To determine the prevalence of loss-of-function variants in the dihydropyrimidine dehydrogenase gene in patients with gastrointestinal neoplasms, assess their clinical relevance, and evaluate the implementation of a multidisciplinary circuit at three months from its implementation.
MethodThis is a descriptive, observational and retrospective study, which included adult patients with gastrointestinal cancer treated at a tertiary university hospital who underwent dihydropyrimidine dehydrogenase genotyping between September 2019 and December 2020. The variables collected were sex, age, type of cancer, location, stage, treatment received, indication of treatment and degree of toxicity developed during the first three cycles. The genotyped variants were rs3918290 (c.1905+1G>A), rs55886062 (c.1679T>G), rs67376798 (c.2846A>T) and rs75017182 (c.1129-5923C>G).
ResultsA total of 115 patients were included. The frequency of heterozygous dihydropyrimidine dehydrogenase variant carriers was 9.6% (11 patients). The most frequently identified variant was rs75017182 (6 patients). The second most common variant was rs67376798 (3 patients), followed by rs3918290 (2 patients). No patients presented with the rs55886062 variant. Two of the dihydropyrimidine dehydrogenase carriers developed grade 3-5 toxicity after the first cycle of a regimen that included fluoropyrimidines. Both received full doses of fluoropyrimidine, since their dihydropyrimidine dehydrogenase genotype was unknown before treatment initiation. None of the dihydropyrimidine dehydrogenase carriers who began treatment with a reduced dose of fluoropyrimidine experienced grade 3-5 toxicity. Since the creation in October 2020 of a multidisciplinary team, with the active participation of hospital pharmacists, the monthly average of dihydropyrimidine dehydrogenase genotyping studies has increased from 6.4 (January-October) to 17.5 (November-December).
ConclusionsThe present study shows a relatively high prevalence of loss-of-function variants in the dihydropyrimidine dehydrogenase gene as well as the importance of genotyping such variants before starting a treatment with fluoropyrimidines. Hospital pharmacists can contribute to the implementation of pharmacogenetics in daily clinical practice in a tertiary hospital.
Determinar la prevalência de variantes de pérdida de función en el gen de la dihidropirimidina deshidrogenasa [DPYD] en pacientes con tumores digestivos, valorar su relevancia clínica y evaluar la implementación de un circuito multidisciplinar tras tres meses de funcionamiento.
MétodoEstudio descriptivo, observacional y retrospectivo donde se incluyeron los pacientes adultos afectos de tumores digestivos, atendidos en un hospital universitario de tercer nivel, a los que se había efectuado el genotipado de DPYD entre septiembre de 2019 y diciembre de 2020. Las variables recogidas fueron sexo, edad, tipo de cáncer, localización, estadio, tratamiento recibido, indicación del tratamiento y grado de toxicidad desarrollado durante los tres primeros ciclos. Se genotiparon las variantes rs3918290 (c.1905+1G>A), rs55886062 (c.1679T>G), rs67376798 (c.2846A>T) y rs75017182 (c.1129-5923C>G).
ResultadosSe incluyeron 115 pacientes. La frecuencia de portadores en heterocigosis de variantes del gen DPYD fue del 9,6% (11 pacientes). La variante más frecuentemente identificada fue el rs75017182 (6 pacientes). La segunda variante más frecuente fue el rs67376798 (3 pacientes), seguida del rs3918290 (2 pacientes). Ningún paciente presentó la variante rs55886062. Dos de los pacientes portadores desarrollaron toxicidad grados 3-5 tras el primer ciclo de un esquema que incluía fluoropirimidinas. Ambos recibieron dosis plenas de fluoropirimidina, puesto que no se conocía el genotipo de DPYD antes de iniciar el tratamiento. Ninguno de los pacientes portadores que empezó el tratamiento con una dosis reducida de fluoropirimidina experimentó toxicidad grados 3-5. Desde la creación en octubre de 2020 de un equipo multidisciplinar, con participación activa del farmacéutico hospitalario, se ha incrementado el número de estudios de genotipado de DPYD de una media de 6,4 estudios mensuales (enero-octubre) a 17,5 (noviembre-diciembre).
ConclusionesNuestro estudio muestra la relativamente elevada prevalencia de variantes de pérdida de función en el gen DPYD, así como la importancia de genotiparlas antes de empezar un esquema de tratamiento que contenga fluoropirimidinas. El farmacéutico hospitalario puede contribuir a la implementación de la farmacogenética en la práctica clínica diaria en un hospital de tercer nivel.
Digestive tumors, especially colorectal cancer, are associated with a high mortality rate throughout the world1. Fluoropyrimidines, particularly 5-fluorouracil and capecitabine, are part of the usual treatment for these types of tumors. Although generally well tolerated, a slight percentage of patients experience severe toxicity (grades 3-4) when treated with these antineoplastic agents2,3, and this has a very significant impact on their quality of life4. Since the preservation of an optimal quality of life is an aspect that patients diagnosed with cancer value very highly, the prevention of severe adverse effects in the context of their treatment is of great importance5.
Several studies have shown that patients with dihydropyrimidine dehydrogenase (DPD) deficiency are at greater risk of experiencing adverse reactions such as diarrhea, mucositis or neutropenia when treated with fluoropyrimidines6–8. A safety alert that was recently published by the Spanish Drug and Healthcare Product Agency (AEMPS) recommends genotype and/or phenotype DPD deficiency testing in candidates to be treated with these drugs9. Specifically, it recommends the genotyping of the most widely studied loss of function variants of the DPYD gene, which are rs3918290 (c.1905+1G>A, DPYD*2A), rs55886062 (c.1679T>G, DPYD*13), rs67376798 (c.2846A>T) and rs56038477 (c.1236G>A/HapB3). Reductions of 25-50% in the initial dose of fluoropyrimidines have already been recommended for patients who are carriers of these variants10–12.
The implementation of these biomarkers in clinical practice can undoubtedly contribute to the prevention of the above-mentioned severe toxicities, at minimal cost to the National Health System13–15. For a year now we have been determining the four variants of the DPYD gene, as part of our center's program of care, in patients who are candidates for treatment with fluoropyrimidines. Few data are as yet available on the prevalence of these variants in the Spanish population and on the clinical importance of genotypic testing in actual practice.
On the basis of the above, the aims of the present study were to determine the prevalence of loss of function variants in the DPYD gene in the population of our reference area and to evaluate the clinical results of genotyping these variants in the daily practice of a level 3 hospital. In addition, variations in the number of DPYD genotyping determinations were evaluated after creating a multidisciplinary team with the hospital pharmacist's active participation.
MethodsStudy populationThis is a descriptive, observational, retrospective and unicentric study carried out at a level 3 university hospital (Hospital de la Santa Creu i Sant Pau, Barcelona). It included all patients of 18 years of age and over who had been diagnosed with gastrointestinal tumors and genotyped for the DPYD gene since the implementation of testing, in September 2019, until December 2020. The study was approved by the center's Ethical Research Committee and informed consent was requested from all the recruited patients.
The clinical data of patients were drawn retrospectively from their electronic clinical histories. Variables included: sex, age at the time of treatment, type of cancer, site of the tumor, stage of the disease, treatment received, and evolution of the treatment and its associated grades of toxicity during the first three cycles of therapy. The registered toxicities were diarrhea, neutropenia, thrombocytopenia, asthenia, nausea/vomiting, mucositis and hand-foot syndrome, and were coded using version 5.0 of the National Cancer Institute's CTCAE (Common Terminology Criteria for Adverse Events).
GenotypingGenomic DNA was extracted from all the patients included in the study after drawing 10 mL blood samples into EDTA tubes using a QiaSymphony® unit (Qiagen, Hilden, Germany).
Variants rs3918290 (c.1905+1G>A), rs55886062 (c.1679T>G), rs67376798 (c.2846A>T) and rs75017182 (c.1129-5923C>G) were genotyped. This last variant was determined instead of rs56038477 (c.1236G>A). The former (rs75017182) is an intronic genetic variant that is in perfect linkage disequilibrium with variant rs56038477 (c.1236G>A) and is responsible for distorting DPYD enzyme functionality. When genotyping of gene DPYD was begun at our center, variants rs75017182 and rs56038477 were determined at the same time, but after confirming that the linkage disequilibrium between them was perfect the decision was made to genotype variant rs75017182 only. In addition to this, a search for controls had been performed in advance, using Sanger sequencing for confirmation purposes. Genotyping of these four variants was carried out using TaqMan® probes (Applied Biosystems, Foster City, CA, USA) and real time PCR with the Applied Biosystems® 7500 Fast Instrument (Applied Biosystems, Foster City, CA, USA).
It should be noted that the DPYD gene analysis was initially only performed on patients who experienced severe toxicity after fluoropyrimidine treatment. The testing is currently carried out on all patients who are candidates for these drugs, given the implications of fluoropyrimidine dosing regimens.
Statistical analysisStatistical analysis was performed using Excel software (Microsoft Office, Redmond, WA, USA, 2010). The continuous variables were expressed as mean and range. Qualitative variables were expressed as frequencies and percentages.
ResultsA total of 115 patients with digestive tumors were included in the study. Most of them (106 patients, 92% of the total number) had been diagnosed with colorectal cancer. One hundred and seven of the patients received fluoropyrimidine-based antineoplastic treatment. The most common drug regimens were FOLFOX (5-fluorouracil, folinic acid and oxaliplatin), XELOX (capecitabine and oxaliplatin) and capecitabine monotherapy. The basal characteristics of the patients included in this study are detailed in Table 1.
Baseline patient characteristics (n = 115)
| n | % | ||
|---|---|---|---|
| Sex | |||
| Males | 71 | 61.7% | |
| Females | 44 | 38.3% | |
| Age | |||
| Mean | 65.8 | ||
| Range | [34-86] | ||
| Location of the primary tumor | |||
| Right colon | 32 | 27.8% | |
| Left colon | 43 | 37.4% | |
| Rectum | 30 | 26.1% | |
| Anal canal | 2 | 1.7% | |
| Pancreas | 2 | 1.7% | |
| Stomach | 5 | 4.3% | |
| Duodenum | 1 | 0.9% | |
| Stage of the tumor | |||
| II | 27 | 23.5% | |
| III | 34 | 29.6% | |
| IV | 54 | 47.0% | |
| Chemotherapy regimen used | |||
| Capecitabine | 32 | 27.8% | |
| XELOX | 15 | 13.0% | |
| FOLFOX (± biological agent) | 50 | 43.5% | |
| FOLFIRI (± biological agent) | 7 | 6.1% | |
| FOLFIRINOX | 2 | 1.7% | |
| Mitomycin-capecitabine | 2 | 1.7% | |
| Trifluridine-tipiracil + bevacizumab (clinical trial) | 1 | 0.9% | |
| Irinotecan + cetuximab | 2 | 1.7% | |
| None | 4 | 3.5% | |
| Type of chemotherapy indicated | |||
| Neoadjuvant treatment | 17 | 14.8% | |
| Adjuvant treatment | 45 | 39.1% | |
| First-line metastatic chemotherapy | 44 | 38.3% | |
| Further metastatic lines | 3 | 2.6% | |
| Primary treatment of localized anal cancer | 2 | 1.7% | |
| No chemotherapy applied | 4 | 3.5% | |
FOLFIRI: combination of 5-fluorouracil, leucovorin and irinotecan; FOLFIRINOX: combination of 5-fluorouracil, leucovorin, irinotecan and oxaliplatin; FOLFOX: combination of 5-fluorouracil, leucovorin and oxaliplatin; XELOX: combination of capecitabine and oxaliplatin.
The four variants of the DPYD gene described above were genotyped in all the patients in the study. The most frequently identified variant was c.1129-5923C>G (rs75017182) (HapB3), which was found in 6 patients (5.2%). The second most frequent variant was c.2846A>T (rs67376798), identified in 3 patients (2.6%). Finally, the variant c.1905+1G>A (rs3918290) was detected in 2 patients (1.7%) (Table 2). The variants were found in heterozygosis in all cases. None of the genotyped patients carried the c.1679T>G (rs55886062) variant. Thus, the frequency of gene DPYD variant carriers in our cohort was 9.6% (11 patients in a total of 115).
Ten of the 11 patients with a partial DPD deficiency were treated with an antineoplastic protocol that included fluoropyrimidines. Five of them began the first cycle on a standard dose, since their DPYD genotype was not known when the treatment was initiated; in contrast, the DPYD genotype of another 5 patients had been ascertained before their first cycle, and these 5 began the treatment on doses of fluoropyrimidine that were reduced by 25-50% in accordance with each individual case (Table 3). Two of the 5 patients that were given standard doses experienced grade 3-5 toxicity during the first three cycles. One of these patients carried the c.1905+1G>A (rs3918290) variant and was treated with FOLFOX-panitumumab at standard doses as a first line treatment for a metastatic left-sided tumor of the colon. Since this patient developed grade 4 neutropenia during the first treatment cycle, the second cycle was delayed for 15 days and administered with a dose reduction of 20%. The other patient, who had a history of high blood pressure, digestive hemorrhage and anemia, carried the variant c.1129-5923C>G (rs75017182) (HapB3), and suffered a cardiorespiratory arrest 12 days after receiving the first cycle of FOLFOX at a standard dose as adjuvant treatment for a stage III right-sided tumor of the colon. None of the 5 patients treated with reduced doses of fluoropyrimidine from the first cycle developed grade 3-5 toxicity. In 3 of them, given their good tolerance to treatment, it was possible to increase the fluoropyrimidine until it reached 75-100% of the standard dose (Table 3).
Fluoropyrimidine dose administered and 3-5 grade toxicity developed by patients with partial DPYD gene activity
| DPYD genetic variant | Number of patients | Chemotherapy regimen administered during the first cycle | Grade 3-5 toxicity observed in the first three cycles |
|---|---|---|---|
| Standard dose of FOLFOX-panitumumab | No | ||
| Standard dose of FOLFOX | Toxic death following the 1st cycle (grade 5 toxicity) | ||
| c.1129-5923C>G (rs75017182) (HapB3) | Standard dose of FOLFOX-bevacizumab (1st cycle). Twenty-five percent reduction of 5-FU during the 2nd cycle following DPYD genotyping | No | |
| 6 | FOLFOX-cetuximab with 5-FU at 75% and a 5-FU bolus at 50% (1st cycle). During the third cycle the regimen consisted of a dose of 5-FU at 50% | No, but grade 2 mucositis in the 2nd cycle | |
| XELOX, consisting of capecitabine at 62.5% and oxaliplatin at 80%. As the treatment was well tolerated during the first two cycles, the dose of 5-FU was raised to 75% in the 3rd cycle | No | ||
| Standard dose of XELOX | No | ||
| FOLFOX at 80% without a 5-FU bolus | No | ||
| c.2846A>T p.D949V (rs67376798) | 3 | FOLFOX with 5-FU at 50% but no 5-FU bolus. As the treatment was well tolerated during the first cycle, the 5-FU dose was raised to 75% in the 2nd cycle. As tolerance was also satisfactory during the 2nd cycle, the 5-FU dose was raised to 100% in the 3rd cycle | No |
| FOLFOX with 5-FU at 50%. As the treatment was well tolerated during the first two cycles, the 5-FU dose was raised to 75% in the 3rd cycle | No | ||
| c.1905+1G>A (rs3918290), DPYD*2A | No fluoropyrimidines administered | − | |
| 2 | Standard dose of FOLFOX-panitumumab | Grade 4 neutropenia in the 1st cycle |
5-FU: 5-fluorouracil; DPYD: dihydropyrimidine dehydrogenase; FOLFOX: combination of 5-fluorouracil, leucovorin and oxaliplatin; XELOX: combination of capecitabine and oxaliplatin.
In October 2020, a multidisciplinary team was created in order to make sure that all patients who were candidates for antineoplastic protocols including fluoropyrimidines were genotyped for the DPYD gene and given doses that were adjusted on the basis of their test results. This team is made up of medical oncologists, hospital pharmacists specialized in oncology, clinical geneticists and laboratory technicians.
The team's flow of work is organized as follows:
- 1.
The oncologist requests DPYD genotyping for patients that are to be treated with fluoropyrimidines.
- 2.
Under the clinical geneticist's supervision, the laboratory technician performs the genetic study and tries to respond in the shortest possible period of time (a week at most). The test's result is entered into the antineoplastic agent prescription application. If the patient is a carrier of any of the loss-of-function variants the geneticist reports the result directly to the multidisciplinary team. In addition, the report with the genotyping results is entered into the patient's medical history. The hospital pharmacists draw up a specific clinical outline in the patient's clinical history, including details regarding the recommended dose adjustments, and the oncologist prescribes the chemotherapy regimen on the basis of such adjustments.
- 3.
At the time of validating the fluoropyrimidine-based treatment, the hospital pharmacist confirms that the DPYD gene analysis has been requested and performed. If this is the case, the pharmacist reviews the prescribed dose of fluoropyrimidine, to make sure it adjusts correctly to the genotypic profile. If the test has not been performed, the pharmacist will request it directly from the clinical geneticist and assess its results at the time of the next cycle of chemotherapy.
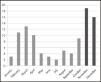
Following the introduction of the above working system, the average monthly number of DPYD analyses has increased from 6.4 (January to October) to 17.5 (November to December) (Figure 1).
DiscussionThe present study's results indicate that about 10% of patients with digestive tumors in our center are carriers of loss-of-function variants of the DPYD gene. This makes them partially DPD deficient and increases their risk of experiencing severe secondary toxicity when treated with fluoropyrimidines.
The percentage of individuals carrying these loss-of-function variants is slightly higher in our series than in other published studies, which report an incidence of 3-8% among the population11. This might be due to the fact that, in the beginning, genotyping of the DPYD gene was performed mainly on patients who had already experienced severe toxicity when treated with these drugs. Regarding the populational incidence of each of the four variants, the relatively high frequency of the rs75017182 variant stands out in our cohort. As previously explained, this variant is in perfect linkage disequilibrium with the rs56038477 variant, whose reported populational frequency is 2.6-6.3%10. Thus, although the frequency of 5.2% that was found for the rs56038477 variant in the present study comes within the range described in the literature for the Caucasian population, further studies of larger cohorts would be required in order to confirm its high prevalence in the Spanish population. On the other hand, our findings revealed a frequency of 2.6% for the variant rs67376798, which is higher than the allelic frequencies of 0.4-1.4% that have been reported for the general Caucasian population16. Finally, the frequency of 1.7% that was found for variant rs3918290 comes within the range described for Caucasians (0.8-2.2%)16. Unsurprisingly, variant rs55886062 was not found in our cohort; with a reported allelic frequency of 0.06-1.00% in the Caucasian population, this variant has the lowest rate of prevalence of the four variants that were analyzed in the study.
No consensus exists as yet in the scientific community regarding the ideal fluoropyrimidine dosing regimen for patients carrying variants of the DPYD gene. Henricks et al. initially recommended that patients with variants rs3918290 and rs55886062 began treatment with fluoropyrimidines at 50% below the standard dose, while carriers of the rs67376798 and rs75017182 variants did so at doses equaling 75% of the standard dose. However, more recent publications recommend a reduction of 50% in the dose of fluoropyrimidine in patients carrying any of the above-described variants of the DPYD gene16,17. In fact, a prospective study evaluating the safety of dose reduction recommendations determined that reductions of 25% in the case of variants rs67376798 and rs75017182 were not enough, and suggested a reduction of 50%, as subsequent publications have confirmed12.
Five of the patients in our study (four who carried variant rs75017182 and one who carried variant rs3918290) began antineoplastic treatment with a fluoropyrimidine regimen that included standard doses of the drug. Of these five patients, two experienced severe toxicity after the first cycle of chemotherapy, which led to the death of one of them. These results reveal the clinical importance of reducing fluoropyrimidine doses in patients carrying any variants of the DPYD gene. As regards the five carriers of DPYD gene variants in whom the first cycle of chemotherapy was begun at doses that were 25-50% below standard, no grade 3-5 toxicity was observed in any of them during the first three cycles of chemotherapy. These data, preliminary and based on a limited number of cases as they are, seem to support the safety of fluoropyrimidine use in patients carrying variants of the DPYD gene, provided the prescribed doses of the drug are adequately reduced. It should be added that tolerance to treatment, in three of these five patients, made it possible to increase the dose of fluoropyrimidine after the first cycle of chemotherapy, reaching 100% of the standard dose, with excellent levels of tolerance, in one case. This interindividual variability reveals the utility of pharmacokinetic monitoring of fluoropyrimidine during the first cycle of chemotherapy, with a view to adjusting the dosing regimen to optimal levels during the second cycle and beyond.
Our results support the clinical importance of DPYD genotyping in all patients that are to be treated with fluoropyrimidines, firstly because of the relatively high number of individuals who carry gene variants (about 10%), and secondly because of the latter's association with severe, and potentially lethal, toxicity. In this respect, we believe that the creation of a multidisciplinary team guarantees the translation of genotyping test results to the clinical setting. Our results, following the team's creation, are still quite preliminary, but they seem to suggest a higher rate of DPYD genotyping determinations.
The present study has several drawbacks. In the first place, the genotyping tests could not always be performed prior to treatment, as the clinical guidelines recommend, due to the limited amount of time available between DNA sample extractions and the beginning of antineoplastic treatment. This meant that, in five of the patients, treatment was administered at full doses in spite of the fact that they were carriers of DPYD gene variants. In this respect, it should be noted that genotypic test results are delivered in significantly shorter periods of time after the implementation of the multidisciplinary approach. Secondly, we must remember that in the case of therapeutic combinations, such as for instance the FOLFOX protocol, the added drugs also contribute to the occurrence of adverse effects. Thirdly, it was not possible for our study to include all patients diagnosed with digestive tumors in our center. Finally, the study's sample size is not large enough to allow for accurate determination of the prevalence of the different DPYD gene variants in our population, and further studies will be needed to validate these results.
In conclusion, the present study reveals a high prevalence of DPYD gene variant carriers in our population, as well as the clinical significance of these genotypic variants and their impact on the occurrence of adverse effects. It also demonstrates the feasibility of implementing a simple approach to DPYD gene genotyping, based on the collaboration of a multidisciplinary team made up of oncologists, hospital pharmacists and geneticists.
FundingNo funding.
Conflict of interestNo conflict of interests.
Contribution to the scientific literature
The present study provides information on the prevalence of the most common variants of the dihydropyrimidine dehydrogenase gene in the Spanish population as well as on the clinical usefulness of genotyping it in patients with digestive tumors.
Participation of hospital pharmacists in multidisciplinary teams favors the implementation of pharmacogenetics in clinical practice.