Inhaled therapy is essential in cystic fibrosis; however, inhalers have a significant environmental impact due to the greenhouse gases (GHGs) emitted. The environmental impact of a product is estimated by its carbon footprint (CF). Pressurized metered-dose inhalers (pMDIs) have a higher CF than dry powder inhalers (DPIs) and soft mist inhalers (SMIs) due to the incorporation of GHGs.
The objectives are to analyze the consumption of inhalers (β2-adrenergic agonist bronchodilators, anticholinergics, and/or corticosteroids) in a cystic fibrosis unit and estimate the generated CF.
MethodRetrospective determination (January 2018–December 2023) of consumption and CF (tCO2eq) by type of inhaler was conducted. Consumption and CF trends were evaluated using linear regression.
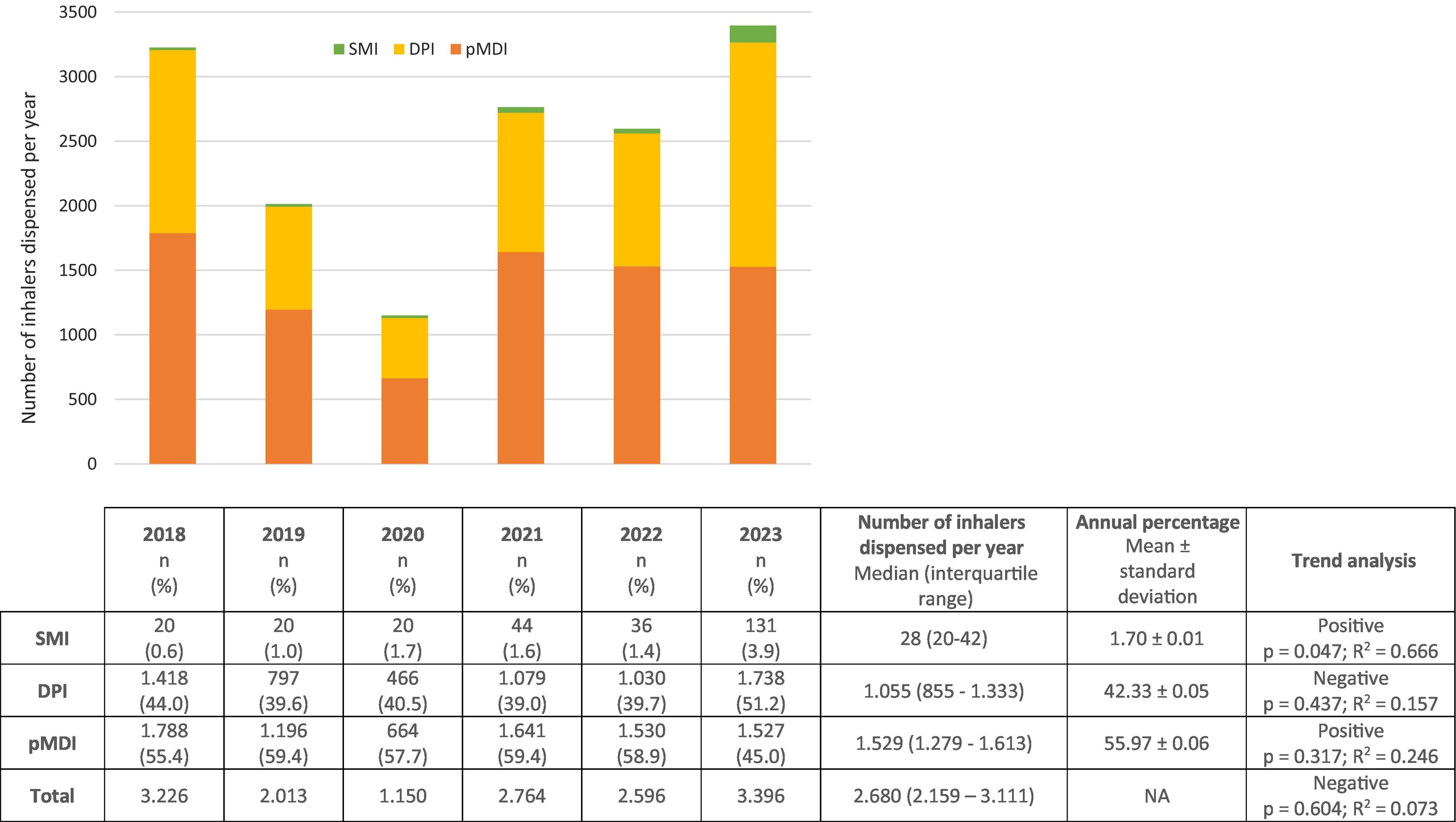
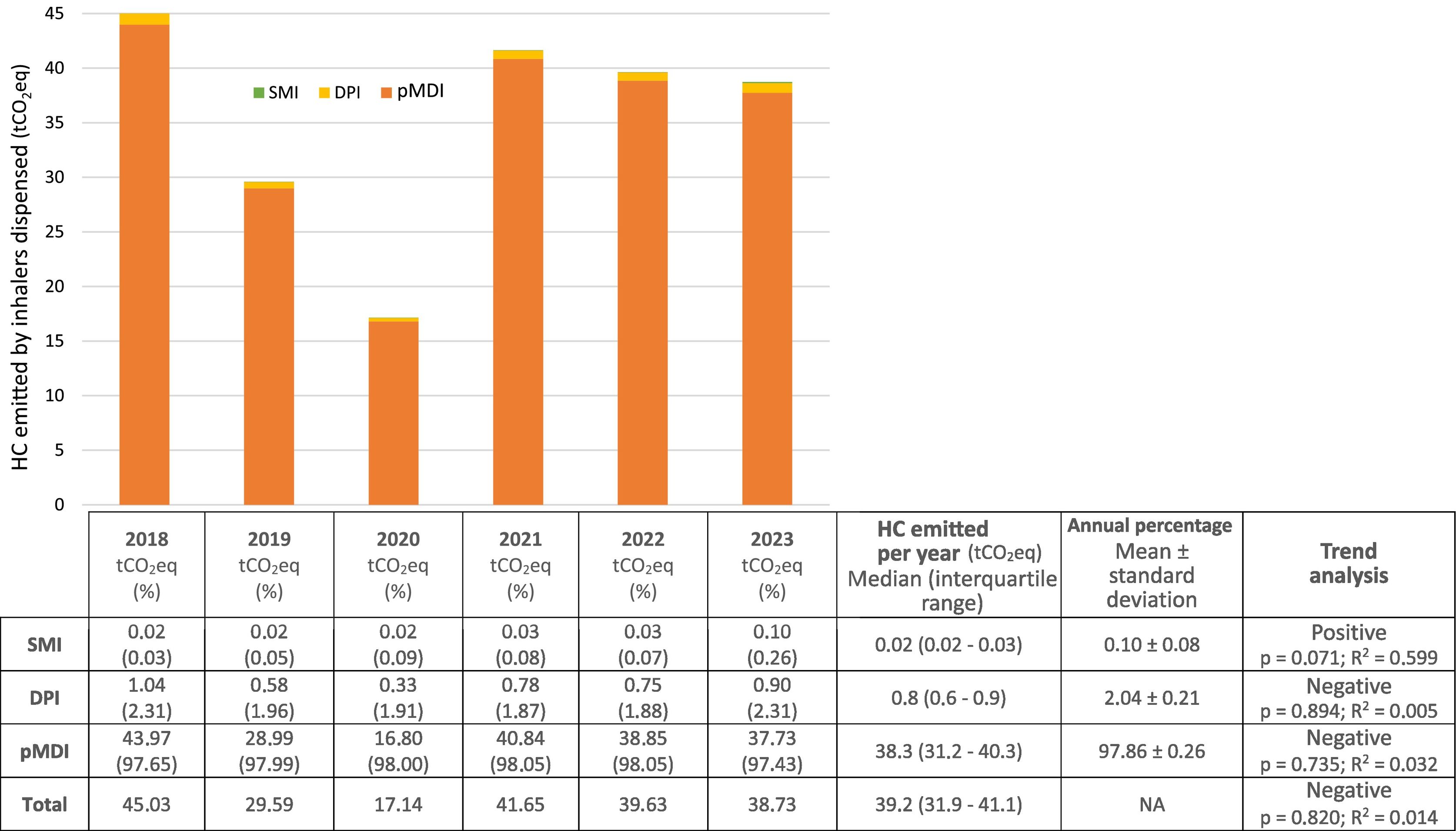
ResultsAnnually, 1.529 (1.279–1.613) pMDIs, 1.055 (855–1.333) DPIs, and 28 (20–42) SMIs were dispensed, representing 55.97%, 42.33%, and 1.70%, respectively. A statistically significant positive trend in the consumption of SMIs was observed. The median annual CF was: pMDIs 38.3 (31.2–40.3) tCO2eq, DPIs 0.8 (0.6–0.9) tCO2eq, and SMIs 0.02 (0.02–0.03) tCO2eq, representing 97.86%, 2.04%, and 0.10%, respectively.
ConclusionspMDIs were the inhalers with the highest consumption and CF, although their consumption appears to be decreasing, with an increase in the consumption of SMIs.
La terapia inhalada es esencial en fibrosis quística; sin embargo, los inhaladores presentan un impacto medioambiental significativo derivado de los gases de efecto invernadero (GEI) emitidos. El impacto medioambiental de un producto se estima mediante su huella de carbono (HC). Los inhaladores de cartucho presurizado (pMDIs) presentan mayor HC que los inhaladores de polvo seco (DPIs) y de nube de vapor suave (SMIs) al incorporar GEI.
Los objetivos son analizar el consumo de inhaladores (broncodilatadores agonistas β2-adrenérgicos, anticolinérgicos y/o corticoides) en una unidad de fibrosis quística y estimar la HC generada.
MétodoSe determinó retrospectivamente (enero 2018-diciembre 2023) el consumo y la HC (tCO2eq) por tipo de inhalador. Se evaluó la tendencia de consumo y HC mediante regresión lineal.
ResultadosSe dispensaron anualmente 1.529 (1.279–1.613) pMDIs, 1.055 (855–1.333) DPIs y 28 (20–42) SMIs, representando el 55,97%; 42,33%; y 1,70%, respectivamente. Se observó una tendencia positiva estadísticamente significativa del consumo de SMIs. La HC mediana anual fue: pMDIs 38,3 (31,2–40,3) tCO2eq, DPIs 0,8 (0,6–0,9) tCO2eq y SMIs 0,02 (0,02–0,03) tCO2eq, representando, respectivamente, el 97,86%, 2,04% y 0,10%.
ConclusionesLos pMDIs fueron los inhaladores con mayor consumo y HC, aunque parece que su consumo tiende a disminuir, incrementándose el de SMIs.
Cystic fibrosis is the most common autosomal-recessive rare disorder among the Caucasian population. Inhaled therapy is essential to treatment as respiratory complications are the leading cause of mortality.1,2 However, its effectiveness depends on factors such as inhalation technique and therapeutic adherence. Adherence is significantly low in cystic fibrosis due to the high complexity of the treatment regimen and its impact on daily life. It is therefore important to consider the patient's preferences when initiating therapy.1,2 The environmental impact of delivery devices is another drawback of this type of therapy.
The term “climate change” includes global warming caused by greenhouse gases (GHGs). The environmental impact of a product is measured by its carbon footprint (CFP), defined as the total amount of GHGs emitted during its life cycle (Fig. 1). This value uses CO2 as a reference, with the most common unit of measurement being ton of CO2-equivalent (tCO2eq), which indicates its GWP.3,4
There are three types of inhalers: pressurized metered-dose inhalers (pMDIs), dry powder inhalers (DPIs), and soft mist inhalers (SMIs).4 In pMDIs, the gases used for drug release are hydrofluorocarbons. These are greenhouse gases with a high global warming potential and therefore a high CFP.4,5 Although pMDIs contribute less than 0.1% of global emissions, a growing number of initiatives aim to reduce their use.4,5 In 2019, pMDIs accounted for more than 90% of emissions from inhaled asthma and COPD devices across 5 European countries, with short-acting β2-adrenergic agonist (SABA) inhalers being the most widely used.4 The Spanish Agency of Medicines and Health Products (AEMPS) reported that 52% of the inhalers used were pMDIs.4,6
The objectives of this study were to analyze the consumption of inhalers in the cystic fibrosis unit (CFU) of the hospital pharmacy service of a Spanish reference centre and to evaluate the environmental impact of these inhalers by calculating their CFP.
MethodsRetrospective study was conducted between January 2018 and December 2023 in the CFU of a hospital pharmacy service of a Spanish cystic fibrosis referral tertiary hospital. We included all patients on inhaled treatment with β2-adrenergic agonist bronchodilators, anticholinergics, and/or corticosteroids. The study was approved by the relevant Clinical Research Ethics Committee.
Inhaler consumptionInhaler consumption was determined based on the number of prescriptions recorded in the electronic prescription program (Silicon, GRIFOLS, Spain). They were classified by group for analysis, and we identified the most dispensed medications for each group.
Carbon footprint estimationThe CFP of each medication and type of device was calculated using measured consumption rates and reference values for CFPs published by the National Health Service of the United Kingdom (supplementary material).7
Results are expressed as absolute value, percentage, mean ± standard deviation, or median (interquartile range) by category and the distribution of values. Trends in consumption and CFP were analyzed by linear regression using Stata v16 (StataCorp LLC, College Station, TX).
ResultsWe included 395 patients (253 ± 21 patients/year) treated with β2-adrenergic agonists, anticholinergics, and/or inhaled corticosteroids (31 medications), representing 66.5% (±4.4%) of the total number of patients in the cystic fibrosis unit.
Inhaler consumptionFig. 2 shows the annual and total consumption by inhaler type and the statistical results. Notable pMDIs were salbutamol Aldo-Union EFG, 100 μg/inh (41.29 ± 0.05%) and Atroaldo (ipratropium), 20 μg/inh (7.91 ± 0.02%). Among the DPIs, we highlight Relvar Ellipta (fluticasone/vilanterol), 92/22 μg/inh (17.32 ± 0.02%), Symbicort Turbuhaler (budesonide/formoterol), 160/4.5 μg/inh (8.80 ± 0.08%), and Spiriva (tiotropium), 18 μg/inh (6.15 ± 0.03%).
Carbon footprint estimationFig. 3 shows the annual CFP for each inhaler type, the total for all inhalers combined, and the statistical results. The pMDIs with higher CFPs were salbutamol Aldo-Union EFG 100, μg/inh (32.1 [25.9–33.3] tCO2eq, 83.82 ± 0.02%) and Atroaldo (ipratropium), 20 μg/inh (3.2 [2.1–3.7] tCO2eq, 8.35 ± 0.02%). Among the DPIs, we highlight Relvar Ellipta (fluticasone/vilanterol), 92/22 μg/inh (0.37 [0.29–0.39] tCO2eq, 45.46 ± 0.05%), Symbicort Turbuhaler (budesonide/formoterol), 160/4.5 μg/inh (0.13 [0.08–0.14] tCO2eq, 16.76 ± 0.08%), and Spiriva (tiotropium), 18 μg/inh (0.04 [0.03–0.05] tCO2eq; 8.92 ± 0.09%).
DiscussionAnesthetic gases and pMDIs account for 5% of emissions from healthcare systems, which in turn account for 5% of national emissions. There is growing interest in the environmental impact of inhalers. Although pMDIs are responsible for a low percentage of emissions, initiatives to reduce their CFP are being developed.4,5
Inhaled therapies are essential in the management of cystic fibrosis, as evidenced by the high percentage of patients on inhaled therapy in this study.1,2 Given the long-term nature of cystic fibrosis treatment, we believe that this population has great potential for intervention.
Our results are consistent with those of the AEMPS report: pMDIs accounted for more than 50% of devices used.6 However, a lower consumption of pMDIs was observed in 2023, along with a statistically significant increasing trend in the consumption of SMIs. The overall lower consumption in 2020 is explained by the SARS-CoV-2 pandemic: confinement and protective measures led to the consumption of accumulated medicines at home and a decrease in other respiratory infections, which reduced the need for treatment.
In 2020 in Spain, the CFPs of pMDIs, DPIs, and SMIs accounted for 0.0909%, 0.0056%, and 0.0005% of total emissions, respectively.8 Likewise, the results of our study show that pMDIs have a larger CFP. They are also consistent with the results of Pernigotti et al.,4 with pMDIs accounting for 98% of the CFP of the cystic fibrosis unit, with SABAs being the most significant contributors. Several scientific organizations and societies have recommended that priority be given to replacing pMDIs with DPIs or SMIs, as this changeover could reduce CFPs by 97%. However, they warn that inappropriate changes in devices could lead to negative clinical impacts and low adherence. Therefore, efficacy, safety, and patient preference should be the priority factors when selecting inhalers.6,8–10 We observed that the CFPs of pMDIs and DPIs, as well as the total CFP of the cystic fibrosis units were decreasing, whereas the CFP of SMIs was increasing. Although none of the results were statistically significant, the trend for SMIs could become statistically significant if it maintains its current trajectory.
Lack of adherence to inhaled cystic fibrosis treatment is one of the main limitations to its effectiveness. The therapeutic complexity, the long-term nature of the disease and its treatment, and the time and effort to administer them, all have a significant impact on patients' lives. It is essential to adapt the devices to the patients' lifestyles to achieve adequate clinical control and correct adherence.4
Furthermore, inadequate adherence leads to the use of rescue treatments. Although guidelines highlight the risks associated with the excessive use of SABAs during exacerbations, in clinical practice, they are overused, particularly salbutamol in pMDIs. It has been estimated that, over its life cycle, one salbutamol pMDIs has a CFP comparable to driving a car 300 km (28 kgCO2eq).11 The use of rescue salbutamol in Spain, Italy, France, Germany, and the UK produces 1,791,312 tCO2eq per year.5 Proper administration, therapeutic optimization, and the use of correct SABAs would significantly reduce emissions.4
Another strategic issue is waste management. The AIRE study found that less than 50% of patients recycle devices at the designated collection point of the integrated packaging management and collection system (SIGRE),12 reflecting the need to raise patient awareness of the importance of correct waste management and reducing its production.6
Pernigotti et al. conducted a predictive study modeling the expected outcomes of applying different sustainable measures over a 10-year period.4 Replacing pMDIs with DPIs/SMIs would reduce annual emissions by 68–84% and 64–71%, respectively; the use of hydrofluorocarbons with lower global warming potential by 78–89%; and therapeutic optimization by 17–48%.
After evaluating the results, and taking into account the essential use of inhaled therapy in cystic fibrosis, the hospital pharmacy service proposed potential strategic measures to be developed in the CFU, which will be presented to the cystic fibrosis specialists to establish a work plan (Table 1). Treatment changes will be made by consensus between the medical team, pharmacists, and patients, thereby ensuring efficacy, safety, and adherence. According to Insalud Circular 8/91, April 23, 1991, since hospital pharmacy services are responsible for dispensing treatments to cystic fibrosis patients, close follow-up and assessment of the results after implementation will be possible.
Potential strategic measures proposed to reduce the environmental impact of inhaled therapies in the cystic fibrosis unit.
| Strategic measures to reduce the environmental impact of inhaled therapies in the cystic fibrosis unit |
|
|
|
|
|
|
|
|
|
DPIs, dry powder inhalers; GWP, global warming potential; pMDIs, pressurized metered-dose inhalers; SABA, short-acting β2-adrenergic agonists; SMIs, soft mist inhalers.
The main limitation of this study is its retrospective nature, which could have led to errors in data collection. Furthermore, the consumption data are based on the devices dispensed from the CFU, which may not coincide with the devices actually administered.
We found that pMDIs were the most commonly used inhalation devices and generated the greatest CFP, with SABAs being the most significant contributors. We also observed a decreasing trend in the consumption of pMDIs, and an increasing trend in the consumption of SMIs. The results highlight the need to develop sustainable strategies to reduce the environmental impact of inhalers.
Contribution to the scientific literatureCurrently, inhalers have a significant environmental impact due to the emission of greenhouse gases, which contribute to global warming. However, inhaled treatments play a fundamental and essential role in the population with cystic fibrosis. Therefore, they represent a target population with a high intervention potential for the implementation of strategic measures aimed at reducing the environmental impact of delivery devices. This is the first published study to assess the environmental impact of inhaled therapies used by cystic fibrosis patients in Spain and provides a starting point for the development of sustainable strategies in the cystic fibrosis unit.
FundingNone declared.
Liability and ceding of rightsAll authors accept the responsibilities defined by the International Committee of Medical Journal Editors (available at: http://www.icmje.org/). In the event of publication, the authors grant exclusive rights of reproduction, distribution, translation, and public communication (by any means or medium, whether sound, audiovisual, or electronic support) of their work to Farmacia Hospitalaria and, by extension, to the SEFH.
CRediT authorship contribution statementLaura Gómez-Ganda: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Formal analysis, Data curation, Conceptualization. Beatriz García-Palop: Writing – review & editing, Writing – original draft, Supervision, Data curation, Conceptualization. Arnau Mariscal-Puig: Validation, Methodology, Investigation, Formal analysis, Data curation. Alejandro Ábalos-Camacho: Validation, Methodology, Data curation, Conceptualization. Aurora Fernández-Polo: Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.