to describe the efficacy and safety of the off-label use of alendronate in the treatment of osteoporosis in children and adolescents.
Methoda retrospective study (2008-2014) of all patients under 18 years who were dispensed alendronate for this indication. The criteria for initiating treatment were: bone mineral density with a Z-score < -2.5 SD, a past history of bone fractures without a previous traumatism, and persistent pain. The variables collected were: demographic, treatment-related, clinical. and safety data. The treatment was considered to be effective when there was an increase in bone mineral density up to a Z-score > -2.5 SD.
Resultsa total of 12 patients, 8 of them male, with a mean age of 11 years (± 3 SD), were treated with alendronate. After a mean time of treatment of 2.15 years (± 1.2 SD), there was an increase in bone mineral density in all patients, 9 of which achieved a Z-score > -2.5 SD, so the drug was considered effective in 75% of cases. No patient had bone fractures or expressed adverse effects during treatment.
Conclusionsalendronate increased bone mineral density and was well tolerated in all patients, therefore it could be considered as a therapeutic option in the treatment of osteoporosis in children.
describir la efectividad y seguridad del uso de ácido alendrónico en el tratamiento de la osteoporosis en ninos y adolescentes, en condiciones distintas a las autorizadas en la ficha técnica.
Métodosestudio retrospectivo (2008-2014) de todos los pa-cientes menores de 18 anos a los que se dispensó ácido alendrónico para esta indicación. Los criterios para iniciar trata-miento fueron: densidad mineral ósea con puntuación Z-score < -2,5 DE, antecedentes de fracturas óseas sin traumatismo previo y dolor persistente. Las variables recogidas fueron: demográficas, de tratamien-to, clínicas y de seguridad. Se consideró efectividad del trata-miento al aumento de la densidad mineral ósea hasta obtener Z-score > -2,5 DE.
Resultadosun total de 12 pacientes, 8 varones, con una media de edad de 11 anos (± 3 DE), fueron tratados con ácido alen-drónico. Tras un tiempo medio de tratamiento de 2,15 anos (± 1,2 DE), se produjo aumento de la densidad mineral ósea en todos los pacientes, 9 de los cuales obtuvieron Z-score > -2,5 DE, por lo que el fármaco se consideró efectivo en el 75% de los ca-sos. Ningün paciente presentó fracturas óseas ni manifesto efec-tos adversos durante el tratamiento.
Conclusionesel ácido alendrónico incrementó la densidad mineral ósea y se toleró bien en todos los pacientes, por lo que se podría considerar como opción terapéutica en el tratamiento de la osteoporosis infantil.
The present article intends to analyze the efficacy and safety of a drug currently positioning itself as first line treatment for paediatric osteoporosis due to its oral administration, but with a still limited experience of use. This series of cases demonstrates that its efficacy is high, without any noteworthy adverse effects, and therefore it appears as a valid therapeutic option.
IntroductionAlendronic acid (AA) is a biphosphonate; its primary mechanism of action consists in bone reabsorption by binding to the hydroxyapatite crystals in the bone matrix, thus turning it more resistant to the action of osteoclasts1. Its indication, according to the product specifications, is for osteoporosis treatment in post-me-nopausal women, in order to prevent fractures2.
Its use in patients under 18-year-of age is conducted outside of its authorization (“off-label”)3 for treatment of osteoporosis caused by a reduction in mobility (Duchenne Muscular Dystrophy4, cerebral palsy in children, “spina bifida”, and bone marrow lesions), bone defect (osteogenesis imperfecta, idiopathic juvenile osteoporosis), chronic inflammation (juvenile idiopathic arthritis, systemic erythematosus lupus, bowel inflammatory disease), malnourishment, and bone toxicity of drugs such as glucocorticoids.
Besides calcium and Vitamin D supplements, there are currently no pharmacological treatments for osteoporosis with efficacy and confirmed safety in paediatric patients. There is limited experience with biphosphonates at this age, because they have been studied in reduced groups of patients4, and good response has been obtained mainly with intravenous pamidronate in osteogenesis imper-fecta, though oral AA has started to be used with similar results and no need for hospitalization5. Its efficacy and long-term safety are not enough, and therefore its widespread use has not been recommended.6. The patients who would benefit from treatment would be those with a reduction in Bone Mineral Density (BMD) (Z-score < -2.5 standard deviations (SD) adjusted according to age and gender), and with previously existing associated symptoms, such as fractures caused by low intensity trauma-tism, vertebral compressions, or disabling bone pain7.
Because this is a use outside its approved indication, it is dispensed through the Hospital Pharmacy Unit, according to Royal Decree 1015/20098 and the rules by the Pharmacy Committee in each centre, following the rules by Organic Law 15/1999 for Personal Data Protection. In our hospital, the Outpatient Area Pharmacist must prepare a report before dispensation, which analyzes the need for the medication according to criteria for treatment initiation; this must then by authorized by the Hospital Management.
The objective of the study was to describe the efficacy and safety of the off-label use of AA for osteoporosis treatment in children and adolescents outside its approved indication.
MethodsAn observational retrospective study in a third level hospital, including all patients under 18-year-old who were administered AA every two months in the Outpatient Area of the Hospital Pharmacy, between March, 2008 (first dispensation conducted) and August, 2014. Criteria for treatment initiation were: BMD with a Z-sco-re < -2,5 SD, a history of bone fractures without previous traumatism, and persistent lumbar and lower limb pain.
The variables collected were:
Demographic: Gender and age at treatment initiation.
Treatment-related: Dosing, treatment duration, treatment compliance, and drugs previously and/or concomitantly used.
Clinical: Besides the basal condition, variables determining efficacy were collected, such as BMD at treatment initiation and completion. BMD was measured through bone densitometry in lumbar spine (L2-L4) through Z-score according to age and gender. Treatment was considered effective when there was a BMD increase up to a Z-score over -2,5 SD was achieved.
Safety-related: Presence of bone fractures, as well as calcium-phosphate metabolism parameters (CPMP) (calcium, phosphate, alkaline phosphatase, parathyroid hormone, plasma levels of 25-hydroxyvitamin D3, and calciuria), for an early detection of any potential hypo-calcemia, the most severe and relevant adverse effect by this drug.
In order to monitor clinical and safety variables, initial clinical and lab test controls were conducted every 4-6 months, and bone densitometry tests every 6-12 months.
The application for dispensation to outpatients (Athos-APD®) was used for identification of patients and anonymized data collection, and the clinical records from the Paediatric Units recorded in the electronic clinical record (Mambrino®) were used for information about patient evolution.
All patients received information at the Pharmacy Care Unit, about the adequate way of administration for the AA tablet, without food and without lying down once taken, in order to prevent the symptoms derived from its ability to cause local irritation in the upper digestive tract mucosa, particularly dyspepsia, abdominal pain, or acid reflux2,3. At each dispensation, follow-up for BMD and phosphate & calcium metabolism was conducted, as well as detection of potential adverse effects. and treatment compliance measurement through the indirect method of dispensation records; treatment compliance was considered if over 90%.
A descriptive analysis was conducted, where the continuous variables with normal distribution were expressed as mean value with SD.
ResultsTwelve (12) patients, 8 of them male, with 11-year-old as mean age (± 3 SD), were treated with AA. The dosing used for all patients was 10 mg/day. The mean treatment duration was 2.15 years (± 1,2 SD). All patients were considered compliant. One patient was previously treated with intravenous pamidronate. Concomitant treatments were: calcium in combination with Vitamin D (3 patients), deflazacort drops (3), phenylbutyrate and carnitine with protein restriction (1), and no treatment in the remaining 5 patients.
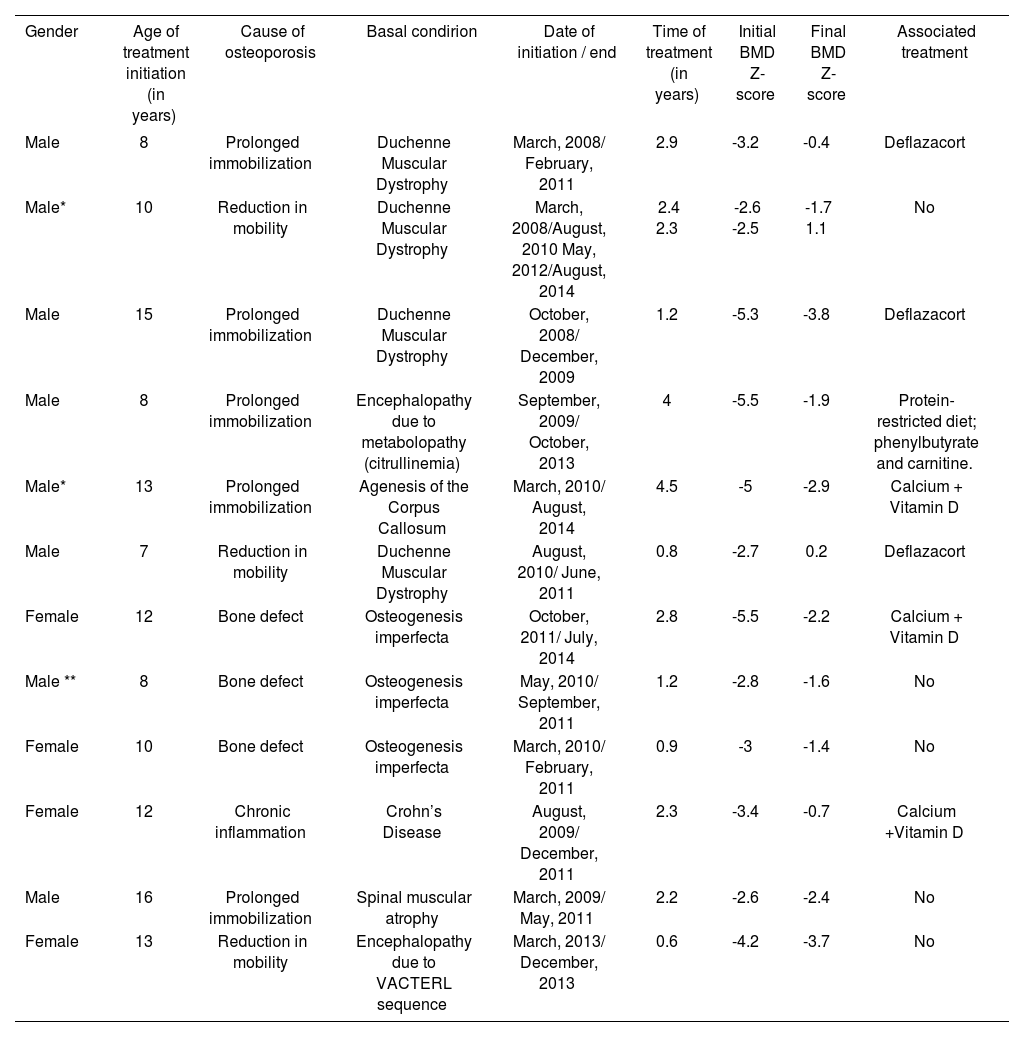
Basal conditions were: Duchenne Muscular Dystrophy (4 patients), osteogenesis imperfecta (3), encephalopa-thy (2), spinal muscular atrophy (1), agenesis of the corpus callosum (1), and Crohn’s Disease (1). (Table 1)
Characteristics of patients and evolution with treatment
| Gender | Age of treatment initiation (in years) | Cause of osteoporosis | Basal condirion | Date of initiation / end | Time of treatment (in years) | Initial BMD Z-score | Final BMD Z-score | Associated treatment |
|---|---|---|---|---|---|---|---|---|
| Male | 8 | Prolonged immobilization | Duchenne Muscular Dystrophy | March, 2008/ February, 2011 | 2.9 | -3.2 | -0.4 | Deflazacort |
| Male* | 10 | Reduction in mobility | Duchenne Muscular Dystrophy | March, 2008/August, 2010 May, 2012/August, 2014 | 2.4 2.3 | -2.6 -2.5 | -1.7 1.1 | No |
| Male | 15 | Prolonged immobilization | Duchenne Muscular Dystrophy | October, 2008/ December, 2009 | 1.2 | -5.3 | -3.8 | Deflazacort |
| Male | 8 | Prolonged immobilization | Encephalopathy due to metabolopathy (citrullinemia) | September, 2009/ October, 2013 | 4 | -5.5 | -1.9 | Protein-restricted diet; phenylbutyrate and carnitine. |
| Male* | 13 | Prolonged immobilization | Agenesis of the Corpus Callosum | March, 2010/ August, 2014 | 4.5 | -5 | -2.9 | Calcium + Vitamin D |
| Male | 7 | Reduction in mobility | Duchenne Muscular Dystrophy | August, 2010/ June, 2011 | 0.8 | -2.7 | 0.2 | Deflazacort |
| Female | 12 | Bone defect | Osteogenesis imperfecta | October, 2011/ July, 2014 | 2.8 | -5.5 | -2.2 | Calcium + Vitamin D |
| Male ** | 8 | Bone defect | Osteogenesis imperfecta | May, 2010/ September, 2011 | 1.2 | -2.8 | -1.6 | No |
| Female | 10 | Bone defect | Osteogenesis imperfecta | March, 2010/ February, 2011 | 0.9 | -3 | -1.4 | No |
| Female | 12 | Chronic inflammation | Crohn’s Disease | August, 2009/ December, 2011 | 2.3 | -3.4 | -0.7 | Calcium +Vitamin D |
| Male | 16 | Prolonged immobilization | Spinal muscular atrophy | March, 2009/ May, 2011 | 2.2 | -2.6 | -2.4 | No |
| Female | 13 | Reduction in mobility | Encephalopathy due to VACTERL sequence | March, 2013/ December, 2013 | 0.6 | -4.2 | -3.7 | No |
Mean age at treatment initiation: 11 years (±3 SD)
Mean time of treatment: 2.15 years (± 1,21 SD)
Initial mean BMD: - 3,7 (± 1,2 SD)
Final mean BMD: -1,7 (±1,4 SD)
Z-score: Number of standard deviations (SD) of a patient based on the mean BMD in the population of the same age and gender.
There was BMD increase in all patients. The initial mean BMD was -3.7 (± 1.2 SD), and the final mean BMD in the collected period was -1,7 (± 1,4 SD). Ten patients ended treatment due to improvement according to clinical report, and 2 continued active at the study completion: one of them had to reinitiate treatment due to worsening. At the end of treatment, 9 out of 12 patients achieved a BMD with a Z-score over -2,5 SD, and therefore the drug was considered effective in 75% of patients.
No patients presented bone fractures during treatment. In lab test controls, CPMPs remained within normality in all cases. AA was well tolerated, and no adverse effects associated were detected.
DiscussionThere are various studies supporting the use of AA in paediatric osteoporosis secondary to neuromuscular conditions4,6,9, osteogenesis imperfecta1,5,10,11, connective tissue diseases12 or Crohn’s Disease13. In all these studies, as well as in this one, a BMD increase has been observed, with a reduction in the number of fractures.
Regarding AA dosing, 10 mg/day1,7,10,11,13 are used in the majority of studies, including ours, with a variation between 0.2 y 0.3 mg/kg/day.
There are no specific recommendations about treatment duration, but its efficacy and safety have been confirmed during a 3-year period14. Even though the mean time for use of biphosphonates in this study has been slightly over two years, patients with over four years of treatment duration were also included.
The limited mobility of the majority of the patients in the study leads them to present BMD reduction, as well as an alteration in the trabecular microarchitecture, particularly at vertebral level15. However, other factors can have an impact on the pathogenesis of osteoporosis in these patients, such as limited exposure to sunlight, a diet with protein restriction (such as the case of the patient with encephalopathy due to citrullinemia), a reduction in the intake of dairy products (and therefore, of calcium), and the use of other associated treatments, such as glucocorticoids4,6. The latter are used in Duchen-ne Muscular Dystrophy in order to improve muscular strength at short-term, though they inhibit osteoblasto-genesis, they stimulate osteoclastogenesis, and cause a negative balance of calcium in the body16. Deflazacort causes a lower loss of bone mass than prednisone or methylpredinisolone, and therefore it is the glucocorti-coid more widely used in these patients4.
Although intravenous pamidronate is the most widely used biphosphonate for paediatric patients, particularly for osteogenesis imperfecta, a similar increase in BMD has been described with AA5. Given that AA is an oral treatment, no hospitalization is required, and this can lead to an improvement in the quality of life of these patients, higher self-sufficiency, and a more efficient use of healthcare resources1,5.
All patients demonstrated treatment compliance, and no adverse effects caused by AA were detected, either of tolerability or alterations in the phosphate & calcium metabolism. However, due to the study methodology (retrospective, with clinical record information and treatment compliance measured though a single method), it is possible that these data might be underestimated, and some cases of lack of compliance and/or mild adverse effects might have gone undetected6.
The study outcomes cannot be extended to all pae-diatric ages, because no < 7-year-old patients were included, and due to the small sample size. It would be necessary to design clinical trials with a higher number of patients, in order to learn about the real efficacy and safety of this drug in paediatric patients, as well as the adequate moment to initiate and interrupt treatment.
As a conclusion, AA increased BMD and was well tolerated in all patients; therefore, it could be considered as a therapeutic option for treatment of paediatric osteoporosis.