were quantified. Finally, given that the findings presented in this study pertain to a single hospital, it is the authors’ intention to extend the research to other centers in order to confirm the results obtained.
No significant differences exist regarding persistence, retention and number of intensifications and deintensifications between patients with biologic-naive ulcerative colitis treated with Remicade® and with Remsima®. Our results also attest to the economic savings that can be made by using biosimilars to treat patients with biologic-naive ulcerative colitis and the wider access to treatment allowed by these drugs as a larger number of patients can be treated at the same, or even a lower, cost.
The results obtained advances the state of the art in the realm of biosimilar-based biologic therapy and its use in the treatment of ulcerative colitis. However, a larger number of multicenter studies is needed to confirm the findings obtained here and to gain a better understanding of the factors that may impact the persistence and the retention of infliximab CT-P13.
FundingNo funding.
Conflict of interestNo conflict of interests.
Presentation at congressesThe paper was accepted as a poster for the 25th Annual Meeting of the Spanish Gastroenterology Society to be held from 23 to 25 March 2022.
Contribution to the scientific literatureThe advent of biosimilar biologic treatments for ulcerative colitis has provided the opportunity to increase access to biologic therapies and enhance the efficiency of health systems.
This article may serve as a reference as it demonstrates similar persistence and retention levels in biologic-naïve ulcerative colitis patients treated with original infliximab and those treated with biosimilar infliximab. The study also demonstrated the considerable economic savings obtained when using biosimilars in patients with ulcerative colitis treated with infliximab.
Early Access date (05/31/2022).