this study analyzes the medication errors reported to a pharmacovigilance system by 26 hospitals for patients in the healthcare system of Colombia.
Methodsthis retrospective study analyzed the medication errors reported to a systematized database between 1 January 2008 and 12 September 2013. The medication is dispensed by the company Audifarma S.A. to hospitals and clinics around Colombia. Data were classified according to the taxonomy of the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP). The data analysis was performed using SPSS 22.0 for Windows, considering p-values < 0.05 significant.
Resultsthere were 9 062 medication errors in 45 hospital pharmacies. Real errors accounted for 51.9% (n = 4 707), of which 12.0% (n = 567) reached the patient (Categories C to I) and caused harm (Categories E to I) to 17 subjects (0.36%). The main process involved in errors that occurred (categories B to I) was prescription (n = 1 758, 37.3%), followed by dispensation (n = 1 737, 36.9%), transcription (n = 970, 20.6%) and administration (n = 242, 5.1%). The errors in the administration process were 45.2 times more likely to reach the patient (CI 95%: 20.2-100.9).
Conclusionsmedication error reporting systems and prevention strategies should be widespread in hospital settings, prioritizing efforts to address the administration process.
analizar los errores de medicación reportados en un sistema de farmacovigilancia en 26 hospitales para pacientes del sistema de salud de Colombia.
Métodosestudio retrospectivo que evaluó las bases de datos sistematizadas de reportes de errores de medicación entre el 1 de enero de 2008 y el 12 de septiembre de 2013 de los me-dicamentos dispensados por la empresa Audifarma S.A a hos-pitales de Colombia. Se utilizó la clasificación taxonómica del National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP). El anâlisis de los datos se realizó mediante SPSS 22.0 para Windows Se determinó como nivel de significación estadística una p < 0,05.
Resultadosse reportaron 9.062 EM en 45 servicios farmacéu-ticos hospitalarios. El 51,9% (n = 4.707) de los errores realmen-te se produjeron, de los cuales el 12,0% (n = 567) afectaron al paciente (categorías C a I) y causaron dano (categorías E a I) a 17 (0,36%). El proceso implicado en los EM ocurridos (catego-rías B a I) con mayor frecuencia fue la prescripción (n = 1.758, 37,3%), seguido por la dispensación (n = 1.737, 36,9%), la transcripción (n = 970, 20,6%) y, por ultimo, la administración (n = 242, 5,1%). Los errores relacionados con los procesos de administración aumentaban 45,2 veces el riesgo de que el me-dicamento erróneo afectara al paciente (IC 95% 20,2-100,9).
Conclusioneses necesario aumentar la cobertura de los siste-mas de reporte de errores de medicación, y crear estrategias para su prevención, especialmente en la etapa de administra-ción del medicamento.
The knowledge about medication errors in South America is quite limited, so this research provides updated information of many hospitals and clinics in Colombia with the determination of the most frequent medication errors identified. It also provides valuable information on the variables associated with the risk that the error occurs.
IntroductionA medication error (ME) is any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the healthcare professional, patient, or consumer. Such events may be related to professional practice, healthcare products, procedures, and systems. They include issues with the following: prescribing; order communication; product labeling, packaging, and nomenclature; compounding; dispensing; distribution; administration; education; monitoring; use1.
Although ME prevalence is unknown, such errors are considered to be the most frequently occurring mistakes in the hospital environment and consequently pose an important threat to patient security with the potential to cause mild to fatal adverse drug reactions (ADRs)2–5. Numerous methods have been proposed for ME detection and reporting, including self-report, review of charts, statistical review, direct observation, and systems for active detection6–8. The latter have been proven to detect the highest rates of ME and are the preferred method, especially for administration errors, but the requirements are high in terms of personnel and monetary expenditure9.
Between 1999 and 2001, 154,816 spontaneous reports of ME in 403 hospitals were registered with the MEDMARX system in the United States (US). Errors that occurred (categories B to I) accounted for 91.3% of the total, 64% reached the patient and 3% caused harm (5). In Spain, at least one ME was registered in 43.0% of patients admitted to an intensive care unit; most of them were mild in severity - 83.0% in categories A and B - and related to the prescription (34.0%) and administration (28.0%) process10.
Since 2001, the National Program of Pharmacovigilan-ce of Colombia has been in charge of the identification, evaluation and prevention of ADRs and negative outcomes associated with medication (NOM)11. However this national organization lacks programs for ME measurement and assessment.
The underestimation of ME puts Colombians in great danger; a study conducted at a primary care hospital in an medium-sized city of Colombia (Pereira) in 2012 found that there were difficulties with the interpretation of 47.9% of prescriptions and all had at least one ME, the most common being the failure to record the duration of the formulation12. Consequently, this study aimed to determine, classify and establish pharmacological variables related to ME reported to a pharmacovigilance system by hospitals in the Colombian healthcare system.
MethodsA retrospective study was performed to process ME reports gathered in a systematized database of medication dispensed in hospital pharmacy settings by the company Audifarma S.A. between January 1, 2008 and September 12, 2013. The MEs were reported in a written form by the professional (physician, pharmacist, nurse) who detected them. Later, with the support of a pharmacoepidemiolo-gy physician, the reports were evaluated by the pharmacist who reviewed and properly registered each case in the database.
The variables considered were: 1) place of occurrence: city and pharmacy; 2) date of occurrence and report; 3) classification according to the taxonomy developed by the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) by error severity, type and cause; 4) contact between the error medication and the patient; 5) consumption of the drug by the patient 6) process involved (administration, dispensing, prescription and transcription); 7) drug ordered; 8) wrong drug; 9) moment of detection. Medicines were grouped according to the Anatomical Therapeutic Chemical (ATC) classification.
Information was gathered into a database in Excel 2010 and analyzed using IBM SPSS Statistics version 22.0 for Windows (IBM, EEUU). Variable comparison was conducted using X2 tests and logistic regression models were designed to assess the relationship between error characteristics and drugs reaching the patient. A p-value of < 0.05 was considered to be significant. The research was reviewed by the Bioethics Committee of the Univer-sidad Tecnológica of Pereira (Pereira, Colombia); it was approved as “research without risk” and guaranteed the anonymity of the patients, following the principles of the Declaration of Helsinki.
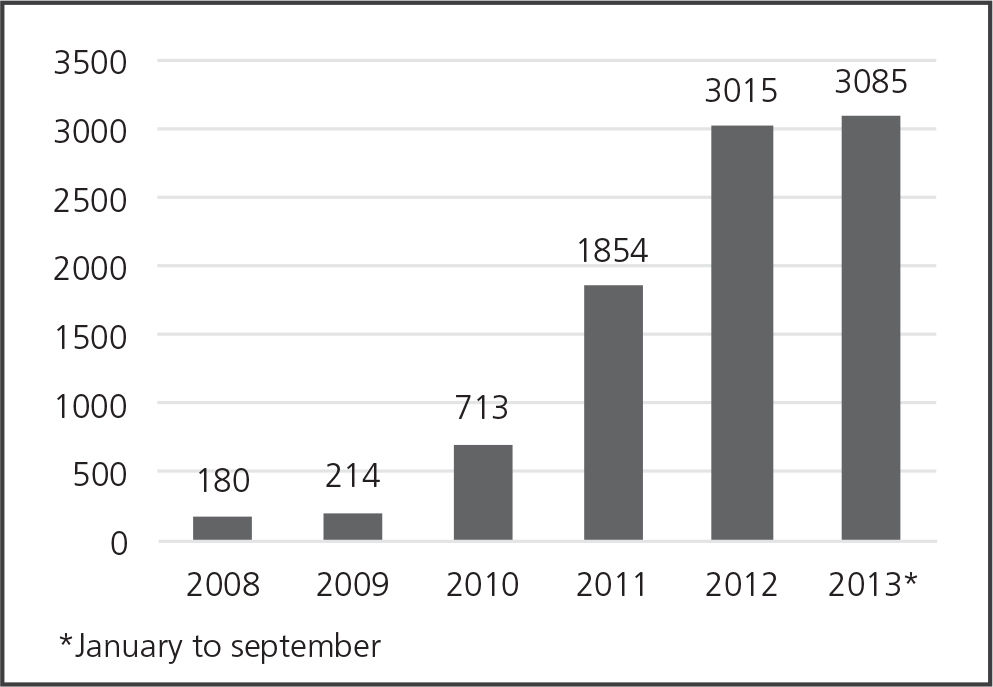
ResultsDuring the period of observation, 9062 MEs were reported in 45 pharmacy settings from 26 clinics and hospitals, located in 15 cities in 13 states of Colombia. Bogota reported the most errors (n = 3540, 39.1%), followed by Antioquia (n = 1698, 18.8%), Valle del Cauca (n = 1476, 16.3%), Cundinamarca (n = 515, 5.7%), and Cauca (n = 466, 5.1%). Errors were reported on the day of occurrence in 21.6% of cases (n = 1955) and 50.0% (n = 4531) between days 1 and 10; the average time between the occurrence of an ME and reporting was 9.6 days (DE: 18.4; range: 0-408 days). The number of reports of ME has been growing for years as can be seen in Figure 1.
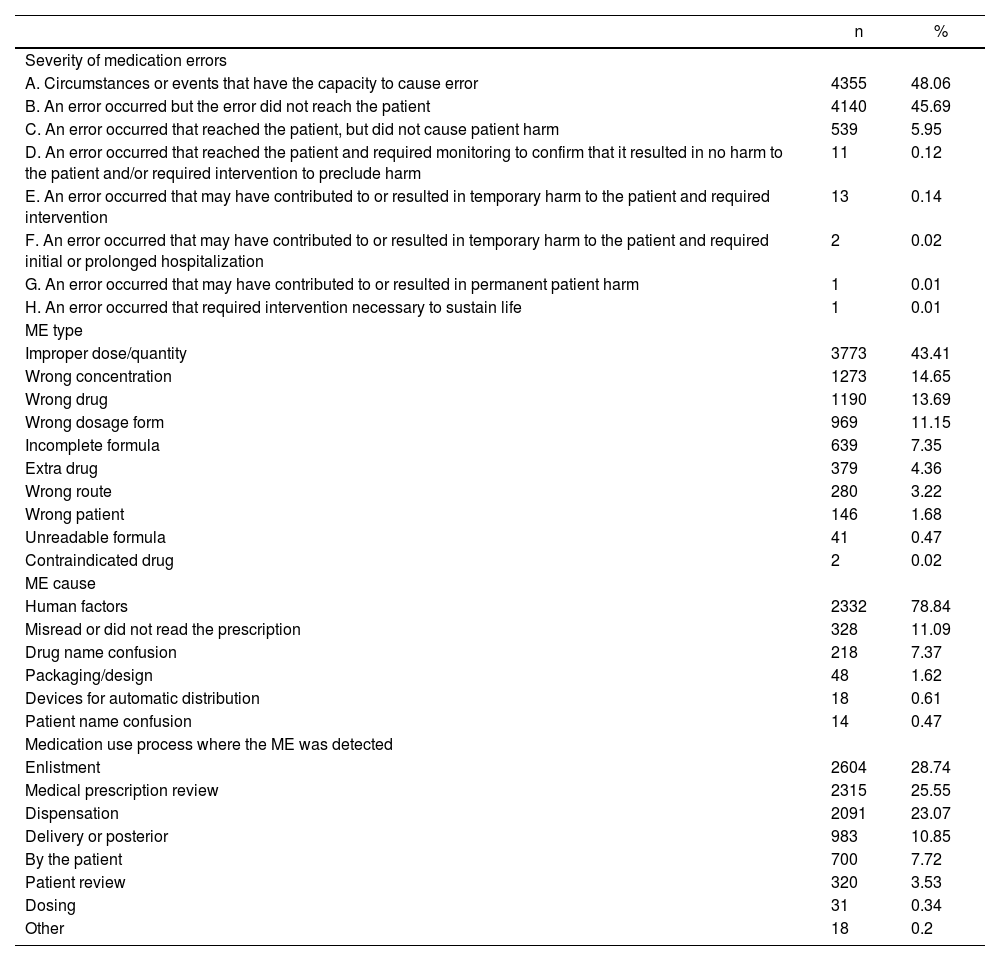
Between September 2012 and August 2013, an average of 1.61 errors per bed/year (DE: 2.35, range: 0.08-9.52) was reported for 2721 beds in the 26 hospitals included in the study. The characteristics of the errors reported are shown in table 1.
Characteristics of medication errors from hospital settings, Colombia 2008-2013
| n | % | |
|---|---|---|
| Severity of medication errors | ||
| A. Circumstances or events that have the capacity to cause error | 4355 | 48.06 |
| B. An error occurred but the error did not reach the patient | 4140 | 45.69 |
| C. An error occurred that reached the patient, but did not cause patient harm | 539 | 5.95 |
| D. An error occurred that reached the patient and required monitoring to confirm that it resulted in no harm to the patient and/or required intervention to preclude harm | 11 | 0.12 |
| E. An error occurred that may have contributed to or resulted in temporary harm to the patient and required intervention | 13 | 0.14 |
| F. An error occurred that may have contributed to or resulted in temporary harm to the patient and required initial or prolonged hospitalization | 2 | 0.02 |
| G. An error occurred that may have contributed to or resulted in permanent patient harm | 1 | 0.01 |
| H. An error occurred that required intervention necessary to sustain life | 1 | 0.01 |
| ME type | ||
| Improper dose/quantity | 3773 | 43.41 |
| Wrong concentration | 1273 | 14.65 |
| Wrong drug | 1190 | 13.69 |
| Wrong dosage form | 969 | 11.15 |
| Incomplete formula | 639 | 7.35 |
| Extra drug | 379 | 4.36 |
| Wrong route | 280 | 3.22 |
| Wrong patient | 146 | 1.68 |
| Unreadable formula | 41 | 0.47 |
| Contraindicated drug | 2 | 0.02 |
| ME cause | ||
| Human factors | 2332 | 78.84 |
| Misread or did not read the prescription | 328 | 11.09 |
| Drug name confusion | 218 | 7.37 |
| Packaging/design | 48 | 1.62 |
| Devices for automatic distribution | 18 | 0.61 |
| Patient name confusion | 14 | 0.47 |
| Medication use process where the ME was detected | ||
| Enlistment | 2604 | 28.74 |
| Medical prescription review | 2315 | 25.55 |
| Dispensation | 2091 | 23.07 |
| Delivery or posterior | 983 | 10.85 |
| By the patient | 700 | 7.72 |
| Patient review | 320 | 3.53 |
| Dosing | 31 | 0.34 |
| Other | 18 | 0.2 |
ME: medication error
Severity categories A, B and C (Table 1) comprised 99.7% of errors (n = 9034). “Real” errors (In NCC MERP terms) occurred in 51.9% (n = 4707) of cases (Categories B-I), of which 12.0% (n = 567) reached the patient (Categories C-I). The medication was consumed by 22.8% (n = 129) of patients who had contact with the incorrect drug, and it caused harm to 17 (0.36%) (Categories E-I), resulting in permanent damage in two cases (Categories G-I); one case could have resulted in death (Category I).
The medication use process most frequently involved in the occurrence of “real” errors was prescription (n = 1758, 37.3%), followed closely by dispensation (n = 1737, 36.9%), transcription (n = 970, 20.6%), and some way behind, administration (n = 242, 5.1%).
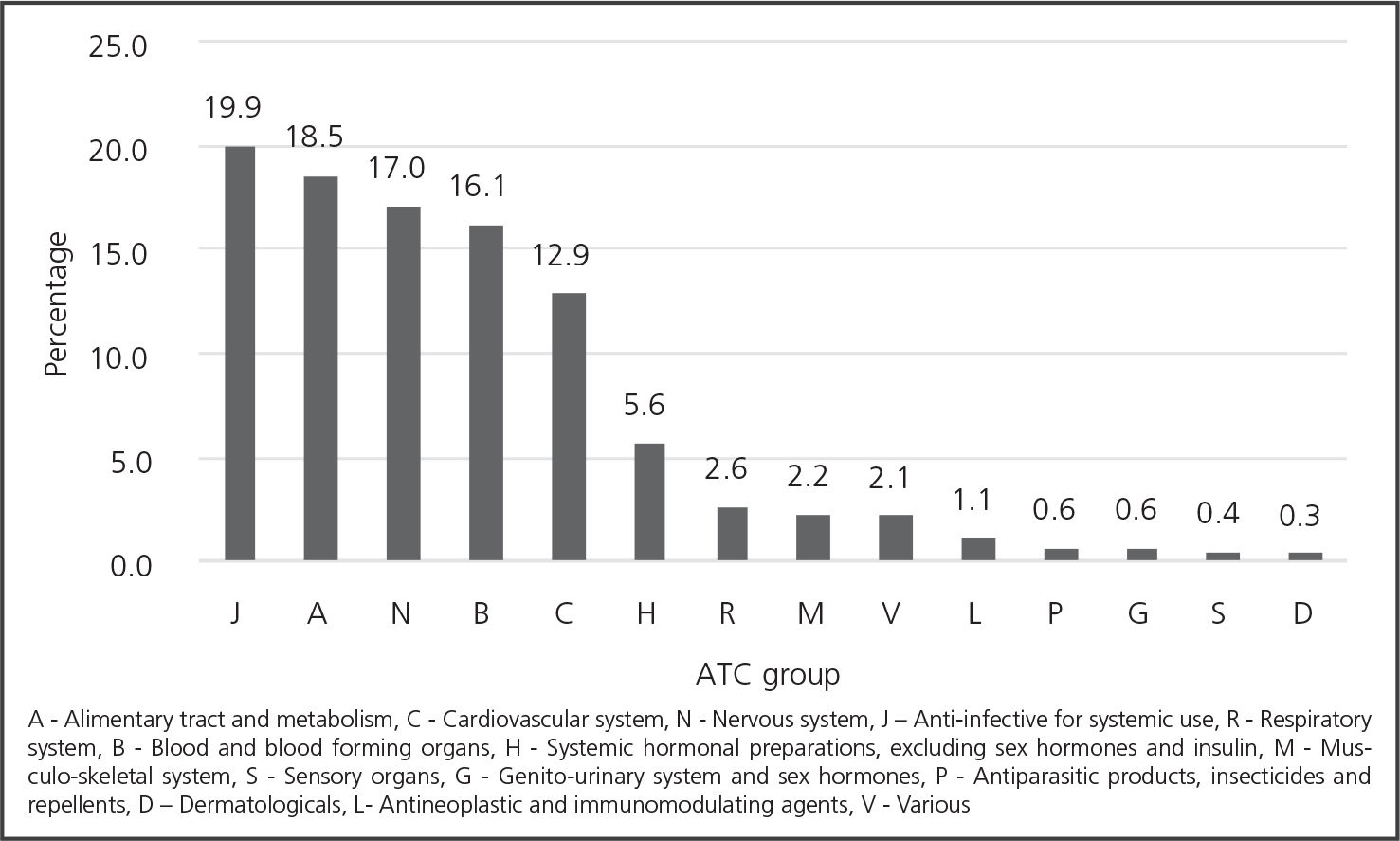
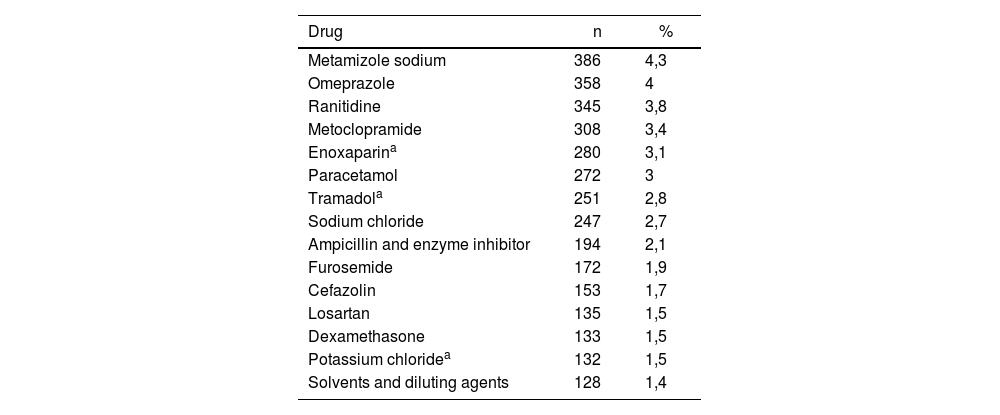
A total of 424 active ingredients were involved in 9058 reports; in four cases, the prescription formula was unreadable. The top medications reported are shown in table 2 and the ATC groups in figure 2. The medication selected was different to that originally ordered in 20.1% of cases (n = 1819), of which 76.1% (n = 1385) came from a different ATC subgroup, and 43.5% (n = 792) from another ATC group. The wrong medication reached the patients in 9.1% (n = 166) of these cases.
Most reported drugs related to hospital medication errors, Colombia 2005-2013
| Drug | n | % |
|---|---|---|
| Metamizole sodium | 386 | 4,3 |
| Omeprazole | 358 | 4 |
| Ranitidine | 345 | 3,8 |
| Metoclopramide | 308 | 3,4 |
| Enoxaparina | 280 | 3,1 |
| Paracetamol | 272 | 3 |
| Tramadola | 251 | 2,8 |
| Sodium chloride | 247 | 2,7 |
| Ampicillin and enzyme inhibitor | 194 | 2,1 |
| Furosemide | 172 | 1,9 |
| Cefazolin | 153 | 1,7 |
| Losartan | 135 | 1,5 |
| Dexamethasone | 133 | 1,5 |
| Potassium chloridea | 132 | 1,5 |
| Solvents and diluting agents | 128 | 1,4 |
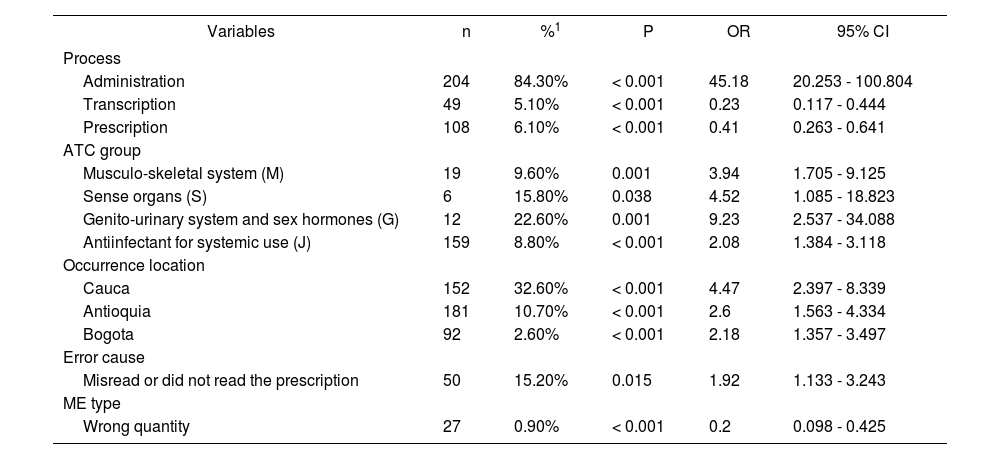
Multivariate analysis found that processes of administration, pharmaceutical groups related to the muscu-lo-skeletal system (M), sensory organs (S), genito-urinary system and sex hormones (G), and anti-infectives for systemic use (J), errors that occurred in Cauca, Antioquia and Bogota, and the incorrect interpretation of the prescription significantly increased the risk of error reaching the patient. In contrast, the processes of transcription and prescription, and issues with wrong quantities were found to decrease this risk (Table 3).
Multivariate analysis with variables associated significantly to occurrence of an error that reaches the patient, Colombia 2005-2013
| Variables | n | %1 | P | OR | 95% CI |
|---|---|---|---|---|---|
| Process | |||||
| Administration | 204 | 84.30% | < 0.001 | 45.18 | 20.253 - 100.804 |
| Transcription | 49 | 5.10% | < 0.001 | 0.23 | 0.117 - 0.444 |
| Prescription | 108 | 6.10% | < 0.001 | 0.41 | 0.263 - 0.641 |
| ATC group | |||||
| Musculo-skeletal system (M) | 19 | 9.60% | 0.001 | 3.94 | 1.705 - 9.125 |
| Sense organs (S) | 6 | 15.80% | 0.038 | 4.52 | 1.085 - 18.823 |
| Genito-urinary system and sex hormones (G) | 12 | 22.60% | 0.001 | 9.23 | 2.537 - 34.088 |
| Antiinfectant for systemic use (J) | 159 | 8.80% | < 0.001 | 2.08 | 1.384 - 3.118 |
| Occurrence location | |||||
| Cauca | 152 | 32.60% | < 0.001 | 4.47 | 2.397 - 8.339 |
| Antioquia | 181 | 10.70% | < 0.001 | 2.6 | 1.563 - 4.334 |
| Bogota | 92 | 2.60% | < 0.001 | 2.18 | 1.357 - 3.497 |
| Error cause | |||||
| Misread or did not read the prescription | 50 | 15.20% | 0.015 | 1.92 | 1.133 - 3.243 |
| ME type | |||||
| Wrong quantity | 27 | 0.90% | < 0.001 | 0.2 | 0.098 - 0.425 |
ME: Medication error, OR: Odds Ratio, 95% CI: 95% confidence interval. Statistical method: lineal logistic regression.
This is the first approach in the study of ME in multiple hospitals in Colombia. Although most errors recorded were mild, they involved medications commonly used in medical practice and the risk of reaching the patient increased significantly during administration. The number of errors reported increased over the years due to the expansion of the number of institutions included in the program and the campaigns carried to increase the awareness of this problem in them. These results will determine the causes on which prevention efforts and ME detection should be focused.
Very few errors reached the patient, half of them (51.9%) were “real” errors (according to NCC MERP), and 17 (0.36%) of these caused damage. In contrast, the United States Pharmacopeia reported that 9.8% of errors were not “real” errors, while a similarly small number of them caused harm (3.0%)5. However, more research is required to clarify if these low rates may be related to fear of reporting severe errors.
Whereas insulin stood out in the US as the main medication group related to ME during three year follow-up, it did not appear among the first in this study. As the administration of this group of medications is usually dynamic through the hospital stay, it requires a different system for registration and delivery, which could mean that errors occur outside the ME prevention programs5. Furosemide, opioids, potassium chloride and heparins were included in both lists; the latter three are considered high-risk medications and should be prioritized in every ME prevention program, as argued by the Institute for Safe Medication Practices (ISMP)1,5. Possible strategies include the standardization of prescribing, storage, preparation, and administration, as well as the improvement of information systems through reducing access limitations, using additional labels, and reducing the use of redundancies during the process of administering these medications13.
Previous studies have estimated that administration and prescription MEs together represent three quarters of the total number of errors in the hospital environment, and patients are exposed to at least one of them for every day of hospital stay1. In comparison, administration errors only accounted for 5.1% of the total in our study, although they were found to be the highest risk factor for an error reaching the patient (OR: 45.2). A literature review found distractions, interruptions, and work overload to be the main causes of administration errors, and thus strategies aimed at improving the workplace environment should be included in prevention efforts14. Overall estimations of the prevalence of administration errors ranged from 14.3% to 70.0% in a literature review of ME reported in Iran. Administration errors were higher among nurse students (17.4-37.7%) than graduate nurses (7.7-27%)15; this is a factor that should be considered for further studies in Colombia, as this survey includes an unknown number of teaching hospitals.
Misinterpretation of prescriptions increased the risk of the drug reaching the patient. Interventions in the prescription habits of physicians and the inclusion of electronic prescribing software have an important part to play in the prevention of prescription and transcription errors16. However, the use of these systems by humans continues to trigger errors, as shown by a study carried out in Hong Kong between 2006 and 2010. In this study, 17.1% of MEs detected were related to technology, of which only 1.9% problems were device errors, whereas the rest (98.1%) were socio-technical errors17.
The low rate of errors per bed/year contrasts with estimations that have been made in previous ME incidence measurements: errors in all prescriptions were reported by a past study held in a primary care hospital in Colombia, while Barker et al. found that nearly one in every five doses administered in their study resulted in an error12,18. The extensive variations in ME incidence across different settings is determined by the wide range of methods for categorization and criteria for measurement in different studies, contributing to the lack of progress towards error prevention15,18.
The taxonomy established by NCC MERP divides errors into “real” errors and circumstances or events that have the capacity to cause error1. However the detection of conditions that increase the risk of contact between the patient and the error, and hence the risk of harm, provide new insights into the matter of ME prevention and the enhancement of patient safety.
In spite of the inclusion of 26 hospitals across the country, the findings of this study cannot be extrapolated to all Colombian hospital settings. Furthermore, it is limited by the use of a spontaneous report system, which may be reflected in the low number of errors registered that caused harm. However, this is the first large-scale approach in Colombia to an issue that is a priority for health authorities in countries such as the US, Australia, the UK and Canada due to the high welfare and economic costs them represent19.
Sources of fundingThis study received funding from the Universidad Tec-nológica de Pereira, Audifarma S.A.
Conflict of interest statementThe authors declare that they have no conflict of interest.